Summary
Background
From humans to frogs, immunoglobulin class switching introduces different effector functions to antibodies through an intrachromsomal DNA recombination process at the heavy chain locus. Although there are two conventional antibody classes (IgM, IgW) in sharks, their heavy chains are encoded by 20 to >100 miniloci. These representatives of the earliest jawed vertebrates possess a primordial immunoglobulin gene organization where each gene cluster is autonomous and contains a few rearranging gene segments (VH-D1-D2-JH) with one constant region, μ or ω.
Results
V(D)J rearrangement always takes place within the μ cluster, but here we show that the VDJ can be expressed with constant regions from different clusters, although IgH genes are spatially distant, at >120 kb. Moreover, reciprocal exchanges take place between Igω and Igμ genes. Switching is augmented with deliberate immunization and is concomitant with somatic hypermutation activity. Since switching occurs independently of the partners’ linkage position, some events involve transchromosomal recombination. The switch sites consist of direct joins between two genes in the 3′ intron flanking JH.
Conclusions
Our data are consistent with a mechanism of cutting/joining of distal DNA lesions initiated by activation-induced cytidine deaminase (AID), in the absence of mammalian-type switch regions. We suggest that, in shark, with its many autonomous IgH targeted by programmed DNA breakage, factors predisposing broken DNA ends to translocate configured the earliest version of class switch recombination.
INTRODUCTION
In mammals a primary antibody response begins with a rising IgM titer that is followed, with a lag, by antibodies of a different class, such as IgG or IgA. The “switched” antibodies preserve the antigen-combining sites previously associated with IgM heavy (H) chain but replace the C-terminal portions which bear other functions such as the recruitment of effector immune cells via binding to Fc receptors, to bring about antigen clearance [1]. In disease states such as hyper-IgM syndrome where there is a deficiency in secreted Ig classes other than IgM, recurrent infections demonstrate the survival value of the absent isotypes. Antibody isotype switching relies on a molecular process called class switch recombination (CSR), where deletional recombination juxtaposes the VDJ combining site to downstream C exon sets. In activated B lymphocytes activation-induced cytidine deaminase (AID) initiates DNA lesions in the VDJ, promoting somatic hypermutation (SHM), and in the highly repetitive switch (S) regions 5′ of the C exons, generating DNA double-stranded breaks (DSB) [2,3]. S regions are critical to CSR, as their sequence and structure enhance targeting by AID, rendering the area recombinogenic [4]. How the DSB are achieved is not clear, but the ends are repaired and become recombined through non-homologous end-joining (NHEJ) pathways [5, 6].
Ig classes exist in all vertebrates, but unambiguous parallels to the mammalian IgM-IgG switch extend only to amphibians [7, 8]. The representatives of the earliest jawed vertebrates, cartilaginous fishes like sharks and skates, are the oldest group to possess an adaptive immune system based on V(D)J recombination. They express two conventional Igs, IgM and IgW, and a third that is a single-domain binder, called IgNAR [9]. The IgM/IgW H chains are encoded by 20 to >100 miniloci or “clusters”, a unique type of organization considered ancestral to the classical Ig locus in higher vertebrates (Fig. 1) [10]. After defining the germline Igμ genes in the nurse shark, we were able to demonstrate that despite the multiple autonomous IgH, H chain exclusion exists and shark antibody heterogeneity was mainly generated by junctional diversity and somatic hypermutation (SHM) [11–15]. Here we report the unanticipated finding that the multiple cluster organization also supports Ig isotype switching. Thus the basic features of humoral immunity -- including V(D)J rearrangement, H chain exclusion, SHM, and now CSR -- emerged in the ancestor of all jawed vertebrates.
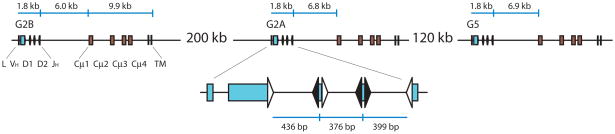
Figure 1.
