Abstract
Background
Cardiac ischemia-reperfusion injury (I/R) represents a major cause of cardiac tissue injury. Adenosine signaling dampens inflammation during cardiac I/R. Here, we investigated the role of the adenosine A2b-receptor (Adora2b) on inflammatory cells during cardiac I/R.
Methods
To study Adora2b signaling on inflammatory cells, we transplanted wild-type (WT) bone marrow (BM) into Adora2b−/− mice or Adora2b−/− BM into WT mice. To study the role of polymorphonuclear leukocytes (PMNs), neutrophil-depleted WT mice were treated with an Adora2b agonist. Following treatments, mice were exposed to 60 min of myocardial ischemia and 120 min of reperfusion. Infarct sizes and Troponin-I levels were determined by triphenyltetrazolium chloride staining and ELISA, respectively.
Results
Transplantation of WT-BM into Adora2b−/− mice decreased infarct sizes by 19 ± 4% and Troponin-I by 87.5 ± 25.3 ng/ml (mean ± SD, n = 6). Transplantation of Adora2b−/− BM into WT mice increased infarct sizes by 20 ±3% and Troponin-I levels by 69.7 ± 17.9 ng/ml (mean ± SD, n = 6). Studies on the reperfused myocardium revealed PMNs as dominant cell type. PMN-depletion or Adora2b agonist treatment reduced infarct sizes by 30 ± 11% or 26 ± 13% (mean ± SD, n = 4), however the combination of both did not reveal further cardioprotection. Cytokine profiling showed significantly higher cardiac tumor-necrosis-factor-α levels in Adora2b−/− compared to WT mice (39.3 ± 5.3 vs. 7.5 ± 1.0 pg/mg protein, mean ± SD, n = 4). Pharmacological studies on human activated PMNs revealed an Adora2b dependent tumor-necrosis-factor-α release.
Conclusion
Adora2b signaling on BM-derived cells such as PMNs represents an endogenous cardioprotective mechanism during cardiac I/R. Our findings suggest that Adora2b agonist treatment during cardiac I/R reduces tumor-necrosis-factor-α release of PMNs, thereby dampening tissue injury.
INTRODUCTION
Although early reperfusion is the only way to prevent further tissue injury after myocardial ischemia (MI), it initiates reversible and irreversible organ damage, which is referred to as ischemia-reperfusion (I/R) injury.1 Postischemic myocardial tissue is a strong stimulus for an inflammatory response which initiates repair processes, but can also damage myocardial tissue thereby increasing the size of the infarct. Thus, attenuation of the inflammatory response may diminish reperfusion damage and maximize the benefit of revascularization.2,3 Numerous exogenous and endogenous agents have been identified to modulate the inflammation response after MI. In particular, adenosine has been shown to be cardioprotective due to antiinflammatory properties.4
Adenosine mediates it’s protective effects through four adenosine receptors (Adora1, Adora2a, Adora2b, and Adora3).5 Although all adenosine receptors have been associated with cardiac tissue protection, Adora1, Adora2a, and Adora3 are the most widely studied.6–8 Recently, Adora2b was also described to elicit strong cardioprotection in I/R injury.9–11 It is expressed on cardiomyocytes, endothelial cells as well as on all main bone marrow-derived inflammatory cells, such as polymorphonuclear leukocytes (PMNs), macrophages or lymphocytes.12 However it is unknown, on which of these cells Adora2b needs to be activated to be cardioprotective. Based on the notion that adenosine elicits cardioprotection through inhibition of inflammation, Adora2b activation on inflammatory cells could represent a possible cardioprotective mechanism. It has been shown that Adora2b−/− mice have higher levels of Tumor necrosis factor α (TNFα).13 TNFα plays a major role in I/R induced apoptosis of cardiomyocytes during reperfusion.14 Bone marrow-derived inflammatory cells such as PMNs could represent a source of TNFα release and we therefore hypothesized that Adora2b-activation on bone marrow-derived cells is responsible for its cardioprotective effect through inhibition of proinflammatory cytokine release in the myocardium upon reperfusion.
To test this hypothesis we first submitted wild-type (WT) or Adora2b−/− mice to an in situ model for myocardial ischemia and reperfusion. Next, we used bone marrow transplantation from WT mice into Adora2b−/− and vice versa (chimeric mice) to identify the contribution of inflammatory cells for Adora2b mediated cardioprotection. This way, Adora2b was either absent on cardiac cells (endothelia, cardiomyocytes) or on bone marrow-derived inflammatory cells (PMNs, lymphocytes or macrophages). Subsequent analysis of cells attracted to the post-ischemic myocardium in WT or Adora2b−/− mice identified PMNs as dominant cell type. To understand Adora2b signaling on PMNs, WT mice with or without PMNs were exposed to in situ MI and treated with a specific Adora2b agonist (BAY 60–6583) upon reperfusion. Finally, to study effects of Adora2b signaling on PMNs, in vivo studies using Adora2b−/− mice or in vitro studies using isolated human PMNs with pharmacological inhibition or activation of the Adora2b were performed.
Using the above described multimodal approach, we found a critical role for Adora2b on bone-marrow derived inflammatory cells in order to elicit its cardioprotective effect. Consistent with these findings, we observed that Adora2b signaling is only cardioprotective when activated on PMNs. Finally, using in vivo and in vitro studies, we identified an Adora2b dependent TNFα release via PMNs.
MATERIALS AND METHODS
Mice
Experimental protocols were approved by the Institutional Review Board at the University of Tübingen, Tübingen, Baden-Wuertemberg, Germany, or the University of Colorado Anschutz Medical Campus, Aurora, Colorado. They were in accordance with the German Law on the Protection of Animals and the National Institutes of Health guidelines for use of live animals. C57BL/6J mice were obtained from Charles River Laboratories (Sulzfeld, Germany) or Jackson Laboratories (Bar Harbor, ME). Adora2b-deficient mice were generated by Deltagen, Inc. (San Carlos, CA).10
Murine Model for cardiac ischemia
Anesthesia was induced (70 mg/kg body weight intraperitoneal) and maintained (10 mg/kg/h) with sodium pentobarbital. Mice were placed on a temperature-controlled heated table (RT, Effenberg, Munich, Germany) with a rectal thermometer probe attached to a thermal feedback controller to maintain body temperature at 37°C. The tracheal tube was connected to a mechanical ventilator (Servo 900C, Siemens, Germany) with pediatric tubing and the animals were ventilated with a pressure controlled ventilation mode (peak ins piratory pressure of 10 mbar, frequency 110 breaths/min, positive end-expiratory pressure of 3 mbar, FiO2 = 0.3). Using this ventilator regime, blood gas analysis revealed normal PaO2 (115 ± 15 mmHg) and PaCO2 (38 ± 6 mmHg) levels during establishment of the method. Therefore this was used as standard setting in the present study. After induction of anesthesia, animals were monitored with a surface electrocardiogram (Hewlett Packard, Böblingen, Germany). Fluid replacement was performed with normal saline, 0.2 ml/h intravenously until the end of ischemia. To avoid cardiovascular collapse and to establish a stable reperfusion after ischemia, infusion rate was increased to 0.6 ml/h during the first hour of reperfusion. To avoid volume overload or dilution of the hematocrit, the rate was reduced to 0.2 ml/h in the second hour of reperfusion. The carotid artery was catheterized for continuous recording of blood pressure with a statham element (WK 280, WKK, Kaltbrunn, Switzerland). Operations were performed under an upright dissecting microscope (Olympus SZX12, Center Valley, PA). Following left anterior thoracotomy, exposure of the heart and dissection of the pericardium, the left coronary artery was visually identified and an 8.0 nylon suture (Prolene, Ethicon, Norderstedt, Germany) was placed around the vessel. Atraumatic left coronary artery occlusion for ischemia studies was performed using a hanging weight system.15 Successful left coronary artery occlusion was confirmed visually by an immediate color change of the vessel from light red to dark violet. The myocardium supplied by the vessel turned from bright red to pale and an immediate onset of ST-elevations in the electrocardiogram was noted. During reperfusion, the change of tissue color was immediately reversed when the hanging weights were lifted and electrocardiogram changes were reversed. To study cardioprotective effects, it is ideal to use an ischemia time associated with infarct sizes of approximately 30 to 40% of the area at risk (AAR). Thus it is possible to demonstrate changes in both directions, e.g., smaller or larger infarct sizes with an experimental therapeutic or a specific gene deletion. An ischemia time of 60 min resulted in a mean infarct size of approximately 45% of the AAR as shown earlier.15,16 Therefore a 60-min ischemia period was chosen in the present study. Infarct sizes were determined by calculating the percentage of infarcted myocardium to the AAR using a double staining technique with Evan’s blue and triphenyltetrazolium chloride. Evan’s blue is excluded from the area of the heart perfused by the left coronary artery and thus allows one to identify the AAR. Triphenyltetrazolium chloride stains tissue red except parts that are depleted in Nicotinamide adenine dinucleotide phosphate. This allows one to visualize infarcted tissue due to its white color. Using planimetry via the NIH software Image 1.0 (National Institutes of Health, Bethesda, MA), the AAR and the infarct size were determined. To measure reliability of infarct size analysis, interobserver variability was tested. Infarct sizes of animals were assessed by two independent investigators both blinded to the experimental protocol. Moreover, infarct size was measured twice on two separate days by the same investigator to reveal intraobserver variability.
