Highlights
► Rhinovirus is responsible for majority of common colds. ► There are no FDA approved drugs are available to treat rhinovirus infection. ► We show that quercetin, a plant flavonoid blocks viral replication in vitro. ► In addition quercetin inhibits virus-stimulated cytokine expression. ► Quercetin also inhibits viral replication and decreasing lung inflammation in vivo.
Keywords: Infection, Translation, Transcription, Inflammation
Abstract
Rhinovirus (RV), which is responsible for the majority of common colds, also causes exacerbations in patients with asthma and chronic obstructive pulmonary disease. So far, there are no drugs available for treatment of rhinovirus infection. We examined the effect of quercetin, a plant flavanol on RV infection in vitro and in vivo. Pretreatment of airway epithelial cells with quercetin decreased Akt phosphosphorylation, viral endocytosis and IL-8 responses. Addition of quercetin 6 h after RV infection (after viral endocytosis) reduced viral load, IL-8 and IFN responses in airway epithelial cells. This was associated with decreased levels of negative and positive strand viral RNA, and RV capsid protein, abrogation of RV-induced eIF4GI cleavage and increased phosphorylation of eIF2α. In mice infected with RV, quercetin treatment decreased viral replication as well as expression of chemokines and cytokines. Quercetin treatment also attenuated RV-induced airway cholinergic hyperresponsiveness. Together, our results suggest that quercetin inhibits RV endocytosis and replication in airway epithelial cells at multiple stages of the RV life cycle. Quercetin also decreases expression of pro-inflammatory cytokines and improves lung function in RV-infected mice. Based on these observations, further studies examining the potential benefits of quercetin in the prevention and treatment of RV infection are warranted.
1. Introduction
Rhinovirus (RV) is a single-stranded RNA virus from the Picornaviridae family which is responsible for the majority of upper respiratory tract infections (Makela et al., 1998, Winther, 2011). Although RV infections cause only mild disease and are self-limiting in normal individuals, they represent a significant economic burden, particularly in loss of working hours and missed school days (Proud, 2005). Accumulating evidence also suggests that RV is an important trigger of acute exacerbations in patients with chronic obstructive pulmonary disease (COPD) and asthma (Johnston et al., 1995, Seemungal et al., 2000, Seemungal et al., 2001). Experimental RV infection of asthma and COPD patients results in the development of lower respiratory disease, airflow obstruction, and systemic and airway inflammation which are greater and more prolonged than the similarly-infected control subjects (Mallia et al., 2006, Mallia et al., 2011, Message et al., 2008). The clinical outcomes of experimental RV infection correlate with viral load and inflammatory cytokines in sputum and bronchoalveolar lavage.
Current therapies for treating virus-related acute exacerbations of asthma and COPD are only partially effective and may also have side effects. Similarly, while currently available therapies may relieve symptoms, no FDA-approved drugs exist for the treatment of RV infections. Therefore, an antiviral drug which possesses anti-inflammatory activity and at the same time effectively inhibits viral replication may be beneficial in treating RV infections.
The RV life cycle offers many opportunities for intervention. The initial stage of the viral life cycle involves binding to a cell surface receptor, endocytosis, acidification of the endosome, viral uncoating, and release of viral RNA (vRNA) to the cytoplasm (Prchla et al., 1994). In the next stage, vRNA is translated as a single large polyprotein from an internal ribosomal entry site (IRES) which is located in the 5′ untranslated region of RV genome. The polyprotein is processed through sequential enzymatic cleavages by two virus-encoded proteinases, 2A and 3C proteinase (Foeger et al., 2003, Hellen et al., 1989). Simultaneously, transcription of viral genome that is catalyzed by virus-encoded RNA polymerase begins (Meredith et al., 1999). In the final stage of viral replication, RNA and capsid proteins are assembled into mature viral particles, which are then released by cell lysis. During the replication process, RNA viruses, including RV, modify the cellular functions to their advantage. For example, RV proteinase 2A attacks the host translational machinery in susceptible HeLa cells by cleaving eukaryotic initiation factor (eIF)-4GI and eIF4GII, thereby shutting off cap-dependent host cell protein synthesis (Glaser and Skern, 2000, Gradi et al., 2003). Drugs which interfere with any of the above steps would be beneficial in treating RV infection.
Viruses also interfere with host defense mechanisms. Upon viral infection, host cells negatively regulate global protein translation in order to limit viral replication. Protein kinase R (PKR), which is activated by viral double-stranded (ds) RNA, phosphorylates eIF2α phosphorylation, thereby inhibiting translation of both host and vRNA (Jacobs and Langland, 1996, Qian et al., 2004). Non-structural proteins of respiratory syncytial virus, influenza virus and hepatitis virus competitively inhibit eIF2α binding to PKR, thereby inhibiting eIF2α phosphorylation (Gale et al., 1997, Groskreutz et al., 2010, Li et al., 2006, Tan and Katze, 2001). Conversely, reversal of such inhibition would augment viral clearance.
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a plant flavonoid present in many plants including broccoli, apples, berries, onions, and tea. Flavonoids share a common chemical structure consisting of two phenol rings linked through three carbons. Quercetin has potent antioxidant effects, combined with free radical species to form considerably less reactive phenoxy radicals. Quercetin also has inhibitory effects on several lipid, protein tyrosine and serine/threonine kinases including phosphatidylinositol (PI)-3-kinase, and induces nitric oxide synthase (Agullo et al., 1997, Davies et al., 2000, Huang et al., 1999, Jiang et al., 2006, Peet and Li, 1999). Quercetin has also been shown to exert antiviral activities in vitro. Quercetin or its derivatives reduce the infectivity or replication of herpes simplex virus type 1, poliovirus type 1, parainfluenza virus type 3, adenovirus, respiratory syncytial virus an influenza virus in vitro (Castrillo and Carrasco, 1987, Chiang et al., 2003, Kaul et al., 1985, Kim et al., 2010b, Neznanov et al., 2008). Among the several quercetin derivates examined, 4′,7-dihydroxy-3-methoxy-5,6-dimethylflavone exhibited potent antirhinoviral effects in human skin fibroblasts (De Meyer et al., 1991). However, quercetin by itself had no antirhinoviral effect in this study. Later, quercetin was shown to inhibit the SARS-CoV protease that is required for SARS virus replication (Chen et al., 2006) as well as hepatitis C viral protein translation and release of newly formed viral particles (Gonzalez et al., 2009). Quercetin supplementation was also shown to reduce susceptibility to influenza A virus infection and severity of disease following stressful exercise in mice (Davis et al., 2008) and symptoms of upper respiratory tract infections in athletes (Nieman et al., 2007). This is of importance, because quercetin supplementation increases skeletal muscle mitochondrial biogenesis modestly in untrained athletes and this is associated with improved exercise performance (Nieman et al., 2010). Since mitochondria orchestrate antiviral signaling following recognition of cytosolic viral components (Tal and Iwasaki, 2011), there may exist a relationship between quercetin-induced mitochondrial biogenesis and reduced susceptibility to influenza infection. Although, association of physical stress/fatigue with susceptibility to RV infection is not established, RV causes severe exacerbations in patients with COPD and asthma (Mallia et al., 2006, Mallia et al., 2011, Message et al., 2008). Understanding the mechanisms by which quercetin inhibits rhinovirus infectivity and replication is necessary to determine the suitability of quercetin to treat RV infections in these patients. In the present study, we examined the effects of quercetin treatment on RV endocytosis, viral genome transcription and translation, and pro-inflammatory responses in vitro. We also assessed the effect of quercetin in vivo using a novel mouse model of RV infection (Newcomb et al., 2008).
2. Methods and materials
2.1. Rhinovirus
RV1B and RV39 were purchased from American Type Culture Collection (Manassas, VA) and viral stocks were generated as described previously (Newcomb et al., 2005). Fifty percent cell culture infectivity dose (CCID50) values of viral stocks were determined by the Spearman–Karber method (Johnston and Tyrrell, 1997). To generate replication-deficient virus (UV-RV), the RV preparation was exposed to ultraviolet light for 15 min on ice at 100 mJ/cm2 (Chattoraj et al., 2011).
2.2. Cell culture and infection
BEAS-2B immortalized human bronchial epithelial cells (American Type Culture Collection) were cultured in bronchial epithelial cell growth medium (BEGM, Lonza, Walkersville, MD) in collagen-coated plates, as described previously (Chattoraj et al., 2011, Wang et al., 2009).