Organization of three linked IgM H chain genes in nurse shark. The IgH clusters were mapped in ref. [13] and distances are indicted. Each cluster consists of a split leader (L) and the rearranging gene segments (VH, D1, D2, JH) depicted as blue boxes (enlarged) flanked by recombination signal sequences (RSS) shown as triangles. The RSS with 23 bp spacers are white, those with 12 bp spacers are black. Brown boxes represent the four C region exons (Cμ1–4) and the transmembrane (TM) exons.
RESULTS
Overview
The experimental results are presented as follows. (1) Screening of cDNA libraries revealed Ig transcripts composed of the VDJ belonging to one IgH gene cluster and the C region to another. (2) Parallel library screening and RT-PCR experiments show that the proportion of switched Ig is highest in immunized adults, less in non-immunized individuals, not detectable in neonates. (3) Every IgH gene studied can switch. Switching to G5 C region and reciprocal switching of G5 VDJ to other C regions were observed. (4) The nature of mutations in productive VDJ of switched Ig suggests the polypeptides were expressed and under selection. (5) Using cDNA primed in the J-C intron, sequences containing switch junctions were isolated. These are transcripts of IgH genes that appear to have undergone recombination.
cDNA sequences not correlating with germline organization
Characterization of nurse shark Igμ genes from bacteriophage and BAC libraries respectively representing 4.5 and 11 genomes’ coverage showed that each cluster consists of a single VH, two D, one JH and one set of Cμ exons (Fig. 1) [12, 13]. G1, G2A, G2B, G3 and G5 are single-copy genes present in all sharks, and their VH, JH and Cμ exons are unique (Fig. 2A). The 6–10 kb J-C intron was sequenced in each gene (accession numbers JQ272838-43) but the highly repetitive S region in tetrapods was not observed.
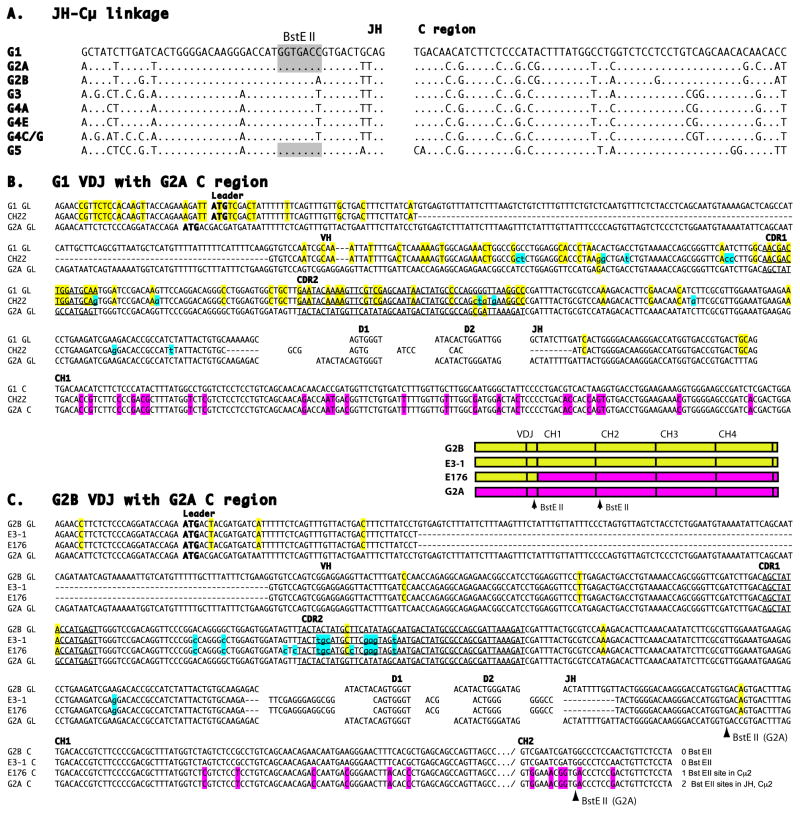
Figure 2.
cDNA sequences compared to germline components
A. Germline JH gene segments and linked Cμ1 exons of the Igμ genes G1-G5, as defined in genomic bacteriophage and BAC libraries [11–13]. BstE II site (shaded) in JH of G1, G2A and G5. Dots indicate identity with G1 reference sequence.
