Abstract
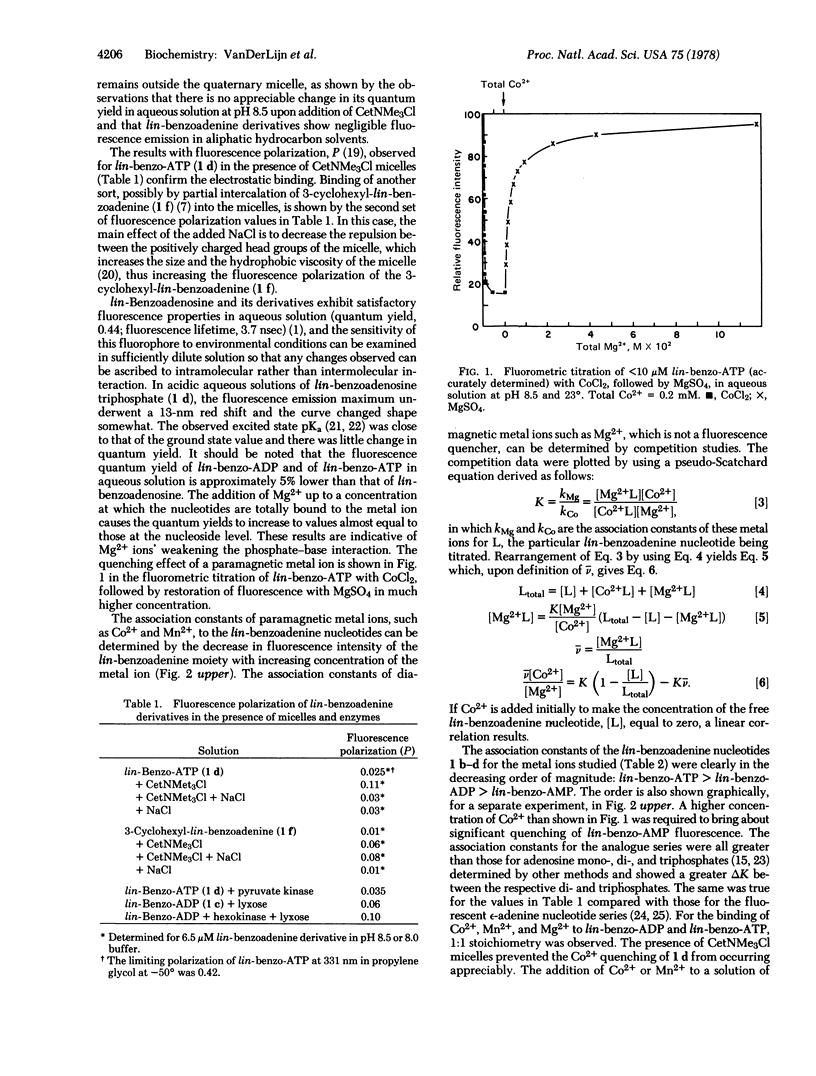
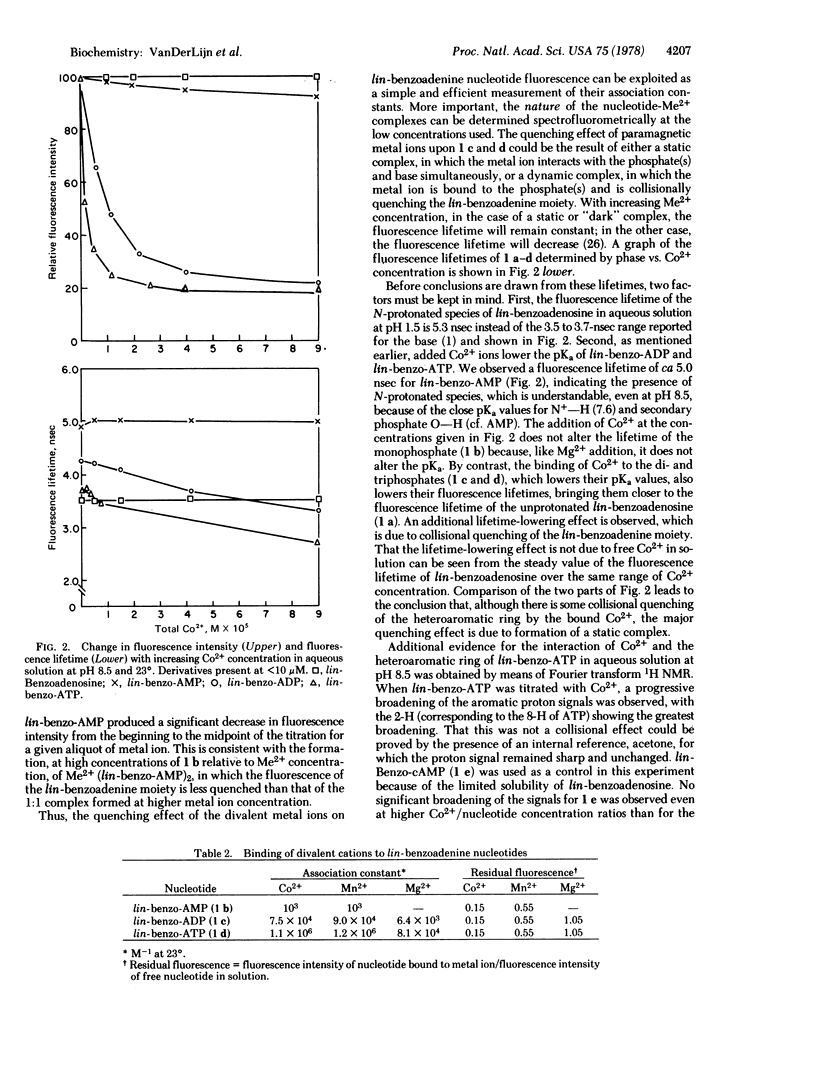
From pKa data for lin-benzoadenosine 5′-mono-, 5′-di-, and 5′-triphosphates, which are fluorescent “stretched-out” analogues of adenine nucleotides, it was possible to designate the cases of interaction of phosphate with the heteroaromatic moiety. The addition of divalent metal cations or quaternary ammonium micelles diminishes the direct intramolecular interaction between the phosphate(s) and base and consequently brings the pKa values close to that of lin-benzoadenosine. Fluorescence spectroscopy was used to investigate the interaction of lin-benzoadenine nucleotides with Mg2+, Mn2+, and Co2+. The association constants for the formation of such complexes were obtained from measurements of steady-state fluorescence quenching. Phase and modulation measurements of the fluorescence lifetimes of lin-benzoadenine nucleotides as a function of Co2+ concentration permitted determination of the static component of the quenching due to intramolecular complex formation. The association constants of the lin-benzoadenine nucleotides with all of the divalent metal ions studied were greater than those observed for the corresponding adenine nucleotides and were in the order: lin-benzo-ATP > lin-benzo-ADP > lin-benzo-AMP. Fourier transform 1H NMR of lin-benzo-ATP in the presence of Co2+ showed broadening of the aromatic proton signals, the 2-H signal (corresponding to the 8-H in ATP) being the most affected. Models are proposed to explain the phosphate-base interaction, the influence of metal ions on base protonation, and the intramo- the intramolecular quenching observed in the complexes due to paramagnetic ion (Co2+, Mn2+) and base interaction.
