Abstract
We describe a Turkish patient with tyrosine kinase 2 (Tyk2) deficiency who suffered from disseminated Bacille Calmette-Guerin infection, neurobrucellosis and cutaneous Herpes zoster infection. Tyk2 deficiency should be considered in patients susceptible to herpes viruses and intramacrophae pathogens even in the absence of atopy, high serum IgE, and staphylococcal disease.
Keywords: Mendelian susceptibility to mycobacterial diseases, Tyk2 deficiency, Hyperimmunoglobulin E syndrome, Brucellosis, Zoster
The first patient with autosomal recessive (AR) tyrosine kinase 2 (Tyk2) deficiency, was reported in 2006 [1]. This 22-year-old Japanese man displayed multiple episodes of staphylococcal disease. Despite the lack of developmental defects typical of hyper IgE syndrome (HIES), a diagnosis of HIES was proposed because the patient also suffered from severe atopic dermatitis and had a high serum immunoglobulin E concentration (2,100 IU/ml) [1]. In 2007, mutations in STAT3 were shown to be a cause of the common, autosomal dominant (AD) form of HIES [2]. In addition to the recurrent staphylococcal infections of the skin and lungs, atopic dermatitis and high serum IgE concentrations (>1000 IU/mL) found in almost all patients with HIES, patients with AD HIES display skeletal, dental and connective tissue abnormalities [3]. By contrast, the patient with Tyk2 deficiency had no bone fracture and his dentition and facial appearence were normal. In addition to cardinal features of HIES, the patient presented viral infections, including molluscum contagiosum (MC) and herpes simplex (HS) infections of the skin and mucosa. Such infections were seen in another form of AR HIES, which is due to mutations in DOCK8 [4]. Moreover, the patient had Bacille Calmette-Guerin (BCG) disease at 22 months of age and non-typhoidal Salmonella gastroenteritis at 15 years, neither of which is typically associated with HIES [1]. We report here the clinical features of a patient with Tyk2 deficiency. Despite some overlap, the clinical features of our patient and the Japanese patient with Tyk2 deficiency are markedly different.
Case report
This now 8 year old male patient was first admitted as a 2 year old in 1994, to Uludag University Hospital with fever, fatigue and recurrent draining cervical, inguinal, and axillary lymph nodes. He was born in 1992 and was the third child of healthy, first-cousin parents. The patient was vaccinated with BCG at two months. At eight months, left axillary lymphadenopathy was detected, and treatment with isoniazid (INH) was initiated. Although initially the regional lymphadenopathy began to regress, the patient developed generalized lymphadenopathy and fatigue at 21 months. On admission, physical examination revealed fever (39°C), and fistulous enlarged bilateral lymp hadenopathy of the cervical, axillary and inguinal regions (Figure). Complete blood count, renal and hepatic function tests were normal. Intradermal skin tests with 5 TU PPD was negative. Chest X-ray showed no lung involvement and skeletal X-ray survey was normal. Immunological assessments, including the determination of serum IgG, IgM and IgA levels and nitroblue tetrazolium test (NBT) results were normal. Serum IgE concentration was 115 IU/ml (normal range: 1.2-52 IU/ml) (Table). Histologic examination of his left axillary lymph node revealed widespread macrophage and polymorphonuclear cell infiltration in the dermis with positive staining for acid-fast bacilli. M. bovis BCG was isolated from the discharging cervical sinuses. The patient was treated with a regimen of INH, rifampicin, streptomycin and pyrazinamide for two months, followed by INH and rifampicin for 16 months. His clinical condition improved, with no relapse.
Figure.
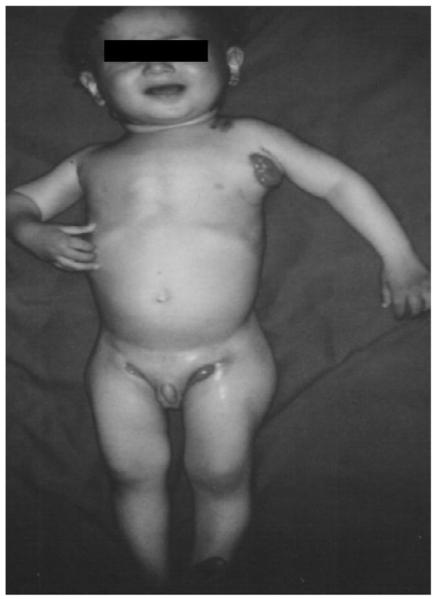
The patient had generalized, bilateral, cervical, axillary, and inguinal lymphadenitis on admission.