Heart Enzyme Measurement
Blood was collected by central venous puncture for Troponin-I (cTnI) measurements using a quantitative rapid cTnI assay (Life Diagnostics, Inc., West Chester, PA).10,15
Generation of Adora2b bone marrow chimeric mice
Bone marrow chimeric mice were generated by radiation of WT (C57BL/6J) or Adora2b−/− mice followed by reconstitution with bone marrow derived from WT or Adora2b−/− mice. Briefly, male donor mice (6–8 week old, 20–22 g) were euthanized, and the marrow from the tibia and femur were harvested by flushing the marrow cavity with sterile isotonic NaCl solution. The bone marrow cells were then centrifuged at 400 g for 5 min, resuspended, and counted. Recipient mice (8–10 week of age, 20–25 g) were irradiated with a total dose of 12 Gy from a 137Cs source. Immediately after irradiation, 107 bone marrow cells/recipient in 0.3 ml 0.9% NaCl were injected into the jugular vein. The resulting chimeric mice were housed in microisolators for at least 8 week before experimentation and fed with water containing tetracycline (100 mg/L) in the first 2 weeks following bone marrow transplantation. Transplantation efficiencies were determined in preliminary experiments using the same conditioning regimen and transplanting CD45.1+ bone marrow into irradiated CD45.1−negative mice. In short, to confirm efficiency of reconstitution, a mutated mouse strain, B6.SJL-Ptprca Pep3b/BoyJ, was used as the source of donor bone marrow. The CD45.1 epitope, absent in cells of recipient mice, was detected by immunofluorescent cell analysis 8 weeks after bone marrow transplantation. The percentage of cells expressing CD45.1 was determined in each population of cells. For this purpose, blood was taken from transplanted recipients; FACSTM lysing solution (BD Pharmingen, San Diego, CA) was added to lysed red blood cells. After centrifugation cells were resuspended in 200 µl of phosphate buffered saline containing 1% bovine serum albumin. Peripheral blood stem cells were incubated with R-phycoerythrin conjugated antimouse CD45.1 monoclonal antibody (clone A20, BD Pharmingen) and either allophycocyanin-conjugated rat anti-mouse CD11b (Mac-1 α-chain, monocytic cells) monoclonal antibody (BD Pharmingen), rat antimouse Ly6G monoclonal antibody (neutrophils, BD Pharmingen), rat antimouse B220 monoclonal antibody (B cells, BD Pharmingen), fluorescein isothiocyanate-conjugated rat antimouse CD8a monoclonal antibody (CD8+ T lymphocytes, BD Pharmingen), or allophyco-cyanin-conjugated rat anti-mouse CD4 monoclonal antibody (CD4+ T lymphocytes, BD Pharmingen) on ice for 30 minutes. CD11b−, CD8a−, and CD4−, B220−, and Ly6G-positive cells were sorted by flow cytometry, and the percentage of cells expressing CD45.1 was determined in each population of cells (FACScan, CellQuest, Becton Dickinson, San Jose, CA).17
Immunohistochemistry
Hearts from untreated controls or from mice subjected to 60 min of ischemia were removed at indicated time points during reperfusion (15, 30, 60, 120 min ischemia) and fixed in 4% buffered formalin (pH 7.4) for 24 h and paraffin-embedded afterwards. The samples were cut in 5 µm slices, placed on SuperFrost Plus slides (Microm International, Walldorf, Germany) and deparaffinized. Histological and enzyme-histochemical evaluation consisted of hematoxylin-eosin staining and chloroacetate esterase staining for visualization of neutrophils. Immunohistochemical staining was performed with rat antimouse antibodies against F4–80 (Serotec, Oxford, United Kingdom), CD-3 (BD Biosciences, Franklin Lakes, NJ), Ly6G (BD Biosciences) as well as Myeloperoxidase (Dako, Hamburg, Germany). In addition, an Adora2b antibody (Millipore, Billerica, MA) was used to confirm Adora2b deficiency at protein level. Immunohistochemistry was performed using DiscoveryXT immunohistochemistry system (Ventana, Tucson, AZ). All histological and immunohistochemical staining were performed on serial sections. Evaluation of the histological and immunohistochemical staining and photographic documentation were performed using an Olympus BX-50 light microscope (Hamburg, Germany). Pictures were taken with a digital camera (Olympus, DP72, Hamburg, Germany).
Neutrophil depletion
In selected experiments, neutrophil depletion was achieved with an anti-GR-1 monoclonal antibody (BD Pharmingen) as described previously. C57BL/6 mice were treated with GR-1 monoclonal antibody 24 h before the experimental procedure, and neutrophil depletion was confirmed by differential blood counts.10
Myeloperoxidase and cytokine multiplex ELISA
To measure Myeloperoxidase and cytokine tissue levels (TNFα, Interferon γ, Interleukin-1β, Interleukin-2, Interleukin-4, Interleukin-5, KC, Interleukin-10, Interleukin-12) after 60 min of ischemia, C57BL/6J or Adora2b−/− mice were euthanized following 120 min of reperfusion. Remaining blood was removed; the myocardial tissue (area at risk) was excised after delineation with Evans' blue and immediately frozen at −80°C. Tissues were homogenized and a Myeloperoxidase ELISA (Signosis, Inc., Sunnyvale, CA) or a cytokine multiplex ELISA (MSD Cytokine Assays, Meso Scale Discovery, Gaithersburg, MA) was performed according to the manufacturer's recommendations.
Isolation of human PMNs
After approval by the Institutional Review Board and obtaining written informed consent from each individual, PMNs were freshly isolated as described previously. Five healthy males (32 yr old, age range: 28–35 yr) were included. The isolation of human neutrophils from whole blood was performed by the gradient density centrifugation method. Briefly, 50 mL of blood was collected with 10 mL of acid citrate dextrose. This was then mixed with 3% Dextran-500 and allowed to sit for sedimentation. The top layer, approximately 25 mL of clear leukocyte rich plasma, was carefully layered on top of 10 mL of Histopaque 1077. The tube was centrifuged at 2,700 rpm for 30 min at 20 °C. Once the centrifugation was complete the top layers were aspirated leaving the final layer containing erythrocytes and neutrophils. The pellet was resuspended and washed with erythrocyte lysis buffer. The sample was centrifuged at 1,000 rpm for 10 min at 20 °C and a white pellet was observed. The neutrophil pellet was resuspended and washed with Hank's balanced salt solution without Ca2+ and Mg2+. The suspension was then centrifuged at 1,000 rpm for 10 min at 20 °C after which the supernatant was decanted and the neutrophil pellet was resuspended in Hank's balanced salt solution with Ca2+ and Mg2+. The time from venipuncture to experimentation with agonist or antagonist was the same in all experiments (2 h). Isolated neutrophils were kept in ice until use. The neutrophils were used from one volunteer per experiment.18
Preparation of activated PMN supernatants and measurement of TNF α
Supernatants from PMNs were harvested 5, 30, 60, and 120 min after activation with formyl-Met-Leu-Phe (10 nM). TNFα was determined in the supernatant using a human TNFα ELISA (Thermo Fisher Scientific Inc., Waltham, MA).