For RV infection, cells were grown in 24- or 6-well plates until 90% confluency. Cells were infected with RV at a multiplicity of infection (MOI) of 1.0 based on CCID50 and incubated for 90 min at 33 °C to allow binding and endocytosis of RV. Infection medium was replaced with fresh medium and incubated for 4.5 h at 33 °C. Quercetin dihydrate (Sigma, St. Louis, MO) or DMSO (vehicle control) was added to the required concentration and further incubated for 16–18 h at 33 °C. In some experiments, cells were pretreated with quercetin for 1 h, infected with RV for 1 h in the presence of quercetin, and the infection medium was replaced with fresh medium containing DMSO or quercetin. In selected experiments, cells were pretreated with quercetin or DMSO overnight, and shifted to quercetin free medium for 8 h. Cells were rinsed with fresh medium and then infected with RV1B in the absence of quercetin.
2.3. Endocytosis of RV
Endocytosis of RV by airway epithelial cells was measured as described previously (Chattoraj et al., 2011, Newcomb et al., 2005). Briefly, BEAS-2B cells were pretreated with quercetin or DMSO and then incubated with RV or equal volumes of sham (cell lysate from uninfected HeLa cells) at 4 °C for 30 min. Cells were washed and then incubated at 37 °C for 30 min to facilitate endocytosis of bound RV. Cells were permeabilized and endocytosed RV was detected by antibody to RV capsid protein, VP2 (Mosser et al., 2002) and Alexafluor 488-conjugated secondary antibody and quantified by flow cytometry, correcting for sham controls. To visualize endocytosed RV, cells were infected, incubated for 30 min, fixed in cold methanol, blocked with 5% normal donkey serum and treated with antibodies to RV capsid protein and early endosomal antigen (EEA)-1 (Santa Cruz Biotechnologies, Santa Cruz, CA). Bound antibodies were detected by Alexafluor 488- (RV) or Alexafluor 594- (EEA-1) conjugated secondary antibody. Cell nuclei were counterstained with DAPI and the cells were visualized by confocal microscopy.
2.4. Animals and treatment
Eight to ten week-old C57BL/6 mice were infected with sham or RV1B through the intranasal route and gavaged with 0.2 mg of quercetin daily for one, or four days. The control group consisted of similarly infected mice treated with vehicle (50% propylene glycol, PG). In some experiments mice were treated with quercetin or vehicle as above for 10 days. Two days after the last treatment, mice were infected with RV or sham. These experiments were approved by the Animal Care and Use Committee of the University of Michigan.
2.5. Viral titer
After appropriate treatment, cells along with medium were subjected to one freeze–thaw cycle and centrifuged. Mice were sacrificed at 1, 4 and 7 days post-infection. Lungs were collected under aseptic conditions and homogenized in 1 ml of PBS. Lung homogenates were subjected to two freeze–thaw cycles and centrifuged. The viral titer (CCID50) in supernatants of cell cultures or lung homogenates was measured as described previously (Sajjan et al., 2009).
2.6. Cytokines
Protein levels of cytokines in cell culture medium or lung homogenate supernatants were measured by multiplex immunoassay (Biorad, Hercules, CA) or ELISA (R&D Systems, Minneapolis, MN).
2.7. Measurement of airways responsiveness
Mice were anesthetized by intraperitoneal injection of ketamine (2.5–5 mg/100 g body weight) and pentobarbital (2 mg/100 g body weight). A steel cannula was inserted into the trachea and connected to a miniature computerized ventilator (Buxco FinePointe Operating system, Wilmington, NC). Airways responsiveness to methacholine was measured by nebulizing increasing doses of methacholine, as described previously (Sajjan et al., 2009).
2.8. Realtime PCR
Total RNA was isolated from the lungs or cell cultures by using RNeasy (Qiagen, Valencia, CA). cDNA was synthesized utilizing a first strand synthesis kit (Applied Biosystems, Carlsbad, CA). mRNA levels of IFN-α, IFN-β, IFN-λ1 and IFN-λ2/3 were quantified by real-time PCR using gene specific primers and expressed as fold increase over the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (G3PDH). Positive strand RV RNA was quantified by qPCR as described previously (Newcomb et al., 2008, Sajjan et al., 2009). To detect negative strand RV RNA, 5 μg of total RNA was converted to cDNA using a primer specific to negative strand RV RNA and superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) as described previously (Newcomb et al., 2008). cDNA was amplified by qPCR using nested primers and the products were analyzed by agarose gel electrophoresis.
2.9. Western blotting
After relevant treatment, cells were lysed in RIPA buffer containing complete protease inhibitors (Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (1 mM sodium fluoride and 1 mM sodium orthovanadate). Cell lysates were centrifuged and total protein in the supernatant was determined. Equal amounts of protein were subjected to SDS–PAGE and proteins transferred to nitrocellulose membranes. The membranes were blocked with either 5% bovine serum albumin or fat-free milk and probed with antibody to phospho-Akt, total Akt, and phospho-eIF2α (all from Cell Signaling Technology, Danvers, MA), eIF4GI (Abcam, Cambridge, MA), or β-actin (Sigma–Aldrich, St. Louis, MO). The bound antibody was detected with appropriate second antibody conjugated with horseradish peroxidase (Biorad) and chemiluminescent substrate (Pierce, Rockford, IL).
2.10. Statistical analysis
Statistical analysis of significance was calculated by either one-way analysis of variance followed by Tukey’s post-hoc test or an unpaired t test with Welch’s correction using Sigma Stat software (Systat Software, San Jose, CA). Results are shown as mean ± SD.
3. Results
3.1. Pretreatment of airway epithelial cells with quercetin decreases RV-induced IL-8 responses
BEAS-2B cells were pretreated with 5, 10 or 25 μM quercetin or an identical volume of DMSO and infected with sham, RV1B, RV39, UV-irradiated (UV)-RV1B or UV-RV39. IL-8 protein levels were determined by ELISA. A concentration of 10 μM quercetin attenuated both RV and UV-RV-stimulated IL-8 responses by 75–80% (Fig. 1 A and B). Treatment of cells with 25 μM quercetin, did not decrease IL-8 further, and therefore in subsequent experiments we used 10 μM quercetin.
Fig. 1.
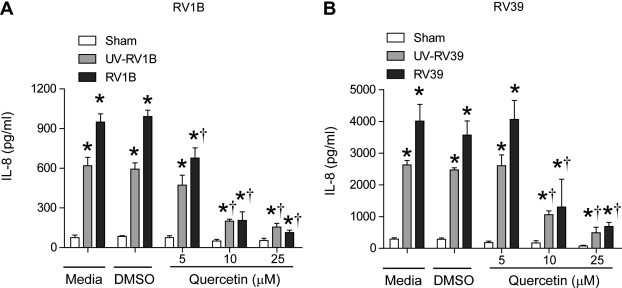
Cells pretreated with quercetin show attenuated RV-stimulated IL-8 responses. BEAS-2B cells were pretreated with DMSO or quercetin for 1 h. Cells were infected with sham, UV-RV1B, RV1B, UV-RV39 or RV39 in the presence of DMSO or quercetin and incubated for 24 h. IL-8 in the media was measured by ELISA. Data represent average and SD calculated from three independent experiments (∗p ⩽ 0.05, different from sham; †p ⩽ 0.05, different from media or DMSO treated cells).
3.2. Quercetin pretreatment inhibits RV endocytosis by airway epithelial cells
Previously, we demonstrated in cultured airway epithelial cells that RV binding and endocytosis is sufficient for the initial IL-8 response to infection (Newcomb et al., 2005). We also showed that endocytosis of RV requires activation of PI-3-kinase. Since quercetin inhibits PI-3-kinase activity (Davies et al., 2000), we determined whether quercetin pretreatment inhibits endocytosis of RV. Quercetin pretreatment significantly decreased endocytosis of both RV1B and RV39 by BEAS-2B cells (Fig. 2 A and B). We observed similar attenuation in the endocytosis of UV-RV. We also examined the colocalization of RV1B and EEA1, an early endosomal marker, by confocal microscopy. Cells inoculated with sham HeLa cell lysate showed diffuse signals for EEA1 in the cytoplasm (data not shown). Cells infected with RV1B showed clustering of RV particles. These cells also showed punctate staining for EEA1 as opposed to diffuse staining, an indication of recruitment of EEA1 to endosomes. Clustered RV particles frequently co-localized with endosomal EEA1, indicating endocytosis of RV in these cells (Fig. 2C). In contrast, cells pretreated with quercetin and infected with RV1B showed an EEA1 distribution similar to sham-treated cells and only faint signals for RV which did not co-localize with EEA1-positive endosomes. (The faint signals for RV may represent the bound, but not endocytosed virus.) These results suggest that pretreatment with quercetin may block endocytosis of RV. Next we examined whether quercetin inhibits RV-induced phosphorylation of Akt, a downstream effector of PI-3-kinase. As observed previously, RV1B treated cells showed increased Akt phosphorylation compared to sham-infected cells (Fig. 2D). Quercetin pretreatment completely inhibited RV-induced Akt phosphorylation. These results indicate that quercetin decreases RV endocytosis and PI-3-kinase/Akt signaling, leading to a reduction in IL-8 expression.