B. G1 VDJ with G2A C region. Comparison of germline VH gene segments and partial Cμ1 exon from shark-33 G1 and G2A IgH with shark-33 cDNA CH22; drawn as the top, bottom and center sequences, respectively. Nucleotide identities restricted between CH22 and one germline (GL) sequence are highlighted in yellow for VH and in pink for Cμ. Mutations are marked by lower case and highlighted in blue. The GL gene segments consist of leader, VH gene segment, two D genes and JH gene segment, as labeled; GL intersegmental sequences not shown. ATG is bolded, the CDR in the V gene segment are underlined and labeled. C is constant region, dashes indicate gapping. Accession number JQ272824.
C. G2B VDJ with G2A C region. Diagram is color-coded to depict the relationship of the four sequences. Comparison of shark-33 cDNA clones E3-1 (non switched G2B sequence) and E176 (switched) that share CDR3. See legend to B. The number and location of BstE II sites in the GL G2A and E176 are indicated at lower right as well as by arrows. Accession numbers JQ272833, JQ272828.
In the course of screening the shark-33 epigonal organ (bone marrow equivalent) cDNA library we isolated a few μ chain sequences whose components did not correlate with the established germline organization [11] in the same individual. One cDNA consisted of VDJ from the Igμ gene G1 but with the C region from G2A (Fig. 2B). Three other cDNAs contained VDJ from G2B but again C region from G2A (not shown). The VDJ were intralocus rearrangements; chimerism began only 3′ of JH.
To ascertain the frequency of chimeric cDNA we looked for switching in all five subfamilies. Three more combinations were found: G3 VDJ to the G4C C region, G4A VDJ and G4CG VDJ to G4E C region (Table 1, shark-33). The C regions were replaced in their entirety. Of six new chimeric sequences obtained from shark-33, one in particular was informative. Clone E176 carried the VDJ of G2B and the C region of G2A, and this VDJ was shared with one of the 11 conventional G2B sequences, E3-1 (Figure 2C). The common CDR3 and mutations shared by these two cDNAs established that both rearrangement and somatic hypermutation (SHM) had occurred in a parental clone previous to the event that produced E176. These cDNA sequences have all the hallmarks of products of Ig H chain class switch recombination in tetrapods.
Table 1.
IgM H chain switch among cDNA clones from epigonal (shark-33, shark-GR) and spleen (shark-JS) libraries
| Clones analyzed by C region | Switched clones | |||
|---|---|---|---|---|
| Nonimmunized Shark-33 | ||||
| Subfamily | G1 | 39 | G1 | <1/39 |
| G2 | 31 | G2A | G2B VDJ to G2A C region, 1 clonea | |
| 11 | G2B | |||
| 1 | switched | |||
| G3 | 1 | G3 | none | |
| G4 | 7 | G4E | G4A VDJ to G4E C region, 2 clonesa | |
| 7 | G4A | G4C/Gb VDJ to G4E C region, 2 clonesa | ||
| 26 | G4Gb | G3 VDJ to G4C C region, 1 clonea | ||
| 14 | G4Cb | |||
| 5 | switched | |||
| G5 | 58 | G5 | <1/58 | |
| Immunized Shark-JS | ||||
| Subfamily | G2 | 30 | G2A | G2B VDJ to G2A C region, 5 clonesa |
| 6 | G2B | |||
| 5 | switched | |||
| G5 | 33 | <1/33 | ||
| Immunized Shark-GR | ||||
| I. IgM C+ | ||||
| Subfamily | G1 | 10 | <1/10 | |
| G2 | 79 | G2A | G2B VDJ to G2A C region, 20 clonesc | |
| 4 | G2B | G4A to G2A C region, 1 clone | ||
| 26 | switched | IgW VDJ to G2A C region, 5 clonesd | ||
| G3 | 12 | <1/12 | ||
| G4 | 188 | not done | ||
| G5 | 58 | <1/58 | ||
| II. IgW | ||||
| IgW VH+/C+ | 63 | all IgW | ||
| IgW VH+/Cneg | 13 | IgW VDJ to IgM G2A, 11 clonesd | ||
| IgW VDJ to IgM G4C, 2 clonesd | ||||
| IgW VHneg/C+ | 1 | IgM G4C VDJ to IgW, 1 clonee | ||
Accession numbers for shark-33 and JS sequences are: JQ272822-JQ272837.
