Abstract
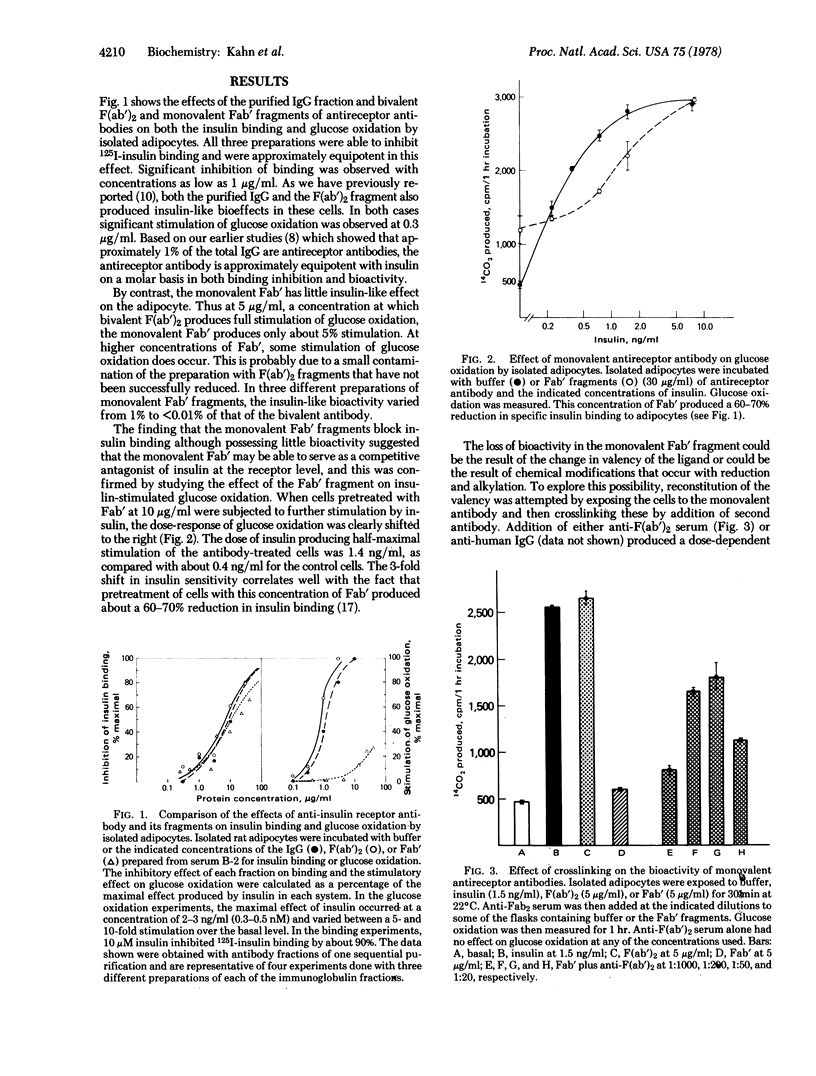
Exposure of adipocytes to antibodies to the insulin receptor results in a blockade of 125I-labeled insulin binding, stimulation of glucose oxidation, and many more insulin-like effects. Allowing for differences in purity, antireceptor antibody is equipotent with insulin on a molar basis. Both the bivalent F(ab′)2 and monovalent Fab′ fragments of the antireceptor antibody are fully active in inhibiting 125I-labeled insulin binding. Bivalent F(ab′)2 also retains its insulin-like effects. In contrast, the monovalent Fab′ loses almost all ability to stimulate glucose oxidation and acts as a competitive antagonist of insulin-stimulated glucose oxidation. Addition of anti-F(ab′)2 antisera, which crosslink the Fab′-receptor complexes, results in a restoration of the insulin-like activity of the antibody. Similarly, when cells are exposed to submaximal doses of insulin, addition of anti-insulin antibodies at low concentration enhances the biological activity of insulin. These data suggest that receptor occupancy by ligand is not sufficient for signal generation and that the insulin-like effects of antireceptor antibody (and perhaps insulin itself) require receptor aggregation or clustering. This aggregation, however, appears to be independent of microfilaments or microtubules because the insulin-like effects of antireceptor antibody, and in fact, of insulin itself, are unaffected by agents that are known to disrupt these structures.