Pharmacological agents
As a specific inhibitor of the Adora2b, PSB1115 (Tocris, Biotren-Chemikalien, Cologne, Germany) was used at a concentration of 100 µM. A highly specific agonist for the Adora2b, BAY 60–6583, developed by Bayer HealthCare AG (Wuppertal, Germany) was used at a concentration of 1µM.10
Statistics
An a priori sample size analysis for infarct sizes and cTnI measurement was based on previous studies suggesting a standard deviation for infarct size and cTnI serum level of 5% and 21 ng/ml, respectively. A biological relevant difference of at least 10 % for infarct size and 40 ng/ml for cTnI between control and experimental groups resulted in four animals per group to obtain statistically significant results with a probability of 0.8. A one-factor ANOVA was used when cell numbers or infarct sizes/cTnI values from different bone-marrow transplanted mice were compared. The dependent variable was the time in regard to bone-marrow ablation and the independent variable were the cell numbers/infarct sizes/ cTnI values. In the case where different treatment regimens were combined, data was compared by two-factor ANOVA. Once a significant interactive effect was established, a one-way ANOVA was performed using the Bonferroni correction. Where appropriate an independent Student’s t test was applied. For all comparisons in the current study, two-tailed testing was performed and P <= 0.05 was considered to be significant. In the current study no 'missing data' occurred. Data are expressed as mean ± SD. For statistical analysis GraphPad Prism 5.0 software for Windows or Bias for Windows® (epsilon-Verlag, Frankfurt, Germany) was used. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Adora2b activation decreases infarct size in vivo
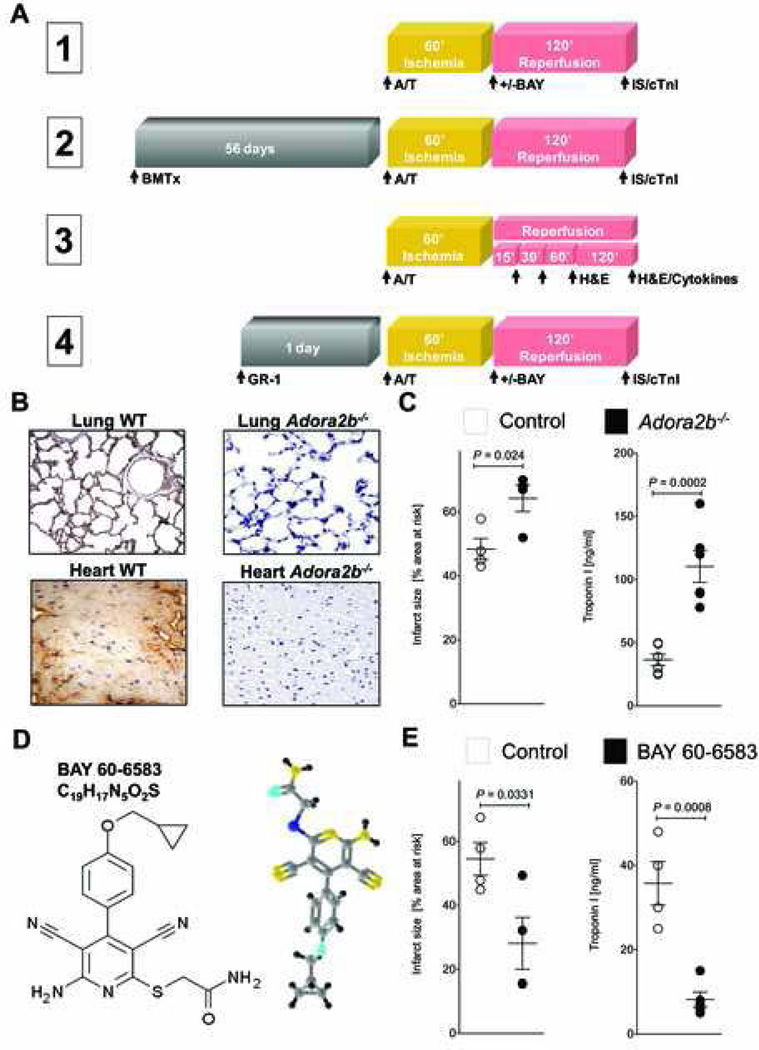
To determine whether Adora2b needs to be activated on cardiac cells (endothelial and cardiomyocytes) or on inflammatory cells, we used different in vivo experimental procedures. An overview of the different experimental designs and timelines is given in figure 1A. We first confirmed that Adora2b protein was indeed absent in commercially available Adora2b−/− mice using real-time polymerase chain reaction analysis10 (data not shown) or immunohistochemistry. No Adora2b transcript or protein was detected when compared to WT controls (Figure 1B). To confirm the cardioprotective effects of Adora2b activation, Adora2b−/− mice or WT controls were subjected to 60 min of ischemia followed by 120 min of reperfusion (Experiment 1 in fig. 1A). Infarct size (IS) staining using Evans Blue and triphenyltetrazolium chloride or serum cTnI analysis showed significantly increased infarct sizes or cTnI levels in Adora2b−/− when compared to WT mice (fig. 1C; ISwild-type:48 ± 4% and cTnIwild-type: 36.5 ± 4.7 ng/ml vs. ISAdora2b−/−: 64 ± 3.5% and cTnI Adora2b−/−:110.5 ± 12.7 ng/ml). Blood pressure and heart rate at baseline (107.3 ± 5mmHg; 492 ± 60 beats/min) or after ischemia (71.3 ± 5mmHg; 485 ± 60 beats/min) were not different between experimental groups. These results show that the absence of Adora2b increases myocardial injury during I/R.
Figure 1. Adora2b in IR injury.
(A) Overview of the 4 different experiment protocols used in this study. Abbreviations: A/T=Anesthesia/Thoracotomy; BMTx=Bone marrow transplantation; +/− BAY= with or without BAY 60–6583; GR-1= anti- (polymorphonuclear leukocyte) PMN antibody; H&E= hematoxylin and eosin stain; IS=Infarct size; cTnI=cardiac Troponin-I; Cytokines=Multiplex ELISA for cytokines. (B) Immunohistochemistry for the adenosine receptor A2b (Adora2b) revealed complete lack of Adora2b staining in Adora2b−/− animals while wild-type (WT) animals exhibit strong Adora2b protein expression in the heart and lung. (C) The absence of the Adora2b led to infarct sizes of 64±4%. Infarct sizes are presented as percentage of the area at risk (AAR). Serum Troponin-I levels were measured by ELISA. Adora2b−/− mice had Troponin-I values of 110±12 ng/ml, whereas WT had Troponin-I values of 36±4 ng/ml. (D) Two and three dimensional structure of BAY 60–6583, a highly specific Adora2b agonist used in this study. (E) WT mice were treated with BAY 60–6583 upon reperfusion and the infarct sizes and Troponin-I values were measured. BAY 60–6583 treatment reduced infarct sizes from 54 ± 5 to 28 ± 8%. Troponin-I values in the serum were reduced from 35 ± 5 to 8 ± 2 ng/ml by Adora2b agonist treatment; mean ±SD; n = 5 per group
We next investigated whether pharmacological activation of Adora2b also reduces myocardial injury in response to I/R. To answer this question, we submitted WT mice to I/R injury (Experiment 1 in fig. 1A). A single bolus of the Adora2b agonist BAY 60–6583 (10 µg/kg body weight) was given intraarterially with the onset of reperfusion after 60 min of ischemia. The chemical structure of BAY 60–6583 is displayed in figure 1D while specificity is published elsewhere.10 BAY 60–6583 treatment significantly decreased infarct sizes as well as cTnI values, when given upon reperfusion (fig. 1E). Thus, pharmacological activation of the Adora2b is feasible and cardioprotective in myocardial I/R injury.
Bone marrow transplantation changes the genotype of leukocytes
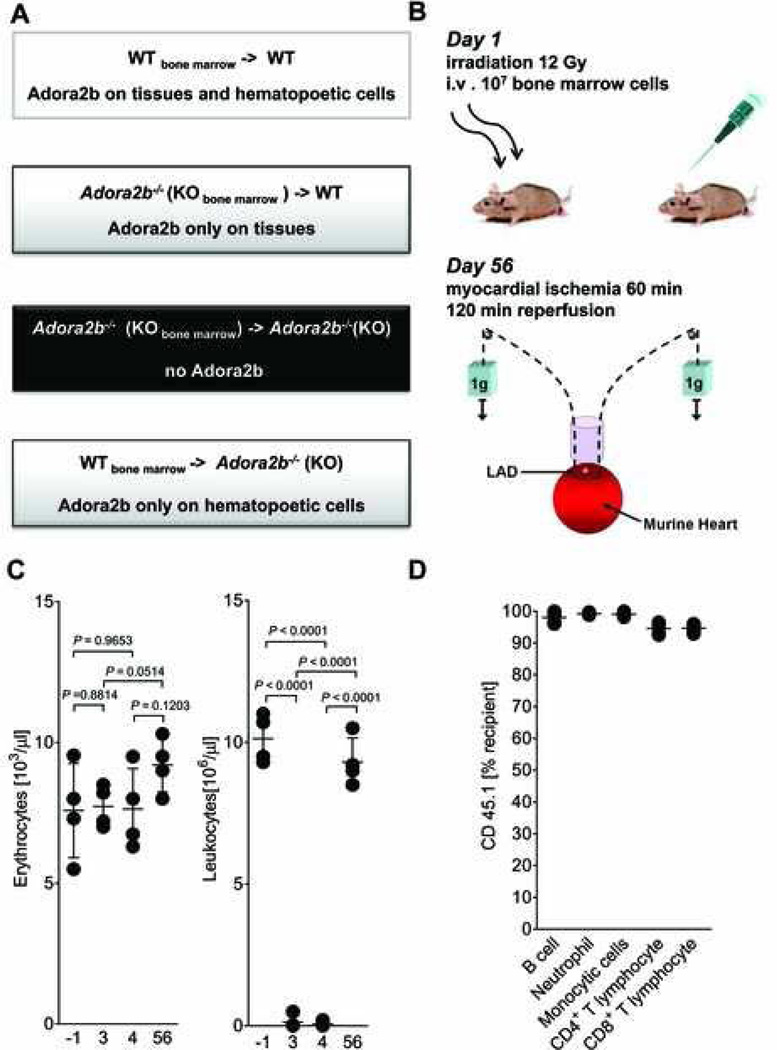
To study the contribution of cardiac cells (endothelial and cardiomyocytes) to Adora2b-mediated cardioprotection, we generated bone marrow - chimeric mice for the Adora2b (figs. 2A and B). This was achieved by whole body irradiation with subsequent bone marrow transplantation. After irradiation treatment, a recovery period of 56 days followed. Then, mice underwent cardiac I/R (fig. 2B, see also Experiment 2 in fig. 1A). The red blood cell count was unchanged by irradiation. In contrast to that, leukocyte numbers significantly decreased on day 3 and 4 after irradiation. At the recovery phase however, numbers of leukocytes were not significantly different when compared to controls (fig. 2C; before radiation 10.1 ± 0.9×103 cells/µl blood; 56 days after radiation 9.3 ± 0.9 ×103 cells/µl blood). Next, we tested the efficiency of leukocyte reconstitution after irradiation. For this purpose a mutated mouse strain (B6.SJL-Ptprca Pep3b/BoyJ) carrying the CD45.1 alloantigen was used as the source of donor bone marrow. The percentage of cells expressing CD45.1 was determined in different leukocyte populations using fluorescence-activated cell sorting analysis as done previously.17 The percentage of cells expressing CD45.1 was between 95% – 98% (fig. 2D). Thus, bone marrow-transplantation changes the genotype of the leukocyte population.