Fig. 2.
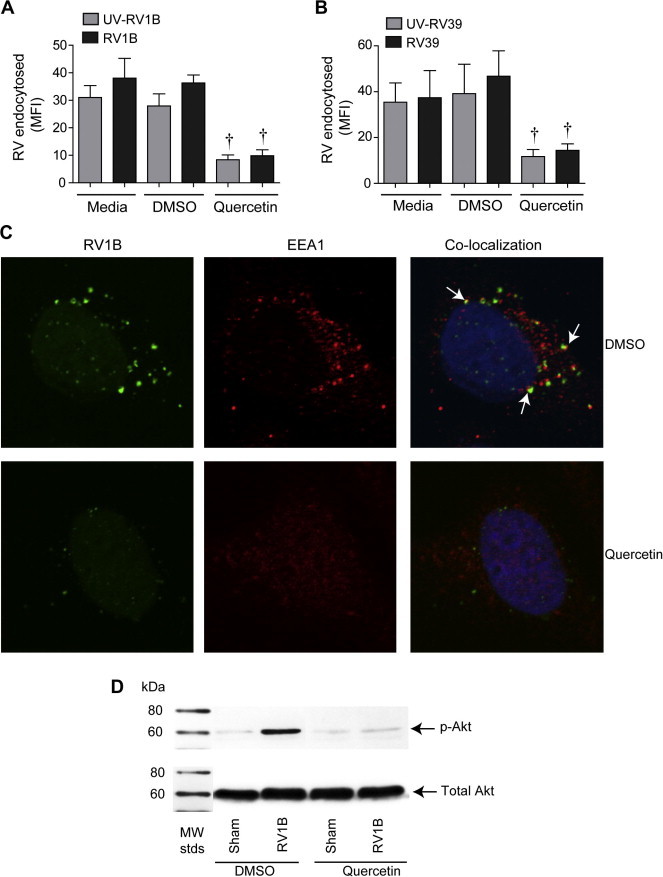
Quercetin pretreatment decreases RV endocytosis and RV-induced Akt phosphorylation. BEAS-2B cells were pretreated with 10 μM quercetin or DMSO and incubated with sham, UV-RV1B, RV1B, UV-RV39 or RV39 for 30 min at 4 °C, washed to remove unbound virus and cells were further incubated for 30 min at 37 °C. Endocytosed virus was detected using antibody to RV followed by flow cytometry (A and B). Data represent average and SD calculated from three independent experiments (∗p ⩽ 0.05, different from sham; †p ⩽ 0.05, different from media or DMSO treated cells). (C) Cells were pretreated with DMSO or quercetin and incubated with RV1B for 30 min. Cells were washed and incubated with antibody to RV capsid protein (green) and EEA1 antibody (red). Nuclei were counter-stained with DAPI (blue) and cells were visualized by confocal microscopy. Arrows represent co-localization of RV1B with EEA1. Cells pretreated with quercetin were infected with sham or RV1B and incubated for 30 min. Total cell lysates were subjected to Western blot analysis with antibodies to p-Akt or total Akt (D). Images and blot are representative of three independent experiments.
3.3. Endocytosis-independent effects of quercetin on RV1B-stimulated IFN and IL-8 responses in airway epithelial cells
Next, to determine endocytosis-independent effects, BEAS-2B airway epithelial cell monolayers were infected with RV1B or RV39 and incubated for 6 h to allow time for viral endocytosis. Quercetin or DMSO was then added to the cell culture medium and incubated for another 18 h. Cells inoculated with sham served as negative controls. Cellular mRNA levels of IFN-β, IFN-λ1 and IFN-λ2/3 and cell supernatant protein levels of IL-8 were determined. As observed earlier (Newcomb et al., 2005, Wang et al., 2009), infection with RV1B and RV39 each stimulated significantly higher IFN-β, IFN-λ1 and IFN-λ2/3 than UV-irradiated virus (Fig. 3 A–C). In contrast, UV-RV1B or UV-RV39-infected cells showed significant increases in IL-8 similar to intact RV (Fig. 3D), suggesting that IFNs are stimulated only by intact, replicating RV. Quercetin treatment inhibited RV-stimulated IFN as well as IL-8 expression, consistent with the notion that quercetin blocks viral replication.
Fig. 3.
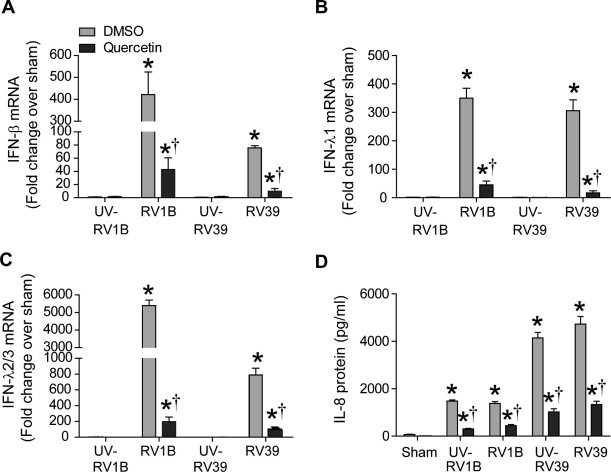
Addition of quercetin 6 h post RV infection attenuates virus-stimulated IFN responses in airway epithelial cells. BEAS-2B cells were infected with sham, UV-RV1B, RV1B, UV-RV39 or RV1B, and incubated for 90 min. Infection medium was replaced with fresh medium and incubated for another 4.5 h. DMSO or 10 μM quercetin was added to the cells and incubation continued for another 18 h. Levels of IFN mRNA (panels A–C) and IL-8 protein (D) were determined by qPCR and ELISA, respectively. Expression of IFN genes were normalized to G3PDH and then expressed as fold change over sham-infected cells. Data represent average and SD calculated from three independent experiments (∗p ⩽ 0.05, different from UV-RV in (A–C); and different from sham in (D); †p ⩽ 0.05, different from media or DMSO treated cells).
3.4. Quercetin-treated cells show decreased viral load
To determine whether decreased IFN and IL-8 responses were due to decreased viral load, we determined viral titer. Cells were infected with RV1B, RV39, UV-RV1B or UV-RV39 and DMSO or quercetin (10 μM) added 6 h later. Cells were incubated for another 18 h. Viral titer was determined at 6 h post-infection (before the addition of quercetin) and 24 h after infection (18 h after adding quercetin). As expected, cells infected with UV-RV1B or UV-RV39 did not show the presence of infectious RV (not shown). Compared to 6 h after infection, cells infected with intact RV1B or RV39 showed an increased load of infectious RV at 24 h post-infection, indicating replication of virus in BEAS2B cells (Fig. 4 A). Twenty-four hours post-infection, cells treated with quercetin showed a viral load 3 logs lower than similarly infected, vehicle-treated cells. We also measured the viral load by assessing vRNA levels. As expected, RV-infected cells showed significantly increased vRNA copy numbers compared to UV-RV-infected cells (Fig. 4B). In contrast, RV-infected cells treated with quercetin showed vRNA copy numbers similar to those infected with UV-RV. These results are again consistent with the notion that quercetin may inhibit viral replication.
Fig. 4.
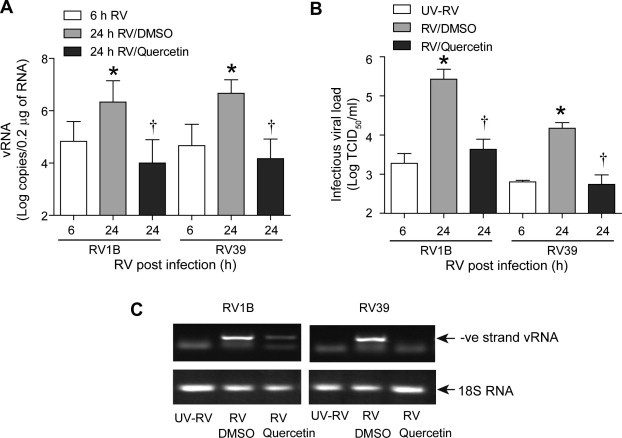
Quercetin added 6 h after RV infection decreases viral load and replication. Cells were infected and treated with quercetin as described in Fig. 3. Cells along with media was collected at 6 (just before adding quercetin) or 24 h (18 h after addition of quercetin or DMSO) after RV infection and infectious viral load was determined (A). Total RNA extracted from cells incubated for a total of 24 h post RV infection was used for determination of vRNA (B). The amplified qPCR product of negative strand RNA was subjected to agarose gel electrophoresis to show the amplified negative-strand vRNA (C). This result was representative of three experiments (∗p ⩽ 0.05, different from UV-RV in (A and B); and different from sham in (D); †p ⩽ 0.05, different from media or DMSO treated cells).
