Abstract
Objectives
To examine longitudinal association of prenatal, infancy, and early childhood lead exposure during sensitive periods with height and BMI.
Study design
The 773 participants were recruited between 1994 and 2005 in Mexico City. We constructed lead exposure history categories for prenatal (maternal patella lead), infancy and childhood periods (mean child blood lead between birth to 24 months and 30 to 48 months, respectively). Linear regression models were used to study lead exposure history with height and BMI at 48 months.
Results
Children with blood lead levels higher than the median during infancy attained a mean height at 48 months that was significantly shorter (−0.84 cm, 95% CI= −1.42 to −0.25) than children with levels lower than the median. Prenatal lead exposure was not associated with height at 48 months. Results for attained BMI were in general in the same direction as for height.
Conclusion
Our study suggests an effect of early life lead exposure on height attainment at 48 months with an exposure window of greatest sensitivity occurring in infancy.
Early life environmental influences on development have been shown to have long-term impacts on growth and later health outcomes.(1) Several studies have related early life exposures to lead and childhood deficits in height(2–6) and body mass index (BMI).(4, 7, 8) Some studies observed a higher BMI among lead-exposed children(5, 9), and other studies did not find an association between lead exposure and BMI.(10) Epidemiologic studies have suggested that the effect of lead may be wholly or partially reversible: early high lead exposure has been associated with decrements in attained length at 15 months(2) but attenuation of exposure at later ages resulted in catch-up growth at 33 and 12 month, respectively.(3, 8) These results suggest that effects of environmental influences, such as lead, on growth is not constant over time, and would be better defined through an exposure-time framework identifying sensitive periods.(11) Sensitive windows of physical development have been portrayed as periods where exposure to environmental influences most detrimentally affects later development.(12–14) Sensitive windows of exposure have been described as: prenatal (from conception to birth), infancy (between birth and 24 months), and early childhood (24 to 72 months).(13, 15, 16) In the present study, we investigated the association of prenatal, infancy, and childhood lead exposure – different sensitive time windows – on attained height and BMI at 48 months.
Methods
The sample population consists of longitudinal, pooled birth cohorts recruited between 1994 and 2005 at maternity hospitals serving low-to-moderate income populations in Mexico City. Similar exclusion criteria were applied to the three cohorts.(17–19) In addition, we excluded premature (<37 weeks of gestation, N=49) and low birth weight (<2500 g, N=28) neonates, and participants with missing data on covariates and extreme outliers (which will be described in the statistical analysis section). Out of 1096 participants who attended the 48 months visit and with a height measure, the final sample size consisted of 773 participants with complete height and 768 BMI measures at 48 months. Children’s weight and height were collected by trained staff at birth and 48 months using standard protocols as described elsewhere.(20) The research protocols were approved by the Ethics and Research Committees of the partnering institutions including the National Institute of Public Health of Mexico, the Harvard School of Public Health, the Brigham and Women’s Hospital, the University of Michigan School of Public Health, and the participating hospitals.