Keywords: insulin receptor, receptor antibody, competitive antagonist, insulin antibody, membranes
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Michelson J. D., Hoffman G. J. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978 May 18;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Autoantibodies to the insulin receptor. Effect on the insulin-receptor interaction in IM-9 lymphocytes. J Clin Invest. 1977 Oct;60(4):784–794. doi: 10.1172/JCI108832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest. 1976 Dec;58(6):1442–1449. doi: 10.1172/JCI108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Freychet P., Brandenburg D., Wollmer A. Receptor-binding assay of chemically modified insulins. Comparison with in vitro and in vivo bioassays. Diabetologia. 1974 Feb;10(1):1–5. doi: 10.1007/BF00421406. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Degradation, receptor binding affinity, and potency of insulin from the Atlantic hagfish (Myxine glutinosa) determined in isolated rat fat cells. J Biol Chem. 1977 Jan 25;252(2):602–608. [PubMed] [Google Scholar]
- Goldfine I. D. Does insulin need a second messenger? Diabetes. 1977 Feb;26(2):148–155. doi: 10.2337/diab.26.2.148. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Jarrett D. B., Roth J., Kahn C. R., Flier J. S. Direct method for detection and characterization of cell surface receptors for insulin by means of 125I-labeled autoantibodies against the insulin receptor. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4115–4119. doi: 10.1073/pnas.73.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. The fate of insulin bound to adipocytes. Evidence for compartmentalization and processing. J Biol Chem. 1978 Jul 25;253(14):4900–4906. [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Akanuma Y., Tsushima T., Suzuki K., Kosaka K., Kibata M. Effects of antiinsulin receptor autoantibody on the metabolism of rat adipocytes. J Clin Endocrinol Metab. 1978 Jul;47(1):66–77. doi: 10.1210/jcem-47-1-66. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F., Springer A. Cytochalasin A and B. Inhibition of sugar uptake in cultured cells. J Biol Chem. 1972 May 10;247(9):2964–2966. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Gorden P., Flier J. S., Kahn C. R., Freychet P. Anti-insulin receptor antibodies inhibit insulin binding and stimulate glucose metabolism in skeletal muscle. Diabetologia. 1978 May;14(5):311–317. doi: 10.1007/BF01223022. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Gurny P. A., Lockwood D. H. Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J Biol Chem. 1977 Jan 25;252(2):560–562. [PubMed] [Google Scholar]
- McDaniel M., Roth C., Fink J., Fyfe G., Lacy P. Effects of cytochalasins B and D on alloxan inhibition of insulin release. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1089–1096. doi: 10.1016/0006-291x(75)90469-6. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., MARKUS G., WISSLER F. C. Separation of univalent fragments of rabbit antibody by reduction of a single, labile disulphide bond. Nature. 1961 Jan 28;189:293–295. doi: 10.1038/189293a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Oneda A., Matsuda K., Sato M., Yamagata S., Sato T. Hypoglycemia due to apparent autoantibodies to insulin. Characterization of insulin-binding protein. Diabetes. 1974 Jan;23(1):41–50. doi: 10.2337/diab.23.1.41. [DOI] [PubMed] [Google Scholar]
- Orci L., Rufener C., Malaisse-Lagae F., Blondel B., Amherdt M., Bataille D., Freychet P., Perrelet A. Symposium III: Hormones and hormone receptors. A morphological approach to surface receptors in islet and liver cells. Isr J Med Sci. 1975 Jul;11(7):639–655. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F. The Banting Memorial Lecture 1976. Insulin today. Diabetes. 1977 Apr;26(4):322–340. doi: 10.2337/diab.26.4.322. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., De Meyts P., Roth J. Cell surface receptors for insulin and human growth hormone. Effect of microtubule and microfilament modifiers. J Biol Chem. 1976 Nov 10;251(21):6844–6851. [PubMed] [Google Scholar]
- Walkenbach R. J., Hazen R., Larner J. Reversible inhibition of cyclic AMP-dependent protein kinase by insulin. Mol Cell Biochem. 1978 Feb 24;19(1):31–41. doi: 10.1007/BF00231232. [DOI] [PubMed] [Google Scholar]