Keywords: adenine binding sites, dimensional probes, fluorescence, phosphate-adenine interaction, pKa
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsler P. E., Buisson D. H., Sigel H. Hydrolysis of nucleoside phosphates, III. The dephosphorylation of adenosine 5'-triphosphate in a binary and ternary Zn2+ complex. Z Naturforsch C. 1974 Nov-Dec;29(11-12):680–682. [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., Chien Y. -h., Jo Taylor P., Robinson J. L., Leonard N. J. Interactions of fluorescent analogs of adenine nucleotides with pyruvate kinase. FEBS Lett. 1973 Feb 1;29(3):215–218. doi: 10.1016/0014-5793(73)80022-5. [DOI] [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J Biol Chem. 1962 Jan;237:176–181. [PubMed] [Google Scholar]
- Chock P. B., Huang C. Y., Timmons R. B., Stadtman E. R. Epsilon-adenylylated glutamine synthetase: an internal fluorescence probe for enzyme conformation. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3134–3138. doi: 10.1073/pnas.70.11.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelaFuente G., Lagunas R., Sols A. Induced fit in yeast hexokinase. Eur J Biochem. 1970 Oct;16(2):226–233. doi: 10.1111/j.1432-1033.1970.tb01075.x. [DOI] [PubMed] [Google Scholar]
- DelaFuente G., Sols A. The kinetics of yeast hexokinase in the light of the induced fit involved in the binding of its sugar substrate. Eur J Biochem. 1970 Oct;16(2):234–239. doi: 10.1111/j.1432-1033.1970.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Höhne W. E., Heitmann P. A new principle for activity measurement of ADP or ATP dependent enzymes: fluorescence quenching of epsilon-ADP and epsilon-ATP by divalent metal ions. Anal Biochem. 1975 Dec;69(2):607–617. doi: 10.1016/0003-2697(75)90165-7. [DOI] [PubMed] [Google Scholar]
- Izatt R. M., Christensen J. J., Rytting J. H. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem Rev. 1971 Oct;71(5):439–481. doi: 10.1021/cr60273a002. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Sprecker M. A., Morrice A. G. Defined dimensional changes in enzyme substrates and cofactors. Synthesis of lin-benzoadenosine and enzymatic evaluation of derivatives of the benzopurines. J Am Chem Soc. 1976 Jun 23;98(13):3987–3994. doi: 10.1021/ja00429a040. [DOI] [PubMed] [Google Scholar]
- Leonard N. L., Morrice A. G., Sprecker M. A. Linear benzoadenine. A stretched-out analog of adenine. J Org Chem. 1975 Feb 7;40(3):356–363. doi: 10.1021/jo00891a021. [DOI] [PubMed] [Google Scholar]
- Matthies M., Zundel G. Phosphate-N base hydrogen bonds involving proton transfer with reference to the non-enzymic hydrolysis of ATP. Biochem Biophys Res Commun. 1977 Jan 24;74(2):831–837. doi: 10.1016/0006-291x(77)90378-3. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Kinetic and magnetic resonance studies of the pyruvate kinase reaction. II. Complexes of enzyme, metal, and substrates. J Biol Chem. 1966 Mar 10;241(5):1178–1193. [PubMed] [Google Scholar]
- Moller J. V., Kragh-Hansen U. Indicator dyes as probes of electrostatic potential changes on macromolecular surfaces. Biochemistry. 1975 Jun 3;14(11):2317–2323. doi: 10.1021/bi00682a007. [DOI] [PubMed] [Google Scholar]
- Perahia D., Pullman B., Saran A. A molecular orbital probe into the conformation of ATP. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1284–1289. doi: 10.1016/0006-291x(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Phillips R., Eisenberg P., George P., Rutman R. J. Thermodynamic data for the secondary phosphate ionizations of adenosine, guanosine, inosine, cytidine, and uridine nucleotides and triphosphate. J Biol Chem. 1965 Nov;240(11):4393–4397. [PubMed] [Google Scholar]
- Plowman K. M., Krall A. R. A kinetic study of nucleotide interactions with pyruvate kinase. Biochemistry. 1965 Dec;4(12):2809–2814. doi: 10.1021/bi00888a035. [DOI] [PubMed] [Google Scholar]
- Roger Phillips S. J. Adenosine and the adenine nucleotides. Ionization, metal complex formation, and conformation in solution. Chem Rev. 1966 Oct;66(5):501–527. doi: 10.1021/cr60243a002. [DOI] [PubMed] [Google Scholar]
- Scopes D. I., Barrio J. R., Leonard N. J. Defined dimensional changes in enzyme cofactors: fluorescent "stretched-out" analogs of adenine nucleotides. Science. 1977 Jan 21;195(4275):296–298. doi: 10.1126/science.188137. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]