Figure 2. Generation of Adora2b bone marrow chimeric mice.
(A) Overview of the different bone-marrow-chimeric mice exposed to in situ myocardial ischemia (fig. 3). (B) Wild-type (WT) or adenosine receptor A2b minus (Adora2b−/−) mice were irradiated with 12 Gy to eliminate all bone-marrow derived cells. Next, 107 cells isolated from the WT or Adora2b−/− bone marrow were injected into mice after irradiation as indicated in (A). After 56 days these mice were submitted to an in vivo model of 60 min myocardial ischemia, followed by 120 min of reperfusion. (C) WT mice were treated with 12 gray (Gy) of irradiation to ablate all bone-marrow derived cells, and then reconstituted with 107 cells isolated from a donor animal bone marrow. Erythrocyte count as well as a Leucocyte count was performed to verify that the bone-marrow ablation was successful. Erythrocyte cell count did not change over time. Leucocytes linage was absent on day 3 (0.1 ± 0.1 × 106 cells/µl; n = 4) and day 4 (0.1 ± 0.1 × 106 cells/µl; n = 4) after irradiation treatment. Leucocytes count was at the same level as preirradiation value at day 56 after bone-marrow transplantation (day -1: 10.1 ± 0.5 × 106 cells/µl; day 56: 9.3 ± 0.5 × 106 cells/µl; mean ± SD; n = 4 per group). (D) WT mice underwent irradiation with 12 Gy to ablate all bone marrow derived cells. Then, irradiated WT mice received bone marrow from donor animal carrying the CD45.1 alloantigen on all bone marrow derived cells to reconstitute the white blood cell linage. Fluorescence-activated cell sorting analysis revealed that between 94 ± 0.6% (CD8+ T lymphocytes) and 98 ± 0.5% (B cell linage) of bone marrow derived cells carried the CD45.1 alloantigen ; mean ± SD; n = 5 per group.
Bone-marrow derived cells require Adora2b to protect from myocardial I/R injury
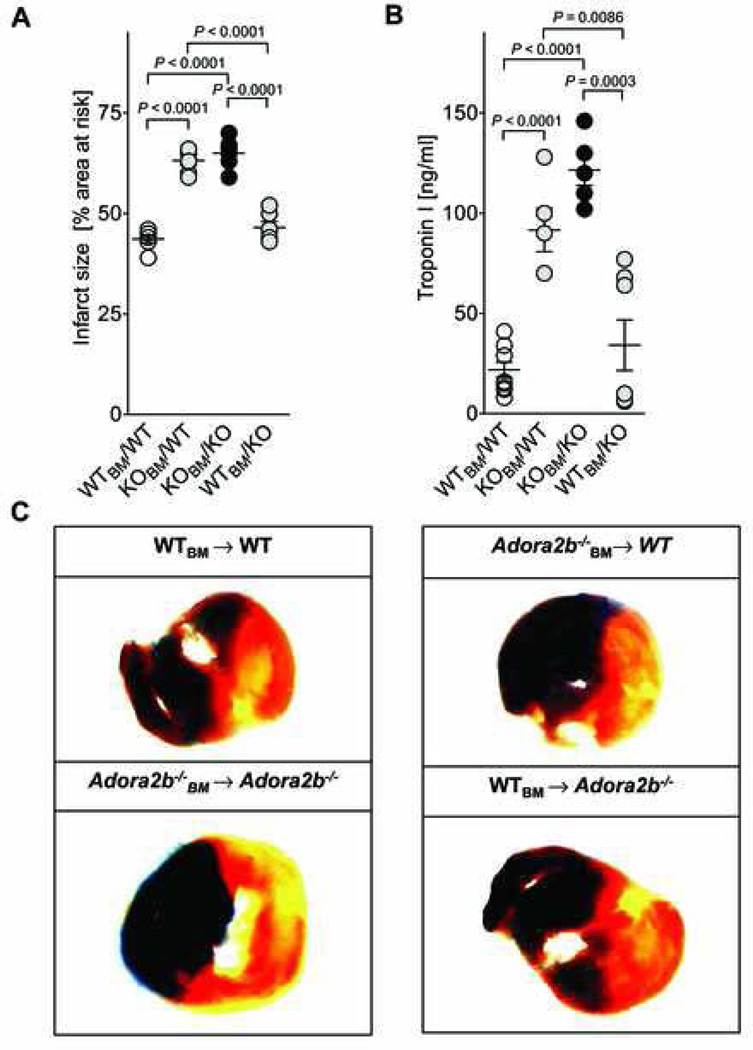
After having generated bone-marrow chimeric mice, we next asked on which cell type Adora2b needs to be activated in order to be cardioprotective: In chimeric mice, Adora2b protein is present either only on cardiac cells and other tissues (Adora2b−/− → WTBM) or only on inflammatory cells (WTBM → Adora2b−/−). These chimeric mice underwent 60 min of ischemia and 120 min of reperfusion (Experiment 2 in fig. 1A), followed by infarct size (IS) and cTnI measurements. When WT mice received Adora2b−/− bone marrow, they developed a phenotype very similar to Adora2b−/− mice and myocardial injury increased significantly (IS: from 42 to 62%; cTnI: 21.9 to 121.6 ng/ml; figs. 3A and B). When Adora2b−/− mice were transplanted with WT bone marrow, they took a phenotype similar to WT mice and myocardial injury was significantly decreased (IS: from 60 to 48%; cTnI: from 91.6 to 34.1 ng/ml; figs. 3A and B). Representative infarct staining is shown in Figure 3C. These results show that Adora2b needs to be present on bone-marrow derived cells in order to elicit its cardioprotective effect.
Figure 3. Infarct sizes and Troponin levels in Adora2b bone marrow–chimeric mice.
(A)–(C) Bone marrow (BM) chimeric mice for the Adora2b were subjected to 60 min of myocardial ischemia and 120 min of reperfusion. (A) Infarct sizes are presented as percentage of the area at risk (AAR). The absence of the Adenosine receptor A2b (Adora2b) on bone marrow derived cells led to infarct sizes of 60±4% [Adora2b−/− (KO)BM → WT (wild-type)] and 63% ± 4% [Adora2b−/− (KO)BM → Adora2b−/−]. Adora2b presence on bone-marrow derived cells led to infarct sizes of 42 ± 2% (WTBM → WT) and 48 ± 1% [WTBM → Adora2b−/− (KO)]. (B) Measurement of serum Troponin-I levels by ELISA. Adora2b absence on bone-marrow derived cells increased Troponin-I values to 122 ± 8 ng/ml [Adora2b−/− (KO)BM → WT] and 92 ± 11 ng/ml [Adora2b−/− (KO)BM → Adora2b−/−(KO)]. Adora2b presence on bone marrow derived cells reduced Troponin-I values to 22 ± 4 ng/ml (WTBM → WT) and 34 ± 13 ng/ml (WTBM → Adora2b−/−(KO)). (C) Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Representative images of infarct sizes from the experiments in (A, B) are displayed; mean ± SD; n = 5 per group.