Table 1.
Immunologic parameters of the patient
|
The patient (at 2 years old) |
Normal values | |
|---|---|---|
| Absolute lymphocyte counts (/mm3) | 4300 | 1100-5900 |
| Absolute eosinophil count (/mm3) | 296/mm3 | 50-350/mm3. |
| Ig G (mg/dl) | 1220 | 764-2134* |
| Ig M(mg/dl) | 234 | 69-387 |
| Ig A(mg/dl) | 79.9 | 70-303 |
| Ig E (IU/ml) | 115 | 1.2-52 |
| T cells CD3+ % (absolute counts /mm3) |
67 (2881) | 55-78 (700-4200) |
| B cells CD19+ % (absolute counts /mm3) |
11 (473) | 10-31 (200-1600) |
| CD4+ % (absolute counts /mm3) |
38 (1634) | 27-53 (300-2000) |
| CD8+ % (absolute counts /mm3) |
22 (946) | 19-34 (300-1800) |
| Natural killer cells | 20 (860) | 4-26 (90-900) |
| CD3-CD16+CD56+% (absolute counts /mm3) |
||
| Antibody response to tetanus toxoid (IU/ml) |
0.69 | 2-20 |
| NBT (Nitroblue tetrazolium), (%) | 100 | 100 |
The normal serum immunoglobulin concentrations for age were defined according to reference values for healthy Turkish children
At eight years, the patient was admitted to a local hospital with a history of 12 h of fever, generalized clonic-tonic seizure, frontal headache and vomiting. At initial presentation, the patient was conscious. His temperature was 38.6°C, with a physical examination, exept for neck stiffness. The patient had a history of unpasteurized cheese consumption. A lumbar puncture performed on admission showed 200 cells/ l, with a predominance of polymorphonuclear leukocytes (80%). The blood and CSF cultures were positive for Brucella spp. Brucellar agglutinins were detected to a dilution of 1/640 and tests for non-agglutinating antibodies (Coombs test) were also positive. The patient received intramuscular streptomycin, oral rifampicin and trimethoprim-sulfamethoxazole for six weeks. A month following completion of the treatment, the patient was admitted to our hospital because his parents reported hearing loss and inarticulate expression. The patient was uncooperative and aggressive. Physical examination disclosed severe cognitive impairment and pneumonia. X-ray and CT scan of the chest showed right pulmonary infiltration, but no abscess. Cefuroxime and clindamycin were administered for 10 days. A magnetic resonance imaging scan demonstrated lacunar infarcts in the left temporal and occipital lobes associated with both parietal lobes. EEG was normal. Audiometric results were 92dB for the right ear and 82dB for the left ear (severe hearing loss, as normal values range between 10 and 15dB). The patient refused to use a hearing aid because of his mental condition.
At 11 years, the patient was hospitalized with herpes zoster of the right maxillary branch of the trigeminal nerve. He weighed 50 kg (95th percentile) and was 149 cm tall (75th percentile). The vesicles contained clear fluid and continued to increase in size for a few days. The cornea was not involved at any stage. Intravenous acyclovir treatment (30 mg/kg) was administered for two weeks.
The clinical features of BCG disease were consistent with a defect in the IFN-γ pathway and further in vitro investigations were therefore performed. AR Tyk2 deficiency was diagnosed at the age of 17 years. The patient is homozygous for a 9bp deletion in exon 16 of TYK2, c.2302_2310del or 2302del9, which creates a premature termination codon at position 767. As a result, there is no full-length TYK2 protein detectable by western blotting in EBV-B cells. The detailed immunological investigation of this patient, and its comparison with the Japanese patient, will be reported elsewhere. Once Tyk2 deficiency was diagnosed in this patient, we looked for skeletal and dental manifestations of HIES, but all investigations were negative. Moreover, the patient’s medical history did not include signs of atopy, asthma or skin problems such as candidiasis, boils, folliculitis or cold abscess. Serum IgE concentration were determined at the ages of 2, 11, 16 and 18 years, and were found to be normal or slightly high [80, 115, 218, 155 IU/ml, respectively], but well below the high levels found in the previously reported patient with Tyk2 deficiency (2,100 IU/ml). The patient has developed no unusual infection in the last six years and is now 18 years old and off all treatment, but his social life is very limited because of developmental challenges and deafness.