3.5. Quercetin interferes with viral genome transcription
During viral genome amplification, negative-strand vRNA is generated as an intermediate. Therefore, we monitored the negative-strand RNA in RV-infected BEAS2B cells by PCR. We observed amplification of negative-strand RNA in cells infected with RV1B or RV39, but not in cells infected with replication-deficient UV-RV (Fig. 4C). In RV-infected, quercetin-treated cells, negative-strand RNA was either decreased or not detected, implying that quercetin may inhibit viral replication at the transcriptional level.
3.6. Quercetin attenuates RV-induced cleavage of eIF4GI
During viral replication, viral proteases cleave eIF4GI to inhibit formation of the initiation complex required for host cap-dependent protein synthesis. This in turn facilitates translation of viral proteins. Cells were infected with RV1B, RV39, UV-RV1B or UV-RV39 as above and quercetin or DMSO was added 6 h post RV infection. After overnight incubation, cells infected with intact RV1B or RV39 showed cleavage of eIF4GI, but not cells infected with sham or UV-RV. Treatment with quercetin completely abolished RV1B- and RV39-induced eIF4GI cleavage (Fig. 5 A).
Fig. 5.
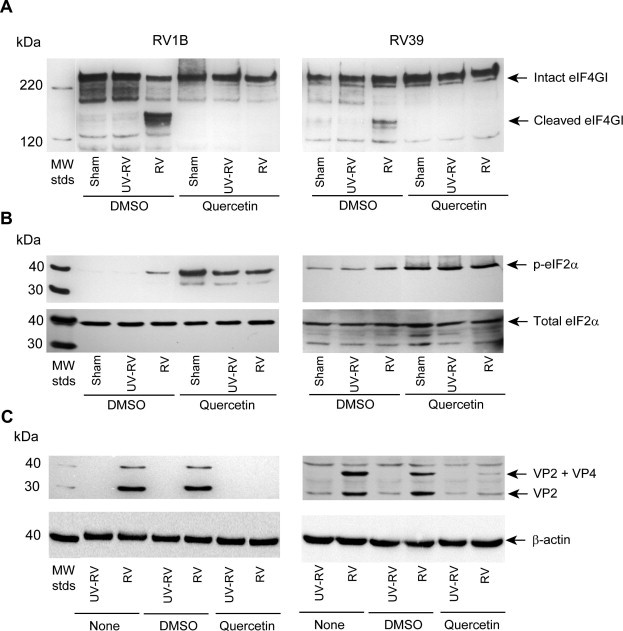
Quercetin inhibits cleavage of eIF4GI, increases phosphorylation of eIF2α and decreases levels of VP2. BEAS-2B cells were infected with RV and incubated for 90 min. Infection medium was replaced with fresh media, incubated for 4.5 h and then quercetin (10 μM) (or DMSO) were added to the cells. After a total of 24 h, total protein was isolated and subjected to Western blot analysis using antibody to eIF4GI (A), eIF2α (B) or VP2 (C). Blots are representative of three independent experiments.
Increased eIF2α phosphorylation limits mRNA translation globally and is essential to inhibit viral replication in infected cells. Therefore, we assessed eIF2α phosphorylation in airway epithelial cells infected with RV1B. Cells infected with intact RV1B showed increased eIF2α phosphorylation compared to cells inoculated with sham or replication-deficient UV-RV (Fig. 5B). As we showed previously (Nanua et al., 2006), quercetin treatment further increased eIF2α phosphorylation. Together these results imply that quercetin may decrease viral genome translation by inhibiting cleavage of eIF4GI and increasing eIF2α phosphorylation.
Next, we determined whether quercetin decreased viral protein levels, examining the levels of the RV capsid protein VP2 by Western blot analysis. While sham-infected cells did not show VP2 capsid protein, RV-infected cells showed two bands corresponding to VP2 and VP2 along with VP4 (VP2 + VP4), a precursor of VP2 (Fig. 5C). Quercetin treated cells showed lower amounts of VP2 and VP2 + VP4 bands, consistent with the notion that quercetin-induced changes in translation initiation may reduce the translation of viral RNA.
3.7. Presence of quercetin during RV infection is necessary to limit viral replication
Next, we examined whether withdrawal of quercetin 8 h prior to viral infection limits viral replication and associated IL-8 and IFN responses. Cells were pretreated with quercetin overnight and shifted to quercetin free medium. Eight hours later, cells were rinsed with medium and infected with RV1B in the absence of quercetin and vRNA, viral titer, IL-8 and IFN production were determined. Although, cells pretreated with quercetin showed a slightly lower viral titer and vRNA than the vehicle-treated cells, the difference was not statistically significant (Fig. 6 A and B). Similarly, no difference in IFNs or IL-8 levels between quercetin and vehicle treated cells was observed (Fig. 6C and D). This is not surprising because quercetin or its metabolite levels in the cells peak at 5 h post treatment and it is slowly reduced with time and completely depleted by 24 h (Kim et al., 2009, Kim et al., 2010a). Therefore addition of quercetin during or immediately after viral infection may be necessary to effectively limit viral infection in vitro.
Fig. 6.
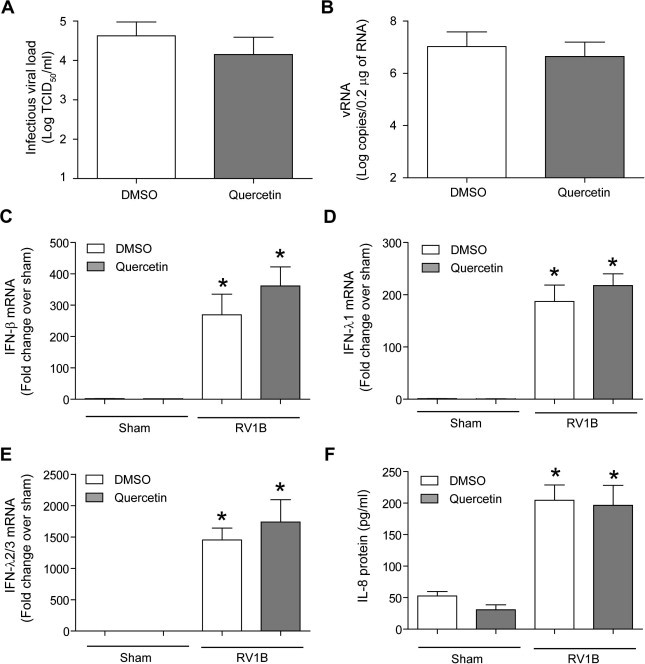
Withdrawal of quercetin several hours before RV infection does not limit rhinovirus infectivity in vitro. BEAS-2B cells were treated with DMSO or 10 μM quercetin overnight. Cells were shifted to fresh media without quercetin or DMSO and incubated for 8 h. Cells were then infected in the absence of quercetin, and incubated for 18 h. Infectious viral load was determined as described in Fig. 4 (A). vRNA and IFN responses (B–E) were determined by qPCR. IL-8 protein levels were determined by ELISA (F). Data represent average and SD values calculated from three independent experiments (∗p ⩽ 0.05, different from sham infected cells).