Polymorphonuclear neutrophils (PMN) are the dominant inflammatory cell type in myocardial I/R
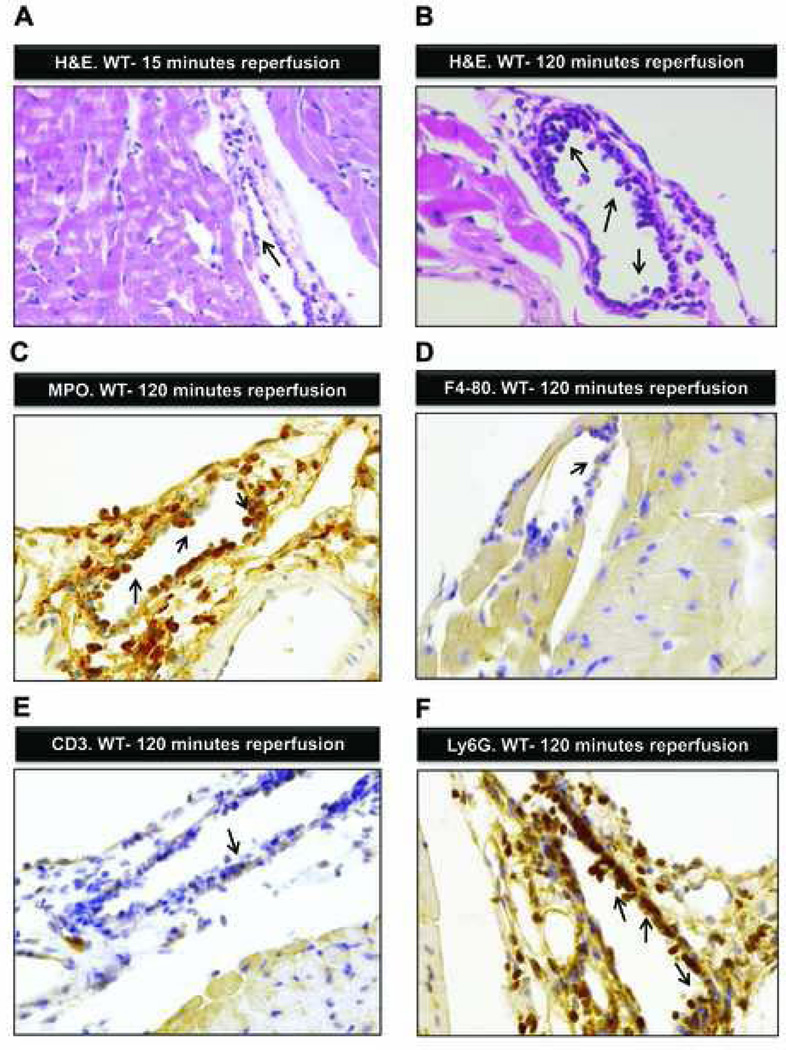
To understand the pathological and cellular changes during reperfusion, we performed hematoxylin and eosin stain staining of myocardial tissue from WT mice exposed to 60 minutes of ischemia followed by 5, 15, 30, 60, or 120 min of reperfusion (Experiment 3 in fig. 1A). Leukocytes adhered sporadically to the vessel wall in the early phase of reperfusion (15 min). The myocardium showed no structural changes (fig. 4A). Morphologically these leukocytes appeared to be PMNs. With longer reperfusion (120 min) significantly more leukocytes adhered to and crossed the vessel wall (fig. 4B). Myocardial cross striations blurred with slight edema of the cardiac tissue as a sign of severe myocardial damage. We next asked which leukocyte population was primarily present at 120 min of reperfusion. To answer this question we performed immunohistochemistry using myeloperoxidase as marker for PMNs, F4–80 as marker for monocytes and CD3 as marker for T cells. We found that most leukocytes adhering to or crossing cardiac vessel walls stained positive for myeloperoxidase (fig. 4C–E). To confirm this finding, we performed additional immunohistochemistry using the other highly specific PMN-marker Ly6G. Again, adherent leukocytes after 120 min of reperfusion stained positive for Ly6G (fig. 4F). Based on this, our results show that PMNs are the dominant bone marrow derived cell population during early and late reperfusion.
Figure 4. Ischemia-reperfusion associated histological changes.
(A) Hematoxylin and eosin stain staining of the myocardial tissue after 60 min of ischemia and 15-min reperfusion. The cardiac structure appears normal with a few inflammatory cells (arrow) adhering to the endothelia. (B) Hematoxylin and eosin staining of the myocardium after 60 min of ischemia followed by 120 min of reperfusion. Multiple inflammatory cells adhering to or passing the endothelial cell layer (arrows; original magnification 400×). (C) Immunohistochemistry for Myeloperoxidase of myocardial tissue after 60 min of ischemia and 120 min of reperfusion. Multiple Myeloperoxidase positive cells adhere to the endothelial cell layer or transmigrate to the perivascular space (arrows; original magnification 200×). (D) Immunohistochemistry for F4–80 surface protein (expressed on monocytes/macrophages) after 60 min of ischemia by 120 min of reperfusion. Single F4–80+ cell adheres to the endothelial cell layer (arrow). (E) Immunohistochemistry for CD3 (T-cell-marker) after 60 min of ischemia and 120 min of reperfusion. Sporadic CD3+ cells attach to the endothelial cell layer (arrow). (F) Immunohistochemistry for Ly6G (polymorphonuclear leukozyte (PMN) marker) after 60 min of ischemia and 120 min of reperfusion. Multiple PMNs adhere to the endothelial cell layer and infiltrate the myocardium (arrows, original magnification 200×); shown are representative images from one experiment out of four mice.
Adora2b-dependent cardioprotection relies on the presence of PMNs
We next asked whether the presence of PMNs increases myocardial injury in reperfusion. PMNs release different cytokines that have been shown to damage myocardial tissue even further in reperfusion. To deplete WT mice from PMNs, they received anti-GR1 antibody intraperitoneally 24 h prior to 60 min of myocardial ischemia and 120 min of reperfusion (Experiment 4 in fig. 1A). This antibody targets specifically PMNs and it reduced the PMNs numbers in the peripheral blood by 98% (fig. 5A). PMN-depletion significantly decreased myocardial damage in response to I/R injury (ISPMN depletion: 28 ± 8% and cTnIPMN depletion: 12.1 ± 3.6 ng/ml, fig. 5B–D). In fact, PMN-depletion was just as effective as Adora2b agonist treatment (fig. 5B–D). Thus, PMNs further enhance myocardial damage in the reperfusion after myocardial ischemia.
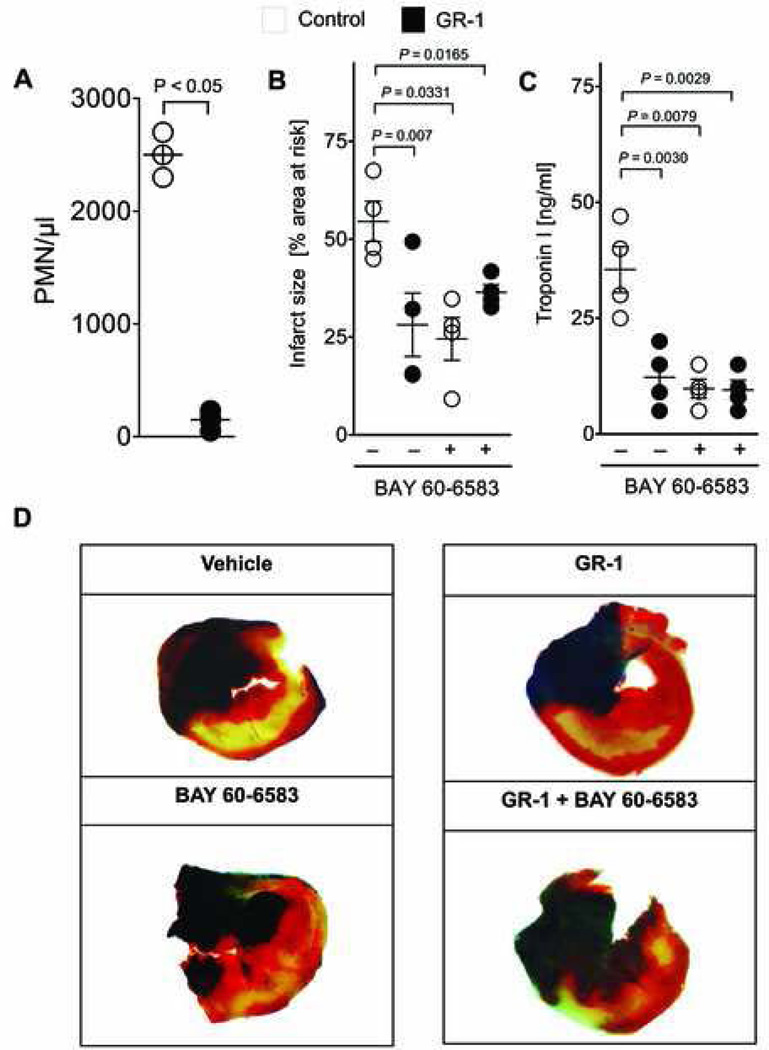
Figure 5. Adora2b signaling in PMN depleted mice.
(A) Wild-type mice were treated with a monoclonal antibody directed against the granulocyte receptor 1 (GR-1) exclusively expressed on polymorphonuclear leukocytes (PMNs) 24 h before the experiment. GR-1 treatment decreased PMNs in peripheral blood from 2,500 ± 150 PMNs/µl (control) to 150 ± 75 PMNs (mean ± SD, n = 4 per group). (B) Infarct sizes are presented as percentage of the area at risk (AAR). The pre-treatment with a PMN-depleting antibody alone led to Infarct sizes of 28 ± 8%. Additional administration of BAY 60–6583 during reperfusion in the presence of PMNs led to infarct sizes of 24 ± 5%, and to 36 ± 2% in the absence of PMNs. (C) Measurement of serum Troponin-I levels by ELISA. Treatment with BAY 60–6583 during reperfusion resulted in Troponin I values to 10±4 ng/ml. GR-1-anitbody treatment resulted Troponin I values to 12 ± 6 ng/ml. PMNs-depletion and subsequent BAY 60–6583 administration in the reperfusion resulted in Troponin I values of 9 ± 4 ng/ml. (D) Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Representative images of infarct sizes from the experiments in (B, C) are displayed; mean ± SD; n = 5 per group.