Lead measurements
Maternal bone lead was assessed at approximately 1-month postpartum using in vivo K-X-ray fluorescence (K-XRF) as Mexican law prohibits non-emergency radiologic procedures among pregnant women. Measures were taken at the mid-tibial shaft (cortical bone) and the patella (trabecular bone). Lead in the bones represents historical exposure and acts as an endogenous source of cumulative fetal lead exposure through mobilization to the plasma and placenta (Chuang, 2001). The instrument and validation have been extensively described.(21) In the present study, analyses included maternal patella lead due to a smaller sample size of available tibia lead measurements. Whole blood was collected from children in trace metal-free tubes (BD Vacutainer® #367734, Becton-Dickinson, Franklin Lakes, NJ) and sampling was conducted at each interview by trained staff using standard protocols as previously described.(20)
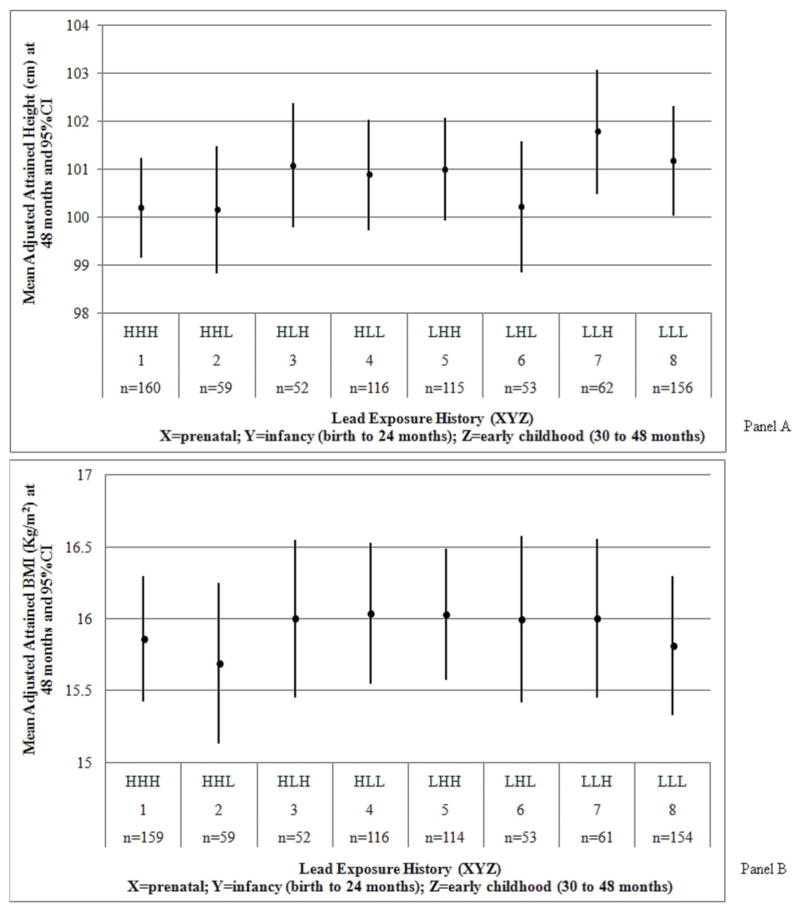
To address the question of exposure windows of sensitivity, we created eight lead history categories based on three windows during development: prenatal lead exposure (maternal patella lead); infancy lead exposure (average blood lead between birth and 24 months of age); early childhood lead exposure (average blood lead between 30 and 48 months of age). Several methods for investigating the effects of time-changing exposure have been described in the life course literature and occupational exposure literature, among others.(22, 23) We chose the critical periods approach as it is most well suited to answering the research question we posed. At each period, a child was classified as having high (H) or low (L) lead exposure defined by the median lead level at each period (for example HHH = high prenatal, high infancy, high childhood), we created the following lead exposure categories: HHH (n=160), HHL (n=59), HLH (n=52), HLL (n=116), LHH (n=115), LHL (n=53), LLH (n=62), and LLL (n=156). Due to a smaller sample size, the number of participants in each lead exposure category slightly differed between height and BMI measures at 48 months (Table I and Figure, B).
Table 1.