Institutional review boards of the Uludag University, Necker Medical School and Rockefeller University reviewed the informed consents of the patient and family members participating in the study.
Discussion
The patient with Tyk2 deficiency described by Minegishi et al [1] presented with relatively mild BCG disease but invasive non-typhoidal salmonellosis, probably because of impaired IL-12 (and possibly IL-23) responses. Our patient also suffered from disseminated BCG infection and neurobrucellosis. Disseminated disease occurs in 1 to 3.4 children per million infants vaccinated. It usually occurs within six months of vaccination and is associated with a high case-fatality ratio of 80 to 83% [5]. Neurobrucellosis is uncommon and may take diverse forms, including encephalitis, meningoencephalitis, radiculitis, myelitis, peripheral and cranial neuropathies [6]. Neurobrucellosis caused severe neurological sequels including sensorineural hearing loss and cognitive impairment in our patient. Tyk2 deficiency probably contributed to the occurrence of neurobrucellosis in this patient as a previous case of brucellosis and impairment of the IL-12-IFNγ circuit has been reported [7]. IFN-γ is a key cytokine for the generation and regulation of the cellular immune response required to eliminate intracellular pathogens from macrophages. The absence of Tyk2 thus resulted in defective IL-12 signaling, leading to impaired IFN-γ production and susceptibility to BCG and Brucella [8]. These infections by intra-macrophagic bacteria are not seen in most patients with STAT3 or DOCK8 mutations and AD or AR HIES, respectively.
MC and HS virus caused skin and mucosal disease in the Japanese case. Our Turkish patient developed herpes zoster infection of the maxillary branch of the trigeminal nerve, which was cured by 14 days of acyclovir treatment and did not recur. These viral illnesses are probably related to an impaired cellular response to IFN-α/β [9], and are not seen in patients with AD HIES. In patients with DOCK8 deficiency, they occur by other mechanisms, involving a profound T cell deficit [10]. Neither of the two patients with Tyk2 deficiency had coarse faces or fragile bones, or any of the developmental features typically seen in patients with AD HIES [1]. However, unlike the Japanese patient with Tyk2 deficiency our patient displayed none of the other three cardinal features of HIES: atopic dermatitis and eczema, staphylococcal infections of the skin and lung, or high serum IgE concentrations. His highest recorded IgE concentration was 218 IU/ml, at the age of 16 years. The surprising discrepancy between the two clinical phenotypes is difficult to explain, but might result from a difference in the immunological impact of Tyk2 in the two patients. In any case, a diagnosis of Tyk2 deficiency should be contemplated in patients with BCG clinical disease, particularly in the presence of unusually severe herpes virus infections, even in the absence of cardinal features of HIES. This may also apply to patients with other mycobacterial diseases, whether tuberculous or atypical, as well as infections caused by other intra-macrophage pathogens, such as Salmonella and Brucella.
Acknowledgments
We appreciate Dr. Yoshiyuki Minegishi’s kind communication of the clinical findings of the first patient with Tyk2 deficiency. This study was approved by the institutional review boards of the Uludag University, Necker Medical School and the Rockefeller University. This work was supported in part by The Rockefeller University and the Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143
List of abbreviations
- Ig
Immunoglobulin
- HIES
hyper immunoglobulin E syndrome
- BCG
Bacille Calmette-Guerin
- Tyk2
tyrosine kinase 2
- AD
autosomal dominant
- MC
molluscum contagiosum
- HS
herpes simplex
- INH
isoniazid
- NBT
nitroblue tetrazolium test
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 3.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Demiraslan H, Metan G, Mese EA, Yildiz O, Aygen B, Sumerkan B, et al. Neurobrucellosis: an evaluation of a rare presentation of brucellosis from a tertiary care centre in Central Anatolia, Turkey. Trop Doct. 2009;39:233–5. doi: 10.1258/td.2009.080430. [DOI] [PubMed] [Google Scholar]
- 7.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–35. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watford WT, O’Shea JJ. Human tyk2 kinase deficiency: another primary immunodeficiency syndrome. Immunity. 2006;25:695–7. doi: 10.1016/j.immuni.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Jouanguy E, Zhang SY, Chapgier A, Sancho-Shimizu V, Puel A, Picard C, et al. Human primary immunodeficiencies of type I interferons. Biochimie. 2007;89:878–83. doi: 10.1016/j.biochi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]