3.8. Quercetin treatment decreases viral load in vivo
RV39, which binds to intercellular adhesion molecule (ICAM)-1, does not infect mouse cells. On the other hand, RV1B, which binds to low density lipoprotein receptor (LDLR) or LDLR-like receptors, infects mouse airway epithelial cells in vitro and in vivo. Mice infected with RV1B show the presence of negative-strand viral RNA in the lungs, transmissibility of RV infection from the lung homogenates of inoculated mice to cultured HeLa cells, and the induction of a robust lung IFN response (Newcomb et al., 2008, Sajjan et al., 2009, Wang et al., 2011). Therefore, we used RV1B to determine the effects of quercetin in vivo. Normal mice were infected with RV1B or sham by intranasal route. After 2 h, mice were treated with quercetin or propylene glycol (vehicle). Mice were sacrificed 1 or 4 days later and assessed for lung viral load. As anticipated, mice infected with sham did not show infectious virus (data not shown). Mice infected with RV1B and treated with vehicle showed significant levels (9 × 104 CCID50/ml) of infectious virus 1 day post-infection (Fig. 7 A). At 4 days after infection, there was no detectable infectious virus, as observed previously (Newcomb et al., 2008). On the other hand, RV1B-infected mice treated with quercetin showed less than 1 log infectious virus/lung, i.e., 4 logs less virus compared to vehicle-treated mice 1 day after infection. Similarly, quercetin-treated mice also showed significantly lower vRNA levels at both 1 and 4 days post-infection compared to vehicle-treated mice (Fig. 7B).
Fig. 7.
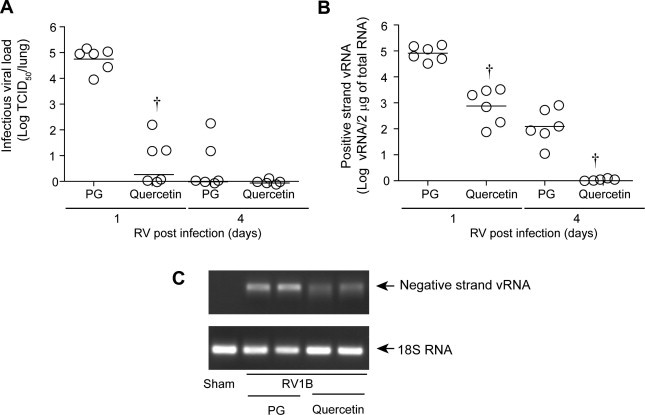
Quercetin decreases viral load in vivo. C57BL/6 mice were infected with RV1B or sham by the intranasal route. Two hours after RV infection, mice were orally gavaged with quercetin (0.2 mg/mouse) or 50% propylene glycol (PG) and then once a day up to 4 days. Mice were sacrificed and infectious viral load (A) was determined by measuring CCID50/ml. Positive strand vRNA (B) was determined by qPCR and negative strand vRNA (C) was detected by qPCR followed by agarose gel electrophoresis. (A and B) Data represent geomean and range of data from two independent experiments carried out in triplicate (†p ⩽ 0.05, different from mice infected with RV1B infected and treated with PG). (C) PCR product from two representative animals from PG and quercetin group showing negative-strand vRNA and 18S RNA.
To determine whether the observed decrease in viral load also depends on the inhibition of viral replication, we determined the level of negative-strand RNA in mouse lungs. RV1B-infected animals treated with vehicle showed detectable levels of negative strand vRNA 1 day after infection (Fig. 7C) which was decreased in similarly infected mice treated with quercetin. At 4 days post-infection, neither vehicle- nor quercetin-treated mice showed detectable levels of negative-strand vRNA (data not shown). These results imply that, in this model, RV replication occurs only within the first 24 h after infection, and that quercetin effectively inhibits this process.
3.9. Quercetin decreases RV-stimulated chemokines and cytokines in vivo
Previously, we have shown that RV infection stimulates lung chemokine and cytokine expression (Newcomb et al., 2008). To determine whether quercetin decreases RV-stimulated chemokine and cytokines levels, we determined the protein levels of CXCL-1 (KC) and CXCL-2 (MIP-2), TNF-α and CCL2 (MCP-1) by single or multiplex ELISA. Mice infected with RV and treated with vehicle showed significantly increased levels of all four cytokines/chemokines compared to mice infected with sham at 1 day but not 4 days post-infection, correlating with the presence of virus in the lungs (Fig. 8 A–D). Quercetin treatment decreased levels of all four cytokines in RV-infected mice, implying that quercetin attenuates RV-stimulated pro-inflammatory responses in mice by decreasing viral load.
Fig. 8.
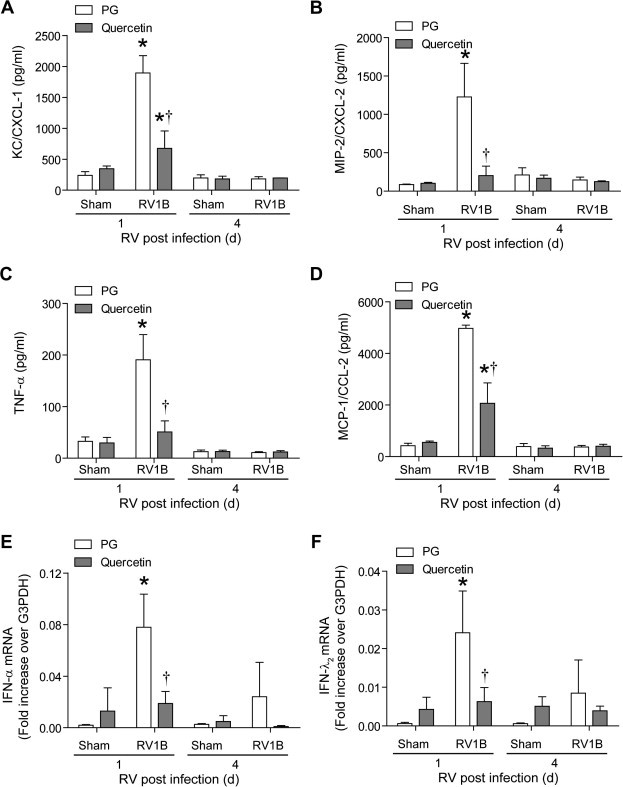
Quercetin decreases RV-stimulated chemokine and cytokine responses in vivo. Mice were infected with RV1B and treated with quercetin or vehicle as described in the Fig. 5 legend. After sacrifice, mouse lungs were excised, homogenized in PBS, centrifuged and supernatant examined by ELISA (A–D) or total RNA was extracted from the lungs and mRNA expression of IFN-α and IFN-λ1 was measured by qPCR (E and F). Data represent mean ± SD calculated from triplicate experiments (∗p ⩽ 0.05, different from sham; †p ⩽ 0.05, different from mice infected with RV and treated with vehicle).
Next, we measured the mRNA levels of representative type I (IFN-α) and III IFN (IFN-λ2), the expression of which depends on generation of the double-stranded RNA intermediate during RV replication. As expected, mice infected with RV1B and treated with vehicle showed significantly higher levels of IFN-α and IFN-λ1 than the sham-infected animals at 1 day post-infection, which returned to normal levels by 4 days after RV infection (Fig. 8E and F). This correlated with the presence of negative-strand vRNA in the lungs. RV1B-infected mice treated with quercetin showed significantly decreased levels of both IFNs compared to RV-infected, vehicle-treated mice, consistent with the decreased negative strand vRNA in quercetin-treated mice.
3.10. Quercetin decreases RV-induced airways cholinergic hyperresponsiveness
In our previous studies, we demonstrated that RV infection increase airways responsiveness to methacholine challenge at 1 day post-infection (Newcomb et al., 2008), which returns to normal by 4 days after RV infection. We therefore determined the effects of quercetin on airways responsiveness to methacholine 1 day after RV infection. As expected, compared to sham-inoculated mice, RV-infected mice treated with vehicle showed increased airways responsiveness, particularly at methacholine doses of 10 and 20 mg/ml (Fig. 9 ). In contrast, RV-infected mice treated with quercetin showed a level of airways responsiveness similar to sham-infected, quercetin-treated animals, which was significantly lower than that observed in RV-infected vehicle treated mice. Together, these results suggest that in vivo quercetin treatment decreases viral load and RV-stimulated chemokine and cytokine responses while improving lung function.
Fig. 9.
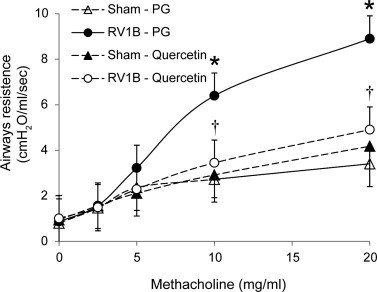
Compared to vehicle-treated animals, RV-infected mice treated with quercetin show decreased airways responsiveness to methacholine. Mice were infected with RV1B and then treated with DMSO or quercetin as described above. After 1 day, mice were anesthetized and airways responsiveness to methacholine challenge was measured (n = 3; ∗p ⩽ 0.05, different from sham infected mice; †p ⩽ 0.05, different from mice infected with RV1B and treated with vehicle, two-way ANOVA).