Comparison of included and excluded participants at baseline
| Included‡ | N | Excluded | ||||
|---|---|---|---|---|---|---|
| Mean or % |
SD | Mean or % |
SD | |||
| Child Characteristics | ||||||
| Sex | 323 | |||||
| Male (%) | 51.4 | 46.8 | ||||
| Gestational age (weeks) | 39.2 | 1.1 | 297 | 38.1 | 2.2 | ** |
| Birth length (cm) | 50.3 | 1.9 | 283 | 49.2 | 3.7 | ** |
| Birth weight (kg) | 3.2 | 0.4 | 296 | 3.0 | 0.6 | ** |
| Height at 48 months (cm) | 100.9 | 3.8 | 323 | 100.7 | 5.2 | |
| BMI at 48 months (kg/m2) | 15.9 | 1.5 | 323 | 16.2 | 3.0 | ** |
| Cohort | 323 | |||||
| 1 | 24.5 | 25.4 | ||||
| 2A | 15.7 | 23.2 | ||||
| 2B | 34.5 | 20.1 | ||||
| 3 | 25.4 | 31.3 | ||||
| Maternal Characteristics | ||||||
| Age at delivery (years) | 25.7 | 5.3 | 298 | 25.7 | 5.6 | |
| Calf circumference at delivery (cm) | 34.2 | 3.0 | 241 | 34.6 | 3.9 | |
| Height (cm) | 154.5 | 5.5 | 290 | 154.2 | 6.5 | |
| Marital status at delivery | 296 | |||||
| Married | 71.3 | 68.2 | ||||
| With partner | 20.4 | 21.0 | ||||
| Single, separated, or divorced | 8.3 | 10.8 | ||||
| Education (years) | 10.5 | 3.0 | 299 | 10.1 | 2.9 | ** |
| Parity | 299 | |||||
| Primiparous | 38.9 | 41.5 | ||||
| 1 previous child | 35.6 | 32.4 | ||||
| 2+ previous children | 25.5 | 24.2 | ||||
| Breastfed for 6 months (%) | 68.3 | 323 | 64.1 | |||
| Lead biomarkers | ||||||
| Median child blood lead from birth to 24 months (μg/dL) | 4.5 | 2.7 | 309 | 4.6 | 2.9 | |
| Median child blood lead from 30 to 48 months (μg/dL) | 5.6 | 2.9 | 303 | 5.7 | 3.6 | |
| Median maternal patella lead (μg Pb/g) | 9.4 | 11.5 | 171 | 10.4 | 16.5 | ** |
| Median maternal tibia lead (μg Pb/g) | 8.2 | 9.7 | 153 | 9.1 | 12.4 | |
Significance level:
<0.05
N= 773 except for BMI at 48 months (N=768), and for tibia (N=650)
Figure 1.
A, Adjusted attained height and B, BMI by lead exposure category. Models were adjusted for: maternal height and calf circumference, number of previous pregnancies, marital status, education level, breastfeeding for 6 months, cohort, calcium treatment group assignment during lactation and pregnancy, age at delivery, and child sex and gestational age at birth. Height models were additionally adjusted for birth length and BMI models for birth weight.
Statistical analyses
Our primary hypotheses of interest were: a) prenatal lead exposure detrimentally affects child height and BMI at 48 months regardless of postnatal lead exposure; i.e. children in the combined categories HHH, HHL, HLH, HLL will have lower height and BMI than children in the combined categories LHH, LHL, LLH, LLL; b) similarly, children exposed to high lead levels during infancy HHH, HHL, LHH, LHL will have lower attained height and BMI than children exposed to low levels HLH, HLL, LLH, LLL; and, c) children exposed to high lead levels during early childhood HHH, HLH, LHH, LLH will have lower attained height and BMI than children exposed to low levels HHL, HLL, LHL, LLL. We also selected secondary hypotheses to explore differences between exposure subgroups (Table II). We hypothesized that HHH children will be shorter than all children and in particular LLL children, due to continual suppressed growth in the sensitive periods. Similarly, low lead exposure during growth will result in LLL children being the tallest. Finally, children exposed to high lead levels both in utero and in infancy (HHH and HHL) will be shorter than LLL and LLH children. The power to detect a 1 cm difference ranged from 71% to 98%; and that of a 0.5 kg/m2 difference from 85% to 99% across hypotheses.
Distributions and descriptive statistics of exposures and outcomes of interest were examined. Extreme outliers of maternal and child anthropometry and lead measures were identified using the generalized extreme studentized deviation (ESD) method.(24) Differences between participants with complete information and participants excluded due to the additional study eligibility criteria were compared using T-test for continuous variables and chi-squared test for categorical variables. The proportion of children overweight was calculated using the SAS macro based on the World Health Organization (WHO) growth standards.(25)
We estimated linear regression models with exposure history groups coded as indicator variables. All models were adjusted for: maternal height and calf circumference, number of previous pregnancies, marital status, education level, breastfeeding for 6 months, cohort, calcium treatment group assignment during lactation and pregnancy, age at delivery, and child sex and gestational age at birth. Height models were additionally adjusted for birth length and BMI models for birth weight. Covariates were chosen based on biological relevance. From the adjusted models, we employed contrasts to compare groups of lead exposure categories depicted by the hypotheses above. In addition, we ran complementary analyses using the three lead exposure measures as continuous variables in the same regression model, to determine if one or more windows were a predictor of our outcomes while correcting for the shared variance among the exposure windows. Data were analyzed using SAS 9.2 (SAS Institute Inc, Cary, NC).