Next, we asked whether cardiac cells (endothelia and cardiomyocytes) also contribute to Adora2b dependent cardioprotection in I/R injury. To answer this question, we injected the Adora2b agonist BAY 60–6583 into PMN-depleted WT mice upon reperfusion and again determined the extent of myocardial injury. Although these animals received a combination of two cardioprotective treatments, the addition of Adora2b agonist to PMN-depletion had no additional effect on IS or cTnI (ISPMN depletion: 28 ± 6.3% vs. ISPMN depletion + BAY 60 6583: 36 ± 4.7 ng/ml, fig. 5B–D). These results indicate that Adora2b signaling is only cardioprotective when activated on PMNs.
Adora2b suppresses TNFα release in myocardial tissue I/R injury
Next, we wanted to determine whether Adora2b activation has an influence in the recruitment of PMNs into the area at risk. We used Myeloperoxidase as a surrogate parameter to quantify PMN numbers in the tissue. WT or Adora2b−/− mice underwent 60 min ischemia followed by 120 min of reperfusion. Next, we harvested the area at risk and measured the myeloperoxidase protein content by ELISA (Experiment 3 in fig. 1A). The Myeloperoxidase content in Adora2b−/− mice was not increased when compared to WT (fig. 6A). Thus, Adora2b activation has no influence on the number of PMNs infiltrating the myocardial tissue after I/R injury.
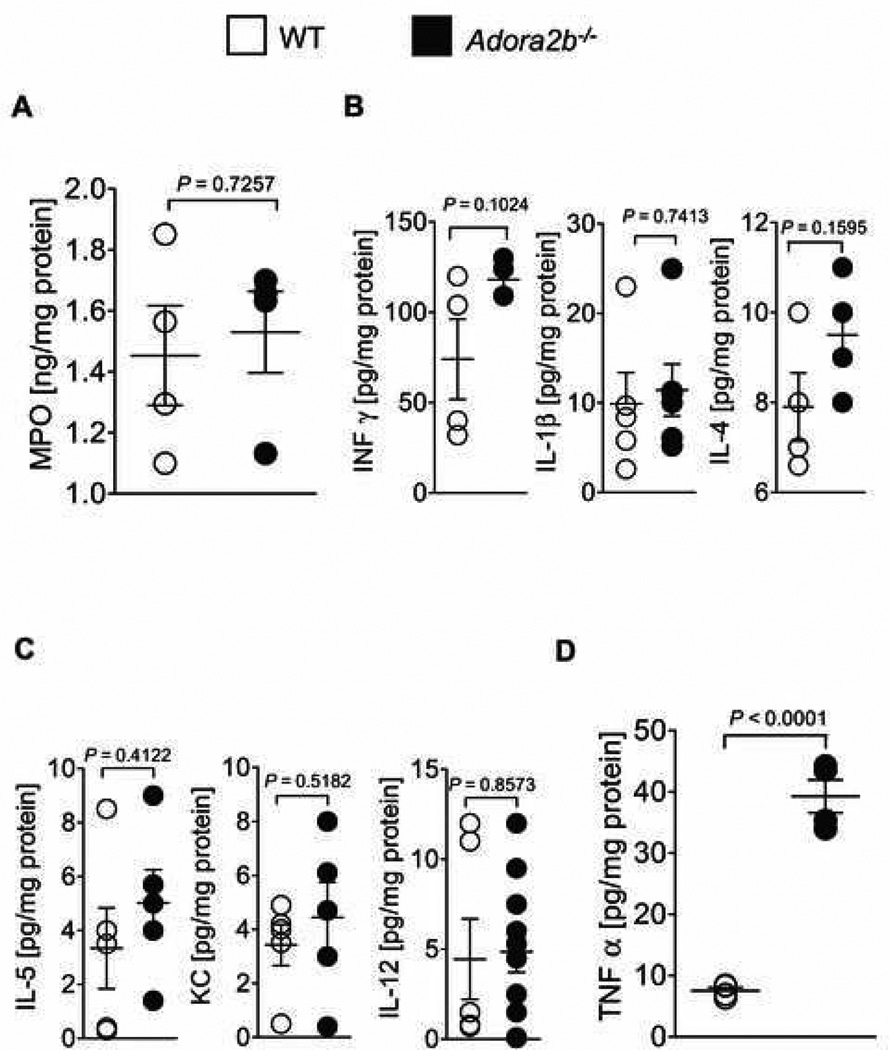
Figure 6. Cytokine profiling during ischemia reperfusion.
(A–D) Wild-type (WT) or Adora2b−/− mice underwent 60 min ischemia followed by 120 min of reperfusion. The area at risk was harvested, homogenized and probed for various cytokines using a muliplex ELISA. Values were normalized to the total protein content of the tissue samples. (A) To quantify polymorphonuclear leukocytes (PMN) sequestration into the myocardium, an ELISA for Myeloperoxidase (MPO) was performed (WT: 1.4 ± 0.2 ng/mg Protein; Adora2b−/−: 1.5 ± 0.1 ng/mg protein; not significant). (B, C) In order to characterize the inflammatory response in WT and Adora2b−/−, Interferon-γ (Inf-γ), Interleukin -1β (IL-1β), Interleukin -2 (IL-2), Interleukin -4 (IL-4), Interleukin -5 (IL-5), Keratinocyte-derived Cytokine (KC), Interleukin - 10 (IL-10) and Interleukin -12 (IL-12) tissue levels were measured; Note: no significant changes were found whereas Interleukin-2 and Interleukin-10 were negative in both groups (data not shown). (D) Tumor necrosis factor-alpha (TNFα) content of the area at risk in WT and Adora2b−/− (WT: 7.5 ± 0.5 pg/mg protein; Adora2b−/−: 39.3 pg/mg protein); mean ± SD; n = 4 per group.
To understand why Adora2b signaling protects the myocardium from I/R despite the fact that it has no influence on PMN numbers, we quantified the protein levels of several proinflammatory cytokines in the area at risk of Adora2b−/−. These proinflammatory cytokines contribute to the damage of the myocardium by enhancing the inflammatory response after myocardial ischemia. We measured TNFα, Interferon-γ, Interleukin-1β, Interleukin-2, Interleukin 4, Interleukin-5, Keratinocyte-derived Cytokine, Interleukin-10 and Interleukin-12 protein content in the area at risk using a multiplex ELISA (Meso Scale; fig. 6B–D). This experiment revealed that TNFα, Interferon-γ, Interleukin-1β, Interleukin-2, Interleukin-4, Interleukin-5, Keratinocyte-derived Cytokine, Interleukin-10 and Interleukin-12 levels were not different between WT and Adora2b−/− mice in the area at risk (figs. 6B and C). However, TNFα protein content was five times higher in the area at risk from Adora2b−/− mice when compared to that from WT (39.3 ± 2.7 vs. 7.5 ± 0.5 pg/mg protein, p < 0.05; fig. 6D). These findings provide evidence that Adora2b activation inhibits TNFα release, while it has no influence on absolute PMN-numbers in the myocardial tissue after I/R injury.