3.11. Quercetin treatment during RV infection is necessary for efficient viral clearance and reduce lung inflammation and improve lung function
To determine whether quercetin pretreatment modulates innate immune responses to subsequent viral infections and thus aid viral clearance, mice were pretreated with quercetin for 10 days, followed by a washout period of 2 days. Mice were then infected with RV1B as above, sacrificed 24 h later and levels of pro-inflammatory cytokines, IFN expression, vRNA, viral titers and lung function were determined. As observed above, mice infected with RV1B showed increased levels of pro-inflammatory cytokines and IFN responses and this was not affected by quercetin pretreatment (Fig. 10 ). In addition, quercetin pretreated mice showed similar levels of vRNA, and viral titer to those mice treated with vehicle (Fig. 11 A and B). Further, quercetin pretreated mice infected with RV1B also showed airways hyperresponsiveness to methacholine challenge similar to mice pretreated with vehicle (Fig. 11D). These results indicate that quercetin treatment during or after viral infection rather than pretreatment is more effective in vivo. These findings are not surprising, because half-life of quercetin ranges between 11 and 28 h (Boots et al., 2008) and therefore one would expect the plasma quercetin levels return to basal levels by 48 h.
Fig. 10.
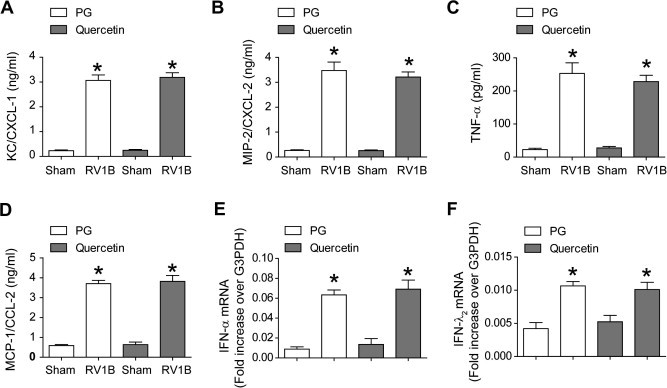
Treatment of quercetin during RV infection is necessary to limit RV-stimulated cytokine responses. Mice were treated with quercetin or vehicle as described in Fig. 7 for 10 days. Forty hours after the last quercetin or vehicle treatment, mice were infected with RV1B or sham and sacrificed 24 h post-infection and lungs harvested for determination of cytokine proteins by ELISA (A–D) or IFN mRNA expression by qPCR (E–G). Data represent mean ± SD calculated from triplicate experiments (n = 5, ∗p ⩽ 0.05, different from sham, ANOVA).
Fig. 11.
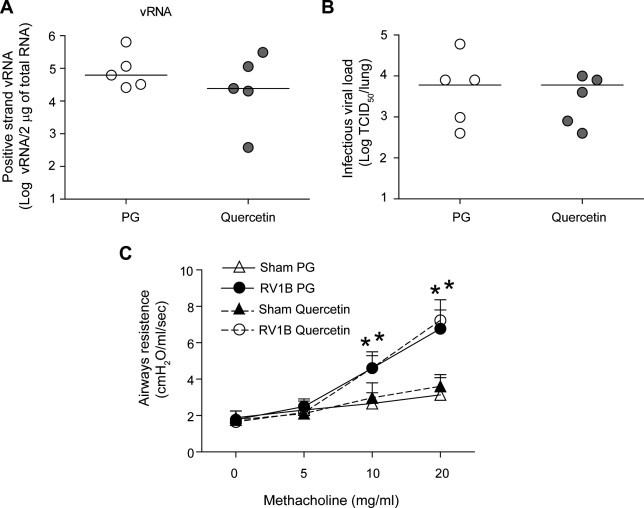
Treatment of quercetin during RV infection is necessary to reduce viral load and improve lung function. Mice were treated with quercetin or vehicle as described in Fig. 7 for 10 days. Forty hours after the last quercetin or vehicle treatment, mice were infected with RV1B or sham. After 1 day, some mice sacrificed, lungs harvested for determination of vRNA (A) by qPCR and infectious viral load (B). Rest of the mice were anesthetized and airways responsiveness to methacholine challenge was measured (C) (n = 3–5, data in (A and B) represents range and median; ∗p ⩽ 0.05, different from sham infected mice, two-way ANOVA).
4. Discussion
In the present study, we demonstrate that quercetin decreases RV infection at multiple steps in the viral life cycle. Quercetin decreases viral endocytosis and inhibits RV-induced IL-8 responses in cultured airway epithelial cells. When quercetin was added after viral endocytosis, it also decreased RV-stimulated IL-8 and IFN responses, consistent with downstream effects. Quercetin inhibited replication of RV by suppressing transcription of RV genome. Further, quercetin decreased RV-induced cleavage of eIF4GI and increased phosphorylation of eIF2α. Finally, we showed that quercetin treatment of RV-infected mice decreased viral replication, expression of pro-inflammatory cytokines and chemokines, and airways hyperresponsiveness to methacholine challenge. Collectively, these results suggest that quercetin may be beneficial in limiting viral replication and reducing symptoms associated with RV infection.
Quercetin is a potent antioxidant and possesses anti-inflammatory properties. It inhibits several lipid, protein tyrosine and serine/threonine kinases by competing for the ATP binding site (reviewed in (Williams et al., 2004)). In fact, a potent inhibitor of PI-3-kinase, LY 294002 was designed based on the structure of quercetin (Vlahos et al., 1994). We and others have shown that PI-3-kinase activity is required for RV endocytosis and also for viral replication-independent IL-8 responses (Bentley et al., 2007, Lau et al., 2008, Newcomb et al., 2005). Therefore, treatment of cells with quercetin prior to or during RV infection could inhibit viral infection by abolishing RV endocytosis, an essential initial step of RV infection. Consistent with this idea, pretreatment of cells with quercetin decreased Akt phosphorylation, endocytosis of RV and attenuated the associated chemokine responses in human airway epithelial cells.
In addition to inhibiting viral entry, quercetin may also inhibit subsequent steps in the picornavirus life cycle. For instance, quercetin and its derivative 3-methylquercetin were shown to inhibit synthesis of poliovirus RNA (Castrillo and Carrasco, 1987, Neznanov et al., 2008) and this was attributed to inhibition of viral RNA polymerase 3Dpol (Castrillo and Carrasco, 1987). Viral RNA polymerase 3Dpol is required for conversion of viral genome to create negative-strand RNA, which serves as a template for generating positive strand viral genome (Castrillo and Carrasco, 1987, Hung et al., 2002). Recently, polyphenols including quercetin were shown to increase zinc uptake in Caco2 cells and zinc ions have been shown to inhibit 3Dpol in vitro (Hung et al., 2002, Krenn et al., 2005, Sreenivasulu et al., 2010). Further, quercetin may also interfere with the processing of RV polyprotein by RV proteases that is required for activation of RNA polymerase (Hellen et al., 1989). In addition to processing viral 3Dpol, viral protease 2A also cleaves eIF4G to inhibit cap-dependent host protein synthesis and promote viral genome translation (Glaser and Skern, 2000, Gradi et al., 2003). Our results demonstrate that quercetin significantly reduced both negative and positive strand viral RNA and this was associated with reduced cleavage of eIFG4II and reduction in viral capsid protein, VP2, suggesting that quercetin may inhibit initial polypeptide processing that is required for both viral RNA polymerase processing and for cleavage of eIFG4II thereby blocking all the downstream steps in RV replication. Another possibility is that quercetin may directly inhibit viral RNA polymerase thereby blocking genome translation and synthesis of new progeny virus.
One of the mechanisms by which host limits viral replication is through shutting down the global protein synthesis by increasing phosphorylation of eIF2α (Jacobs and Langland, 1996, Qian et al., 2004). We observed that quercetin increases phosphorylation of eIF2α, beyond that stimulated by RV infection itself and therefore it is likely that quercetin also limits viral replication by enhancing host innate immune responses. Recently, replication of enterovirus, a member of picornaviridae family was shown to occur in specialized organelles enriched in phosphoinositide-4-phosphate (PI4P) lipids generated by PI-4-kinase IIIβ (Hsu et al., 2010). If RV replication requires PI4P-enriched orgenelles, it is possible that quercetin which inhibits PI-4-kinases (Prajda et al., 1995) may interfere with formation of these orgenelles thus blocking viral replication. Together, these observations suggest that quercetin may interfere with viral replication by more than one mechanism.