Results
Characteristics of the sample population (N=773) are presented in Table I. Maternal patella lead was somewhat correlated with infancy blood lead (r= 0.19, P<0.05) and with early childhood blood lead (r= 0.16, P<0.05). Infancy and early childhood blood lead were moderately correlated (r= 0.50, P<0.05). Certain characteristics (gestational age, birth length and weight, BMI at 48 months, and maternal patella lead) differed between included and excluded participants and were mainly due to the additional eligibility criteria of excluding low birth weight and premature infants, and to the outliers of anthropometry and lead measures. The proportion of children overweight (≥ 85th and < 95th percentile-for-age) was 9.31% and obese (≥ 95th percentile-for-age) was 4.79% at 48 months.
The Figure shows the adjusted attained height and BMI at 48 months, respectively, by lead history categories. The greatest heights were observed among the low lead group for the infancy period. Similarly, the lowest heights were in the high infancy lead level. The highest proportion of overweight and obese children was found in group LHH; the lowest proportion in group HHL. Table II shows the unadjusted and adjusted results of hypothesis testing using general linear models and contrasts. Children with high prenatal lead exposure (maternal patella lead higher than the median) did not differ in attained height at 48 months (−0.46, 95% CI= −1.03 to 0.10) from children with low prenatal lead exposure (maternal patella lead lower than the median), regardless of postnatal exposure and adjusting for covariates. Children with blood lead levels higher than the median during infancy (birth to 24 months) attained a mean height at 48 months that was significantly shorter (−0.84 cm, 95% CI= −1.42 to −0.25) than children with levels lower than the median. In comparison, children with high lead levels during early childhood (30 to 48 months) were not significantly shorter at 48 months than children with low levels (0.41, 95% CI= −0.17 to 0.98), regardless of prior lead exposure. When we included all three lead exposure measures in the same model to account for their shared variance, we found that the most sensitive time window of lead exposure associated with height at 48 months was infancy. None of the windows was related to BMI (results not shown). The secondary hypotheses consisted of comparing pairs or combinations of groups based on the general hypothesis that higher lead concentrations earlier would lead to growth deficits. We found that children with consistently high lead levels (HHH) were 0.98 cm (95% CI= −1.86 to −0.10) shorter than children with consistently low lead levels (LLL) throughout preschool years (Table II). HHH children were marginally significantly shorter by 0.66 cm (95% CI= −1.34 to 0.02) than all other children in our study population. Children in LLL were not significantly taller compared with all other children. High lead levels during infancy (HHH and HHL) were significantly associated with a 1.30 cm (95% CI= −2.09 to −0.51) decrease in attained height among 48 months-old children compared with low lead levels (LLH and LLL). Results for attained BMI at 48 months were in general in the same direction as for height. However, none of the groups compared were significantly different (Table II). For example, children with high prenatal lead levels (HHH, HHL, HLH, HLL) had a non-significant smaller attained BMI at 48 months (−0.07 kg/m2, 95% CI= −0.30 to 0. 17) compared with children with low lead levels (LHH, LHL, LLH, LLL).