Adora2b agonist (Bay 60–6583) dampens TNFα release from activated human PMNs
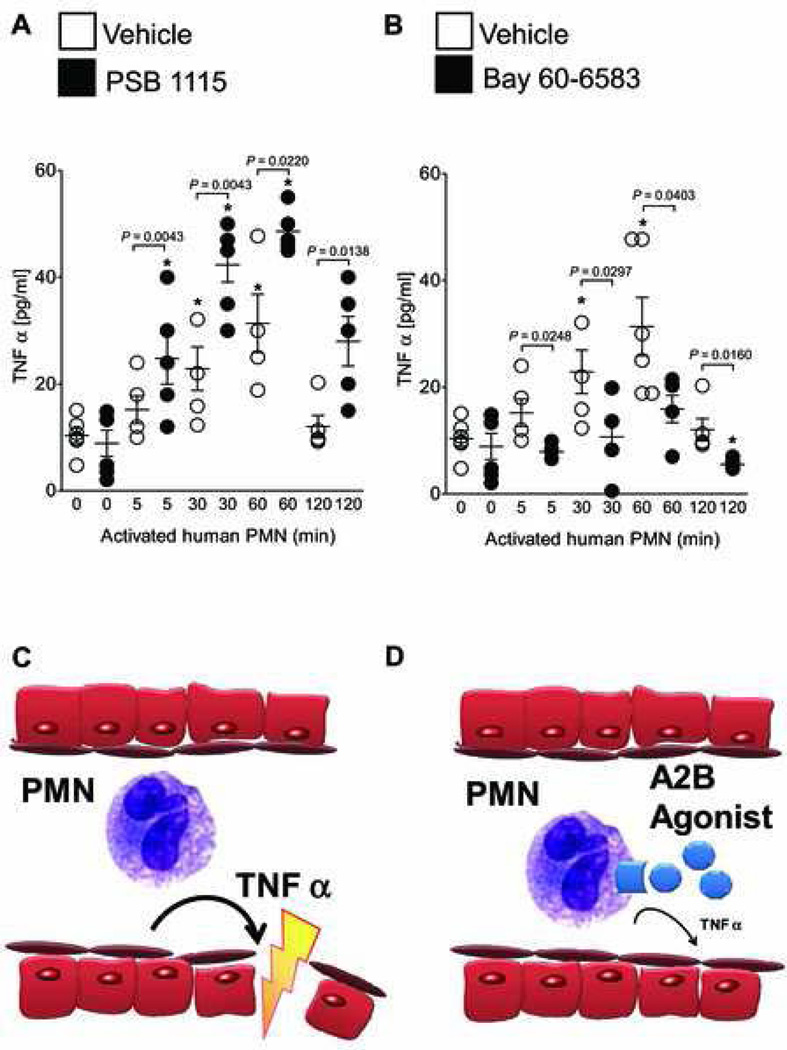
We next asked whether Adora2b directly inhibits the release of TNFα from PMNs. To this end, we isolated PMNs and activated the cells with formyl-Met-Leu-Phe (10 nM). This was done in the presence of an Adora2b antagonist (PSB 1115) or agonist (BAY 60–6583). To eventually translate such findings into a clinical setting we used human PMNs. Then we measured the content of TNFα in the supernatant over time by ELISA. Activated PMNs increased their TNFα release 2.3 ± 0.4 and 3.2 ± 0.3 fold (p < 0.05, n = 5) at 30 and 60 min, respectively. Pretreatment with a specific Adora2b antagonist PSB 1115 (100 µM) significantly amplified the TNFα release throughout the experiment (fig. 7A). In contrast to that, pretreatment of human PMNs with a specific Adora2b agonist (BAY 60–6583, 1µM) abrogated the TNFα release at any time point throughout the experiment (fig. 7B). Moreover, pretreatment with BAY 60–6583 suppressed TNFα release from PMNs below baseline at 120 min (fig. 7B).
Figure 7. Adora2b signaling in human PMNs.
(A, B) Human polymorphonuclear leukocytes (PMNs) were isolated from healthy individuals and activated using formyl-Met-Leu-Phe (fMLP 10 nM). Cells were either treated with a specific Adora2b antagonist (PSB 1115, 100 µM, a) or an Adora2b agonist (BAY 60–6583,1µM, b) or vehicle. Tumor necrosis factor-alpha (TNFα) release into the supernatant was measured by ELISA. Vehicle treatment of PMNs significantly increased TNFα with a maximal response after 30 and 60 min following activation (22.88 ± 4.0 pg/ml and 31.4 ± 5.4 pg/ml, respectively). (A) Pretreatment with an Adora2b antagonist enhances TNFα secretion significantly (24.8 ± 4.8, 42.3 ± 3.2, 48.6 ± 4.0 and 28 ± 4.6 pg/ml at 5, 30, 60, or 120 min, respectively). (B) Pretreatment with an Adora2b agonist significantly dampens TNFα release (7.89 ± 1.3, 10.70 ± 2.6, 15.9 ± 2.5 and 5.5 ± 0.9 pg/ml at 5, 30, 60, and 120 min, respectively; n = 5 per group). (C, D) Proposed role and potential therapeutic intervention: Cardiac ischemia reperfusion injury leads to PMN adhesion to the endothelial cells in the area at risk. (C) PMNs release TNFα, which further damages the myocardial tissue and enhances reperfusion injury. (D) Pharmacological treatment upon reperfusion using an Adora2b agonist activates A2B adenosine receptors on PMNs, which inhibits TNFα release and thereby protects the myocardium from further damage.
Based on our findings we propose that Adora2b activation suppresses TNFα release by PMNs in I/R injury. Therefore we suggest Adora2b treatment to reduce PMN dependent injury and TNFα release and thereby ameliorating I/R injury (figs. 7C and D).
DISCUSSION
The question addressed in this study was to identify the contribution of Adora2b on inflammatory cells to cardioprotection in I/R injury. The main finding of our study is that Adora2b mediated cardioprotection is dominantly through inflammatory cells, most likely due to an inhibition of TNFα release from invading PMNs.
Adora2b is expressed on cardiac, vascular, hematopoietic cells12,19 and its activation protects the myocardium from I/R injury.10,20 Theoretically, all these cell types could be responsible for Adora2b dependent cardioprotection. In this study we provide evidence that Adora2b is cardioprotective through activation of PMNs during reperfusion after myocardial ischemia. Two findings from our experiments support this concept. First, when Adora2b is present on inflammatory cells (but absent on all other cells), infarct sizes are identical to WT controls. This effect is reversed when Adora2b is absent on inflammatory cells. Then, myocardial injury is very similar to whole-body Adora2b-deficient mice. Second, a highly specific agonist for Adora2b reduces infarct sizes to the same extent as depletion of PMNs. The combination of both treatments (Adora2b agonist and PMN depletion) has no additional protective effect. Therefore the activation of Adora2b on endothelial cells or cardiomyocytes does not contribute to the cardioprotective effects. Our observation that Adora2b is cardioprotective when activated on PMNs is in line with other studies. Adenosine receptor blockade on PMNs drastically increases I/R injury.21 Stimulation of adenosine receptors with AMP-579 is highly cardioprotective, primarily through inhibition of PMN activation and adhesion to vascular wall.22,23 Unfortunately, AMP-579 is an unspecific Adora1, Adora2a, and Adora2b agonist, which makes it difficult to draw conclusions about the adenosine receptor subtype involved.24,25 Studies in gene targeted mice suggest that primarily signaling through Adora2b is responsible for this effect on PMNs.13
I/R injury elicits a strong inflammatory response in the heart leading to the recruitment of leukocytes to the myocardium. The vast majority of leukocytes are PMNs in our study. This is in line with several animal and clinical studies indicating that the principal effector cells of reperfusion injury are blood borne cells such as PMNs.26–28 PMNs mediate myocardial necrosis during ischemia, reperfusion, or both. In our experiments, PMN depletion or treatment with an Adora2b agonist decrease infarct sizes to a similar extent. The protective effect of the two interventions however is not cumulative. When Adora2b is activated, it inhibits neutrophil transmigration and the expression of adhesion molecules.13,29 Based on this, we suspected that the number of PMNs in the area at risk in Adora2b−/− mice should be higher than in WT animals. However, we were suprised to find identical myeloperoxidase levels in the postischemic tissue of WT and Adora2b−/− mice. This means that the number of PMNs in the area at risk is identical in the two genotypes. Since Adora2b-deficient mice have an enhanced inflammatory response and activation of Adora2b elicits antiinflammatory responses12,13,17,29, we subsequently assessed whether Adora2b activation changes PMNs activity rather than recruitment in I/R injury.
A screening of seven cytokines revealed significantly higher TNFα levels in Adora2b deficient mice when compared to WT after I/R injury. This is consistent with previous reports, showing that Adora2b−/− mice have higher plasma level of the proinflammatory cytokine TNFα at baseline and after vascular injury.13,30 The suppression of TNFα might be the primary mechanism how Adora2b is protective in myocardial I/R injury. Several findings in this study support that. Adora2b−/− mice have higher levels of TNFα. Many cell types, such as mast cells, endothelia, cardiomyocytes and PMNs could be the source of this TNFα release during I/R.31 In our model of myocardial I/R injury Adora2b mainly mediated cardioprotection through activation on PMNs. The pharmacological blockade of the receptor increases TNFα release from PMNs. Vice versa activation of Adora2b suppresses TNFα release from PMNs. In patients with ischemic heart disease leukocytes are the primary source of TNFα.32,33 TNFα can damage the myocardium in two ways. First, it directly induces apoptosis of cardiomyocytes in reperfusion.14,34 Second, it enhances the activation and recruitment of neutrophils into the postischemic myocardium that further damage the myocardium.35 A suppression of TNFα, is beneficial in acute myocardial I/R injury36,37 and we suggest Adora2b signaling as a different route to achieve this.