In our previous studies, we demonstrated that RV1B infects normal mice, transiently replicates, increases the levels of pro-inflammatory cytokines and IFNs in the lung, and increases airways responsiveness to methacholine challenge (Newcomb et al., 2008, Sajjan et al., 2009, Wang et al., 2011). Using this model system, we show for the first time that quercetin not only inhibits RV replication in vitro, but also inhibits RV replication, RV-induced pro-inflammatory cytokine and chemokine expression, and RV-induced airway hyperresponsiveness in vivo. Mice treated with quercetin showed a significantly decreased viral load, diminished levels of negative-strand RNA, and decreased replication-dependent expression of type I and type III IFNs (Wang et al., 2009, Wang et al., 2011), providing direct evidence that quercetin effectively inhibits viral replication in vivo. In addition, quercetin treatment decreased RV-induced lung inflammation, as evidenced by reduced expression of CXCL-1, CXCL-2, CCL2 and TNF-α. We have previously shown that, in addition to their role in lung inflammation, CXCR2 ligands and TNF-α contribute to RV-induced airways hyperresponsiveness (Nagarkar et al., 2009). Neutrophils, attracted to the airways by CXCR2 ligands, induce a state of hyperresponsiveness by elaboration of TNF-α (Nagarkar et al., 2009). Therefore, decreased airways responsiveness observed in RV-infected mice treated with quercetin may be due to decreased lung CXCL1, CXCL2 and TNF-α levels.
Quercetin dihydrate is an aglycon and is passively absorbed by small intestine. Only 24% of the ingested quercetin aglycon is absorbed as oppose to 52% of naturally occurring quercetin glycosides (Hollman et al., 1995). Plasma quercetin levels are in the low nanomolar range (<100 nM) in humans consuming normal diet which is estimated to provide 5–40 mg/day quercetin (Boots et al., 2007, Harwood et al., 2007). Upon supplementation with 1 g of quercetin aglycone/day increases plasma levels to 1.5 μM (Boots et al., 2007, Conquer et al., 1998). Previously we have shown that treatment of mice with 0.2 mg of quercetin yield total plasma quercetin (including both quercetin and its primary conjugates) levels on the order of 150 nM (Ganesan et al., 2010, Nanua et al., 2006). This dose of quercetin was sufficient to reduce lung inflammation in a mouse model of chronic obstructive pulmonary disease, and also an allergic mouse model of asthma (Ganesan et al., 2010, Nanua et al., 2006, Rogerio et al., 2007). Based on the present study, it appears that a nanomolar range of plasma quercetin is also sufficient to inhibit viral replication in vivo, even though a higher concentration (10 μM) of quercetin is required in vitro. Although, the precise explanation for this is unclear, it may depend on the differences in the stability and bioavailability of quercetin. One possibility is that quercetin may be absorbed better in vivo via small intestine compared to respiratory epithelial cells in vitro. Another possibility is that quercetin metabolites generated in vivo may be more potent as an antiviral agent than the parent compound. Quercetin dihydrate is metabolized in different organs including small intestine and liver similar to quercetin glycosides (Harwood et al., 2007). Quercetin glycosides are in fact precursors of quercetin aglycon in vivo. Quercetin glycosides are initially hydrolyzed to form quercetin aglycon in the epithelial cells of small intestine (Murota and Terao, 2003) and the aglycon generated is metabolized by phase II enzymes present in the small intestine, liver and other organs to methylated, sulfated and glycosylated forms. One hour after intragastric administration of quercetin to rats, the majority of the absorbed quercetin was found in the form of quercetin glucuronides, sulfoglucuronides and sulfates as well as isorhamnetic conjugates (Justino et al., 2004). Quercetin glucuronide was proposed to be a more potent modulator of reactive oxygen-generating enzymes than quercetin (Terao et al., 2011). Further studies are required to assess the antiviral effects of known quercetin metabolites.
5. Conclusion
We showed that quercetin, a potent antioxidant and anti-inflammatory agent, also possesses anti-rhinoviral effects. Quercetin inhibits viral infection at multiple stages, including endocytosis, transcription of the viral genome and viral protein synthesis. Further studies are needed to determine whether quercetin is beneficial in preventing or treating rhinovirus infections, or reducing symptoms related to viral infection in patients with chronic lung disease including chronic obstructive pulmonary disease, asthma or cystic fibrosis.
Acknowledgments
This work was supported by NIH Grants AT4793 and HL897720 (to U.S.) and HL81420 (to M.B.H). S.G. conducted the study, analyzed the data and prepared the first draft of the manuscript; A.N.F., A.T.C., Q.W. and N.A. provided assistance with the experiments; M.B.H. performed critical review of the manuscript; M.B.H. and U.S. obtained the funding and conceived the study, and U.S. supervised and finalized the manuscript.
References
- Agullo G., Gamet-Payrastre L., Manenti S., Viala C., Remesy C., Chap H., Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem. Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- Bentley J.K., Newcomb D.C., Goldsmith A.M., Jia Y., Sajjan U.S., Hershenson M.B. Rhinovirus activates interleukin-8 expression via a Src/p110beta phosphatidylinositol 3-kinase/Akt pathway in human airway epithelial cells. J. Virol. 2007;81:1186–1194. doi: 10.1128/JVI.02309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Boots A.W., Li H., Schins R.P., Duffin R., Heemskerk J.W., Bast A., Haenen G.R. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007;222:89–96. doi: 10.1016/j.taap.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Castrillo J.L., Carrasco L. Action of 3-methylquercetin on poliovirus RNA replication. J. Virol. 1987;61:3319–3321. doi: 10.1128/jvi.61.10.3319-3321.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj S.S., Ganesan S., Faris A., Comstock A., Lee W.M., Sajjan U.S. Pseudomonas aeruginosa suppresses interferon response to rhinovirus infection in cystic fibrosis, but not in normal bronchial epithelial cells. Infect. Immun. 2011;79:4131–4145. doi: 10.1128/IAI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li J., Luo C., Liu H., Xu W., Chen G., Liew O.W., Zhu W., Puah C.M., Shen X., Jiang H. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure–activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006;14:8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.C., Chiang W., Liu M.C., Lin C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother. 2003;52:194–198. doi: 10.1093/jac/dkg291. [DOI] [PubMed] [Google Scholar]
- Conquer J.A., Maiani G., Azzini E., Raguzzini A., Holub B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- Davies S.P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.M., Murphy E.A., McClellan J.L., Carmichael M.D., Gangemi J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- De Meyer N., Haemers A., Mishra L., Pandey H.K., Pieters L.A., Vanden Berghe D.A., Vlietinck A.J. 4′-Hydroxy-3-methoxyflavones with potent antipicornavirus activity. J. Med. Chem. 1991;34:736–746. doi: 10.1021/jm00106a039. [DOI] [PubMed] [Google Scholar]
- Foeger N., Schmid E.M., Skern T. Human rhinovirus 2 2Apro recognition of eukaryotic initiation factor 4GI. Involvement of an exosite. J. Biol. Chem. 2003;278:33200–33207. doi: 10.1074/jbc.M304007200. [DOI] [PubMed] [Google Scholar]
- Gale M.J., Jr., Korth M.J., Tang N.M., Tan S.L., Hopkins D.A., Dever T.E., Polyak S.J., Gretch D.R., Katze M.G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Faris A.N., Comstock A.T., Chattoraj S.S., Chattoraj A., Burgess J.R., Curtis J.L., Martinez F.J., Zick S., Hershenson M.B., Sajjan U.S. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010;11:131. doi: 10.1186/1465-9921-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser W., Skern T. Extremely efficient cleavage of eIF4G by picornaviral proteinases L and 2A in vitro. FEBS Lett. 2000;480:151–155. doi: 10.1016/s0014-5793(00)01928-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez O., Fontanes V., Raychaudhuri S., Loo R., Loo J., Arumugaswami V., Sun R., Dasgupta A., French S.W. The heat shock protein inhibitor quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Svitkin Y.V., Sommergruber W., Imataka H., Morino S., Skern T., Sonenberg N. Human rhinovirus 2A proteinase cleavage sites in eukaryotic initiation factors (eIF) 4GI and eIF4GII are different. J. Virol. 2003;77:5026–5029. doi: 10.1128/JVI.77.8.5026-5029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskreutz D.J., Babor E.C., Monick M.M., Varga S.M., Hunninghake G.W. Respiratory syncytial virus limits alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) phosphorylation to maintain translation and viral replication. J. Biol. Chem. 2010;285:24023–24031. doi: 10.1074/jbc.M109.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood M., Danielewska-Nikiel B., Borzelleca J.F., Flamm G.W., Williams G.M., Lines T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Hellen C.U., Krausslich H.G., Wimmer E. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry. 1989;28:9881–9890. doi: 10.1021/bi00452a001. [DOI] [PubMed] [Google Scholar]