Discussion
Shukla et al (3) found modest results similar to ours, where the average difference in height between the two extreme exposure groups was 0.99 cm. These workers defined slightly different lead exposure time windows: mean blood lead concentration between 3 and 15 months for infancy and between 18 and 33 months for early childhood.(3) Depending on where children are on the reference growth chart, a 1-cm decrement in height at 48 months could have different implications. For example, if the mean height of a sample of boys was in the 50th percentile-forage, a 1-cm height decrement could place them instead approximately at the 40th percentile-for-age.(25) Similarly, if girls aged 48 months were in the 25th percentile-for-age, a 1-cm decrement in their height could correspond to approximately a height-for-age on the 17th percentile, which may entail considerable public health implications(25) as height is reflective of population health.(26) Other studies have found anthropometry to be most affected by lead exposure during the infancy period. Shukla et al (2) found a 2-cm decreased length at 15 months attributable to a 10 μg/dL increase in 3 to 15 months blood lead levels and a high (≥7 μg/dL) maternal blood lead during pregnancy. In another study, Schell et al (8) found that infants with high lead levels in utero (≥ 3 μg/dL) followed by high postnatal lead levels (≥ 6 μg/dL) had lower attained weight, arm and head circumference-for-age at 12 months, but not length-for-age, than infants in all other exposure groups.(8)
Lead has inconsistently been associated with BMI, with some studies reporting BMI deficits,(4, 7) other reporting increases in BMI.(5, 9) However, these studies have been limited by their cross-sectional design and lack of chronic exposure biomarkers such as bone lead. We previously reported that prenatal lead exposure was associated with a sustained decrease in longitudinal weight status of girls between 24 and 60 months.(20) Although height and BMI measure different aspects of growth(27) and the effect of lead might occur through distinct mechanisms, sensitive periods are likely to be the same for both outcomes given that they are wide in age range and that exposure and absorbed dose considerably change between those periods. Lead is thought to affect bone growth by impairing bone activity (osteoblasts) and/or bone cartilage by influencing epiphyseal growth plate chondrocytes.(7, 28, 29)
A number of subjects were excluded from the analytical sample due to exclusion of outliers or missing demographic and anthropometric data (Table I). Excluding low birth weight and premature children could have underestimated the effect of early lead exposure. It is unlikely but possible that selection bias could have resulted in an artifactual result given that the samples of included and excluded participants differed. These results are generalizable to urban Mexican children of low-to-moderate income reproductive-aged mothers.
The analytical approach employed has some limitations. Although exposure may have changed during each time window, our method assumes it is constant.(3, 8) This method nonetheless allows us to disentangle more specific periods of sensitivity than other methods using overall average or peak exposures. More refined periods would allow for an increased number of lead exposure history windows, but would also result in a smaller sample size in each group, which could limit the ability to make valid inferences. The lead exposure measures in this study were partially, but not prohibitively, correlated. An explanation for the results obtained could be that children’s blood lead levels peak around 24 months.(30) If this were the case in our study, then this might be why we observed a significant effect for infancy lead exposure. However, in our study, the median blood lead level of children from birth to 24 months was lower than from 30 to 48 months. Finally, we were not able to take into account child nutritional status, air pollution or other exposures, or maternal smoking during pregnancy. However, our objective was less to predict variance in physical growth than to analyze the relationship of lead exposure in critical windows to growth outcomes. Only 4% of mothers reported pregnancy smoking, which was quite low. In addition, the lack of air pollution measures would be an unmeasured confounder.
Conclusion
Our study suggests that early life lead exposure has a negative impact on skeletal growth that remains evident at 48 months of age, with an exposure window of greatest sensitivity occurring in infancy (birth to 24 months). Prenatal lead exposure was not associated with height at 48 months as originally hypothesized. In addition, none of the lead exposure time windows was related to BMI at age 48 months.
Table 2.
Hypotheses and estimates for height and BMI differences at 48 months
| Estimates for height differences (cm) | Estimates for BMI differences (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Hypothesis | Unadjusted
|
Adjusted‡† |
Unadjusted
|
Adjustedঠ|
||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95%CI | |
| Primary hypotheses | ||||||||
| High vs. low prenatal | −0.47 | −1.05, 0.11 | −0.46 | −1.03, 0.10 | −0.12 | −0.36, 0.12 | −0.07 | −0.30, 0.17 |
| High vs. low infancy | −1.21 | −1.79, −0.63 | −0.84 | −1.42, −0.25 | −0.03 | −0.27, 0.21 | −0.07 | −0.31, 0.18 |
| High vs. low early childhood | 0.37 | −0.21, 0.95 | 0.41 | −0.17, 0.98 | 0.09 | −0.15, 0.32 | 0.09 | −0.15, 0.33 |
| Secondary Hypotheses | ||||||||
| HHH vs. LLL | −1.39 | −2.21, −0.57 | −0.98 | −1.86, −0.10 | 0.01 | −0.33, 0.34 | 0.05 | −0.32, 0.41 |
| HHH vs. all others | −0.91 | −1.57, −0.25 | −0.66 | −1.34, 0.02 | −0.03 | −0.30, 0.24 | −0.07 | −0.36, 0.21 |
| LLL vs. all others | 0.68 | 0.02, 1.35 | 0.46 | −0.21, 1.13 | −0.04 | −0.31, 0.24 | −0.13 | −0.41, 0.16 |
| HHH+HHL vs. LLL+LLH | −1.68 | −2.46, −0.90 | −1.30 | −2.09, −0.51 | −0.15 | −0.47, 0.17 | −0.13 | −0.47, 0.20 |
N=773 for height models, and N=768 for BMI models
Adjusted for maternal age at delivery, parity, marital status, education, breastfeeding, calcium treatment group, cohort, calf circumference, height, and child sex and gestational age at birth.