Several considerations have to be kept in mind when interpreting data from our study. First, in adenosine-receptor deficient mice, compensatory upregulation of other adenosine receptors has been described.38 However, this cannot explain the observed phenotype in our pharmacological studies on WT mice treated with the Adora2b agonist BAY 60–6583 upon reperfusion. Moreover, previous studies on transcript levels of other adenosine receptors (Adora1, Adora2a, Adora3) were unaltered in the Adora2b−/− mouse strain used in the current study.10 Based on this, we believe that all observations made in Adora2b−/− are a consequence of the gene deletion. Second, we used a coronary artery ligation model in mice. Collateral blood flow during the coronary occlusion can change the size of an infarction significantly. Certain species, e.g., guinea pigs and dogs, have an extensive collateral flow and a single vessel ligation is less effective than in other species.39 In contrast to that, mice and rats only have limited collateral flow.40 However only mice with the same genetic background should be used, since there are differences in collateral flow between different mouse strains.41 All mice used in this study have a C57J background. Therefore, we believe that a difference in collateral blood flow did not contribute significantly to observed changes in infarcts sizes. Third, there are obvious differences between mouse and human physiology, despite the fact that 99% of mouse genes have an equivalent in humans.42 Mice have a heart rate 10 times higher than humans. As a consequence, murine cardiomyocytes contraction relies almost completely on calcium release from the sarcoplasmatic reticulum. Thus, caution must be exercised while extrapolating findings from these models to the human setting. Nevertheless genetically engineered mouse models are the mainstay to study cardiac structure-function relationships.
In summary, this study shows that Adora2b is dominantly protective when activated on PMNs in myocardial I/R injury. It decreases the release of the proinflammatory cytokine TNFα from PMNs and limits inflammatory responses after I/R injury. We believe that Adora2b agonist administration may provide a novel therapeutic option in the treatment of myocardial I/R injury.
What We Already Know about This Topic
Adenosine receptor(Adora)2b has been shown to be protective during myocardial ischemia-reperfusion (I/R) injury
Based on the notion that adenosine elicits cardioprotection through inhibition of inflammation, the present study investigated whether Adora2b activation on bone marrow-derived cells such as polymorphonuclear leukocytes (PMNs) inhibits proinflammatory cytokine release following myocardial I/R
What This Article Tells Us That Is New
Adora2b activation on PMNs is cardioprotective during myocardial I/R injury by limiting the release of the proinflammatory cytokine tumor necrosis factor-α
Acknowledgments
Sources of financial support for the work:
Institutional support: University of Tübingen, Tübingen, Baden-Württemberg, Germany, University of Colorado Anschutz Medical Campus, Aurora, Colorado; Edinger- Institute, Frankfurt, Frankfurt, Hesse, Germany; Governmental funding: National Heart, Lung, and Blood Institute (NIH-NHLBI). Bethesda, Maryland, Grant 1K08HL102267-01 to T.E.; Foundation for Anesthesia Education and Research, Rochester, Minnesota to T. E. the American Heart Association Scientist Development Grant (AHA SDG), Dallas, Texas to T. E. and a Deutsche Forschungsgemeinschaft research fellowship, Bonn, North Rhine-Westphalia, Germany to M. K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings at which the work has been presented:
Molecular Cardiology: Disease Mechanisms and Experimental Therapeutics, February 22 – 27, 2011, Keystone, Colorado; IARS 2011 Annual Meeting May 21–24, 2011, Vancouver, British Columbia, Canada
Summary statement: Cardiac ischemia-reperfusion injury represents a major cause of tissue damage. The current study aims to understand the contribution of adenosine A2b-receptor signaling on inflammatory cells to cardiac ischemia-reperfusion injury.
REFERENCES
- 1.Hausenloy DJ, Yellon DM. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359:518–520. doi: 10.1056/NEJMe0803746. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Jennewein C, Paulus P, Zacharowski K. Linking inflammation and coagulation: Novel drug targets to treat organ ischemia. Curr Opin Anaesthesiol. 2011;24:375–380. doi: 10.1097/ACO.0b013e3283489ac0. [DOI] [PubMed] [Google Scholar]
- 4.Peart JN, Headrick JP. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Eltzschig HK. Adenosine: An old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neely CF, DiPierro FV, Kong M, Greelish JP, Gardner TJ. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the heart. Circulation. 1996;94:II376–II380. [PubMed] [Google Scholar]
- 7.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 8.Cerniway RJ, Yang Z, Jacobson MA, Linden J, Matherne GP. Targeted deletion of A(3) adenosine receptors improves tolerance to ischemia-reperfusion injury in mouse myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1751–H1758. doi: 10.1152/ajpheart.2001.281.4.H1751. [DOI] [PubMed] [Google Scholar]
- 9.Kuno A, Solenkova NV, Solodushko V, Dost T, Liu Y, Yang XM, Cohen MV, Downey JM. Infarct limitation by a protein kinase G activator at reperfusion in rabbit hearts is dependent on sensitizing the heart to A2b agonists by protein kinase C. Am J Physiol Heart Circ Physiol. 2008;295:H1288–H1295. doi: 10.1152/ajpheart.00209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 11.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A(2B) adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–H2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 16.Warth A, Eckle T, Kohler D, Faigle M, Zug S, Klingel K, Eltzschig HK, Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology. 2007;107:402–410. doi: 10.1159/000099060. [DOI] [PubMed] [Google Scholar]
- 17.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas M, Porto G, Lima JL, Fernandes E. Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection. Clin Biochem. 2008;41:570–575. doi: 10.1016/j.clinbiochem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 21.Hu G, Salem MR, Crystal GJ. Role of adenosine receptors in volatile anesthetic preconditioning against neutrophil-induced contractile dysfunction in isolated rat hearts. Anesthesiology. 2005;103:287–295. doi: 10.1097/00000542-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Budde JM, Velez DA, Zhao Z, Clark KL, Morris CD, Muraki S, Guyton RA, Vinten-Johansen J. Comparative study of AMP579 and adenosine in inhibition of neutrophil-mediated vascular and myocardial injury during 24 h of reperfusion. Cardiovasc Res. 2000;47:294–305. doi: 10.1016/s0008-6363(00)00115-2. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Zhao ZQ, Clark KL, Velez DV, Guyton RA, Vinten-Johansen J. A novel adenosine analog, AMP579, inhibits neutrophil activation, adherence and neutrophil-mediated injury to coronary vascular endothelium. Eur J Pharmacol. 2000;397:197–205. doi: 10.1016/s0014-2999(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 24.Kristo G, Yoshimura Y, Keith BJ, Stevens RM, Jahania SA, Mentzer RM, Jr, Lasley RD. Adenosine A1/A2a receptor agonist AMP-579 induces acute and delayed preconditioning against in vivo myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287:H2746–H2753. doi: 10.1152/ajpheart.00493.2004. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Yang X, Yang XM, Walker S, Forster K, Cohen MV, Krieg T, Downey JM. AMP579 is revealed to be a potent A2b-adenosine receptor agonist in human 293 cells and rabbit hearts. Basic Res Cardiol. 2010;105:129–137. doi: 10.1007/s00395-009-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 27.Mehta J, Dinerman J, Mehta P, Saldeen TG, Lawson D, Donnelly WH, Wallin R. Neutrophil function in ischemic heart disease. Circulation. 1989;79:549–556. doi: 10.1161/01.cir.79.3.549. [DOI] [PubMed] [Google Scholar]
- 28.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80:1816–1827. doi: 10.1161/01.cir.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: The holy grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 32.Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, Khaghani A, Barton PJ, Polak JM, Pepper JR, Banner NR, Yacoub MH. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001;104:I233–I240. doi: 10.1161/hc37t1.094872. [DOI] [PubMed] [Google Scholar]
- 33.Vaddi K, Nicolini FA, Mehta P, Mehta JL. Increased secretion of tumor necrosis factor-alpha and interferon-gamma by mononuclear leukocytes in patients with ischemic heart disease. Relevance in superoxide anion generation. Circulation. 1994;90:694–699. doi: 10.1161/01.cir.90.2.694. [DOI] [PubMed] [Google Scholar]
- 34.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJE, Sabbadini RA. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes - Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz R, Aker S, Belosjorow S, Heusch G. TNFalpha in ischemia/reperfusion injury and heart failure. Basic Res Cardiol. 2004;99:8–11. doi: 10.1007/s00395-003-0431-x. [DOI] [PubMed] [Google Scholar]
- 36.Kupatt C, Habazettl H, Goedecke A, Wolf DA, Zahler S, Boekstegers P, Kelly RA, Becker BF. Tumor necrosis factor-alpha contributes to ischemia- and reperfusion-induced endothelial activation in isolated hearts. Circ Res. 1999;84:392–400. doi: 10.1161/01.res.84.4.392. [DOI] [PubMed] [Google Scholar]
- 37.Niemann JT, Youngquist S, Rosborough JP, Shah AP, Phan QT, Filler SG. Infliximab attenuates early myocardial dysfunction after resuscitation in a swine cardiac arrest model. Crit Care Med. 2010;38:1162–1167. doi: 10.1097/CCM.0b013e3181d44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng B, Ledent C, Mustafa SJ. Up-regulation of A 2B adenosine receptor in A 2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol. 2008;44:905–914. doi: 10.1016/j.yjmcc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: A critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovas Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 40.Hearse DJ. The elusive coypu: The importance of collateral flow and the search for an alternative to the dog. Cardiovasc Res. 2000;45:215–219. [Google Scholar]
- 41.Schaper W. Collateral circulation: Past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]