- Hollman P.C., de Vries J.H., van Leeuwen S.D., Mengelers M.J., Katan M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T., Cameron C.E., Ehrenfeld E., van Kuppeveld F.J., Altan-Bonnet N. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.T., Hwang J.J., Lee P.P., Ke F.C., Huang J.H., Huang C.J., Kandaswami C., Middleton E., Jr., Lee M.T. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 1999;128:999–1010. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M., Gibbs C.S., Tsiang M. Biochemical characterization of rhinovirus RNA-dependent RNA polymerase. Antiviral Res. 2002;56:99–114. doi: 10.1016/s0166-3542(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Jacobs B.L., Langland J.O. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- Jiang J.S., Shih C.M., Wang S.H., Chen T.T., Lin C.N., Ko W.C. Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells. J. Ethnopharmacol. 2006;103:281–287. doi: 10.1016/j.jep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L., Symington P., O’Toole S., Myint S.H., Tyrrell D.A. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br. Med. J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.L., Tyrrell D.A.J. Rhinoviruses. In: Lennette E.H., Schmidt N.J., editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. American Public Health Association; Washington DC: 1997. pp. 533–563. [Google Scholar]
- Justino G.C., Santos M.R., Canario S., Borges C., Florencio M.H., Mira L. Plasma quercetin metabolites: structure–antioxidant activity relationships. Arch. Biochem. Biophys. 2004;432:109–121. doi: 10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kaul T.N., Middleton E., Jr., Ogra P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Park K.S., Yeo W.S., Choo H., Chong Y. In vitro solubility, stability and permeability of novel quercetin–amino acid conjugates. Bioorg. Med. Chem. 2009;17:1164–1171. doi: 10.1016/j.bmc.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Park K.S., Lee C., Park H.R., Choo H., Chong Y. Enhanced stability and intracellular accumulation of quercetin by protection of the chemically or metabolically susceptible hydroxyl groups with a pivaloxymethyl (POM) promoiety. J. Med. Chem. 2010;53:8597–8607. doi: 10.1021/jm101252m. [DOI] [PubMed] [Google Scholar]
- Kim Y., Narayanan S., Chang K.O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Krenn B.M., Holzer B., Gaudernak E., Triendl A., van Kuppeveld F.J., Seipelt J. Inhibition of polyprotein processing and RNA replication of human rhinovirus by pyrrolidine dithiocarbamate involves metal ions. J. Virol. 2005;79:13892–13899. doi: 10.1128/JVI.79.22.13892-13899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C., Wang X., Song L., North M., Wiehler S., Proud D., Chow C.W. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J. Immunol. 2008;180:870–880. doi: 10.4049/jimmunol.180.2.870. [DOI] [PubMed] [Google Scholar]
- Li S., Min J.Y., Krug R.M., Sen G.C. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology. 2006;349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Makela M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimaki M., Blomqvist S., Hyypia T., Arstila P. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P., Message S.D., Gielen V., Contoli M., Gray K., Kebadze T., Aniscenko J., Laza-Stanca V., Edwards M.R., Slater L., Papi A., Stanciu L.A., Kon O.M., Johnson M., Johnston S.L. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P., Message S.D., Kebadze T., Parker H.L., Kon O.M., Johnston S.L. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir. Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith J.M., Rohll J.B., Almond J.W., Evans D.J. Similar interactions of the poliovirus and rhinovirus 3D polymerases with the 3′ untranslated region of rhinovirus 14. J. Virol. 1999;73:9952–9958. doi: 10.1128/jvi.73.12.9952-9958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T., Contoli M., Sanderson G., Kon O.M., Papi A., Jeffery P.K., Stanciu L.A., Johnston S.L. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser A.G., Brockman-Schneider R., Amineva S., Burchell L., Sedgwick J.B., Busse W.W., Gern J.E. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J. Infect. Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- Murota K., Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Nagarkar D.R., Wang Q., Shim J., Zhao Y., Tsai W.C., Lukacs N.W., Sajjan U., Hershenson M.B. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J. Immunol. 2009;183:6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanua S., Zick S.M., Andrade J.E., Sajjan U.S., Burgess J.R., Lukacs N.W., Hershenson M.B. Quercetin blocks airway epithelial cell chemokine expression. Am. J. Respir. Cell Mol. Biol. 2006;35:602–610. doi: 10.1165/rcmb.2006-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb D.C., Sajjan U., Nanua S., Jia Y., Goldsmith A.M., Bentley J.K., Hershenson M.B. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J. Biol. Chem. 2005;280:36952–36961. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- Newcomb D.C., Sajjan U.S., Nagarkar D.R., Wang Q., Nanua S., Zhou Y., McHenry C.L., Hennrick K.T., Tsai W.C., Bentley J.K., Lukacs N.W., Johnston S.L., Hershenson M.B. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N., Kondratova A., Chumakov K.M., Neznanova L., Kondratov R., Banerjee A.K., Gudkov A.V. Quercetinase pirin makes poliovirus replication resistant to flavonoid quercetin. DNA Cell Biol. 2008;27:191–198. doi: 10.1089/dna.2007.0682. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., McAnulty L.S., Mayer E.P. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Williams A.S., Shanely R.A., Jin F., McAnulty S.R., Triplett N.T., Austin M.D., Henson D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010;42:338–345. doi: 10.1249/MSS.0b013e3181b18fa3. [DOI] [PubMed] [Google Scholar]
- Peet G.W., Li J. IkappaB kinases alpha and beta show a random sequential kinetic mechanism and are inhibited by staurosporine and quercetin. J. Biol. Chem. 1999;274:32655–32661. doi: 10.1074/jbc.274.46.32655. [DOI] [PubMed] [Google Scholar]
- Prajda N., Singhal R.L., Yeh Y.A., Olah E., Look K.Y., Weber G. Linkage of reduction in 1-phosphatidylinositol 4-kinase activity and inositol 1,4,5-trisphosphate concentration in human ovarian carcinoma cells treated with quercetin. Life Sci. 1995;56:1587–1593. doi: 10.1016/0024-3205(95)00125-p. [DOI] [PubMed] [Google Scholar]
- Prchla E., Kuechler E., Blaas D., Fuchs R. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 1994;68:3713–3723. doi: 10.1128/jvi.68.6.3713-3723.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud C.G. EIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Qian X., Karpova T., Sheppard A.M., McNally J., Lowy D.R. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerio A.P., Kanashiro A., Fontanari C., da Silva E.V., Lucisano-Valim Y.M., Soares E.G., Faccioli L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007;56:402–408. doi: 10.1007/s00011-007-7005-6. [DOI] [PubMed] [Google Scholar]
- Sajjan U., Ganesan S., Comstock A.T., Shim J., Wang Q., Nagarkar D.R., Zhao Y., Goldsmith A.M., Sonstein J., Linn M.J., Curtis J.L., Hershenson M.B. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am. J. Physiol. 2009;297:L931–944. doi: 10.1152/ajplung.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S., Maccallum P., Meade T.W., Jeffries D.J., Johnston S.L., Wedzicha J.A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- Seemungal T.A., Harper-Owen R., Bhowmik A., Jeffries D.J., Wedzicha J.A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasulu K., Raghu P., Nair K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010;75:H123–128. doi: 10.1111/j.1750-3841.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- Tal M.C., Iwasaki A. Mitoxosome: a mitochondrial platform for cross-talk between cellular stress and antiviral signaling. Immunol. Rev. 2011;243:215–234. doi: 10.1111/j.1600-065X.2011.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.L., Katze M.G. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology. 2001;284:1–12. doi: 10.1006/viro.2001.0885. [DOI] [PubMed] [Google Scholar]
- Terao J., Murota K., Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wang Q., Miller D.J., Bowman E.R., Nagarkar D.R., Schneider D., Zhao Y., Linn M.J., Goldsmith A.M., Bentley J.K., Sajjan U.S., Hershenson M.B. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 2011;7:e1002070. doi: 10.1371/journal.ppat.1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Nagarkar D.R., Bowman E.R., Schneider D., Gosangi B., Lei J., Zhao Y., McHenry C.L., Burgens R.V., Miller D.J., Sajjan U., Hershenson M.B. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Winther B. Rhinovirus infections in the upper airway. Proc. Am. Thor. Soc. 2011;8:79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]