Additionnally adjusted for child length at birth
Additionnally adjusted for child weight at birth
Acknowledgments
Supported by U.S. National Institute of Environmental Health Sciences (NIEHS) (grants R01 ES007821, R01 ES014930, R01 ES013744, P42 ES05947, P42 ES016454, K23ES000381, T32 ES007018), Consejo Nacional de Ciencia y Tecnología (CONACyT) (grants 4150M9405 and 41912-M), American British Cowdray Hospital, Mexico City, CONSERVA, Department of Federal District, México, NIEHS P01 ES012874 (STAR Research Assistance Agreement RD-83172501 awarded by the U.S. Environmental Protection Agency [EPA]), NIEHS/EPA Formative Children’s Environmental Health and Disease Prevention Center (P20 ES018171/RD834800), and the University of Michigan NIEHS P30 Core Center in Environmental Health (P30ES017885). M.A. was supported by a University of Michigan Risk Science Center Fellowship and a Ruth L. Kirschstein National Research Service Award (T32 DK 007703-16). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, or the U.S. EPA.
List of Abbreviations Used in this Manuscript
- BMI
body mass index
- ESD
extreme studentized deviation
- K-XRF
K shell X-ray Fluorescence
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 2.Shukla R, Bornschein RL, Dietrich KN, Buncher CR, Berger OG, Hammond PB, Succop PA. Fetal and infant lead exposure: effects on growth in stature. Pediatrics. 1989;84:604–12. [PubMed] [Google Scholar]
- 3.Shukla R, Dietrich KN, Bornschein RL, Berger O, Hammond PB. Lead exposure and growth in the early preschool child: a follow-up report from the Cincinnati Lead Study. Pediatrics. 1991;88:886–92. [PubMed] [Google Scholar]
- 4.Little BB, Spalding S, Walsh B, Keyes DC, Wainer J, Pickens S, Royster M, Villanacci J, Gratton T. Blood lead levels and growth status among African-American and Hispanic children in Dallas, Texas -1980 and 2002: Dallas Lead Project II. Ann Hum Biol. 2009;36:331–41. doi: 10.1080/03014460902806615. [DOI] [PubMed] [Google Scholar]
- 5.Lamb MR, Janevic T, Liu X, Cooper T, Kline J, Factor-Litvak P. Environmental lead exposure, maternal thyroid function, and childhood growth. Environ Res. 2008;106:195–202. doi: 10.1016/j.envres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Avila M, Peterson KE, Gonzalez-Cossío T, Sanín LH, Aro A, Schnaas L, Hu H. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57:482–8. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- 7.Ignasiak Z, Sławińska T, Rozek K, Little BB, Malina RM. Lead and growth status of school children living in the copper basin of south-western Poland: differential effects on bone growth. Ann Hum Biol. 2006;33:401–14. doi: 10.1080/03014460600730752. [DOI] [PubMed] [Google Scholar]
- 8.Schell L, Denham M, Stark A, Parsons P, Schulte E. Growth of infants’ length, weight, head and arm circumferences in relation to low levels of blood lead measured serially. Am J Hum Biol. 2009;21:180–7. doi: 10.1002/ajhb.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ Health Perspect. 1995;103:952–7. doi: 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballew C, Khan LK, Kaufmann R, Mokdad A, Miller DT, Gunter EW. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Pediatr. 1999;134:623–30. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical Methods to Study Timing of Vulnerability with Sparsely Sampled Data on Environmental Toxicants. Environ Health Perspect. 2010;119:409–15. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner JM. Foetus into man: physical growth from conception to maturity. Cambridge, Massachusetts: Harvard University Press; 1990. [Google Scholar]
- 13.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–93. [PubMed] [Google Scholar]
- 14.Lemasters GK, Perreault SD, Hales BF, Hatch M, Hirshfield AN, Hughes CL, Kimmel GL, Lamb JC, Jon LP, Rubin C, Seed JG. Workshop to Identify Critical Windows of Exposure for Children’s Health: Reproductive Health in Children and Adolescents Work Group Summary. Environ Health Perspect. 2000;108:505–9. doi: 10.1289/ehp.00108s3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EPA. Guidance on Selecting Age Groups for Monitoring and Assessing Child-Hood Exposures to Environmental Contaminants (Final) Washington, DC: EPA/630/P-03/003F2005. [Google Scholar]
- 16.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108 (Suppl 3):451–5. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Cossío T, Peterson KE, Sanín H, Fishbein E, Palazuelos E, Aro A, Hernández-Avila M, Hu H. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–62. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Avila M, Gonzalez-Cossío T, Hernández-Avila JE, Romieu I, Peterson KE, Aro A, Palazuelos E, Hu H. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology. 2003;14:206–12. doi: 10.1097/01.EDE.0000038520.66094.34. [DOI] [PubMed] [Google Scholar]
- 19.Téllez-Rojo MM, Hernández-Avila M, Gonzalez-Cossío T, Romieu I, Aro A, Palazuelos E, Schwartz J, Hu H. Impact of breastfeeding on the mobilization of lead from bone. Am J Epidemiol. 2002;155:420–8. doi: 10.1093/aje/155.5.420. [DOI] [PubMed] [Google Scholar]
- 20.Afeiche M, Peterson KE, Sánchez BN, Cantonwine D, Lamadrid-Figueroa H, Schnaas L, Ettinger AS, Hernández-Avila M, Hu H, Téllez-Rojo MM. Prenatal Lead Exposure and Weight of 0 to 5 Year-Old Children in Mexico City. Environ Health. 2011;119:1436–41. doi: 10.1289/ehp.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Aro A, Rotnitzky A. Bone lead measured by X-ray fluorescence: epidemiologic methods. Environ Health Perspect. 1995;103 (Suppl 1):105–10. doi: 10.1289/ehp.95103s1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson DB. Latency models for analyses of protracted exposures. Epidemiology. 2009;20:395–9. doi: 10.1097/EDE.0b013e318194646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallqvist J, Lynch J, Bartley M, Lang T, Blane D. Can we disentangle life course processes of accumulation, critical period and social mobility? An analysis of disadvantaged socio-economic positions and myocardial infarction in the Stockholm Heart Epidemiology Program. Soc Sci Med. 2004;58:1555–62. doi: 10.1016/S0277-9536(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 24.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 25.WHO. Methods and development. Geneva: World Health Organization; 2006. Multicentre Growth Reference Study Group, WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. [Google Scholar]
- 26.Subramanian SV, Özaltin E, Finlay JE. Height of Nations: A Socioeconomic Analysis of Cohort Differences and Patterns among Women in 54 Low- to Middle-Income Countries. PLoS ONE. 2011;6:e18962. doi: 10.1371/journal.pone.0018962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. Physical Status: The Use and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 28.Klein RF, Wiren KM. Regulation of osteoblastic gene expression by lead. Endocrinology. 1993;132:2531–7. doi: 10.1210/endo.132.6.8504755. [DOI] [PubMed] [Google Scholar]
- 29.Puzas JE, Sickel MJ, Felter ME. Osteoblasts and chondrocytes are important target cells for the toxic effects of lead. Neurotoxicology. 1992;13:783–8. [PubMed] [Google Scholar]
- 30.Bellinger DC. Lead. Pediatrics. 2004;113:S1016–22. [PubMed] [Google Scholar]