The VH gene segments of germline G4C and G4G are indistinguishable; it is the C regions that differ.
clones n=n identical, n/n share CDR3 but differ by mutation: GR 9=305, 12, 23, 47, 63, 99, 102, 157, 208, 51/154/214, 158/164/304/215/218, 36/235 (12 CDR3 among 19 unique sequences)
clones n=n identical, n/n share CDR3 but differ by mutation: IgW VDJ to IgM G2A, w-2/3w=4w/47w=22w=17w =40w; 1w=14w/7w/15w/52w/65w/6w/20w; 246w (3 CDR3 among 16 sequences, 11 unique). IgW VDJ to IgM G4C, 58w=61w. Some clones shown in Fig. S5 (accession numbers JQ272792-JQ272796).
Switch frequency is higher in immunized sharks
Shark-33 was not deliberately immunized, therefore its mutant Ig sequences were produced during natural exposure to environmental antigens. Two cDNA libraries were constructed using mRNA from immunized sharks-JS and GR (Table 1). Whereas in shark-33 1/12 μ cDNA bearing G2B VDJ have switched, in shark-JS it was 5/11 and in shark-GR 20/23. For sequences bearing the C region of the G2 subfamily, 1/43 (2.3%) were switched in shark-33, compared to 5/41 (12%) in shark-JS and 25/108 (23%) in shark-GR. In view of these differences we focused on G2 switches.
G2 sequences were amplified by RT-PCR from six animals, including immunized (sharks-JS, GR, PI) and non-immunized individuals (sharks-AQ, 33, 626). The reverse primer targeted the G2A/B C region but the universal forward primer allowed amplification of any VH subfamily (G1-G5). Most of the VDJ switching to G2A C region would be G2B, as observed in the cDNA libraries (Table 1). The two isotypes are differentiated by two BstEII sites found in G2A but not in G2B C region (sites shown in Fig. 2C). VH-hybridizing fragments with a single BstEII (marking the G2A C region) are ~647 bp (Fig. 3A); the absence of BstEII in JH means either a mutated G2A JH or a switched G2B VDJ. Individuals display varying amounts of the ~647 bp fragment.
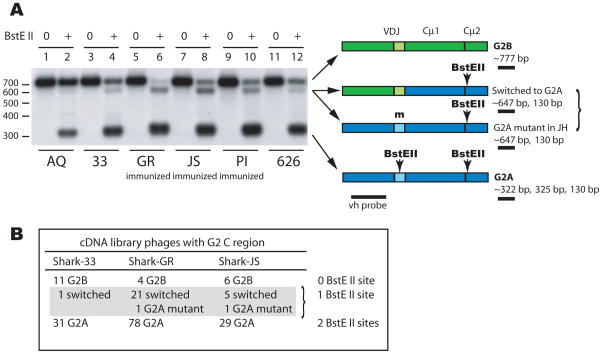
Figure 3. Frequency of switch to G2A C region varies among individuals.
Panel A. Left, RNA was isolated from non-immunized sharks-AQ (lane 1, 2), 626 (11, 12), and 33 (3, 4) and from immunized sharks GR (lane 5, 6), JS (lane 7, 8), and PI (lane 9, 10). First strand cDNA was primed with a universal CH2 primer (CH2-3′) and PCR was performed with G2-specific reverse primer (G2CH2R) but universal forward primers (V1-5) for 30 cycles. The PCR products were incubated with (+) and without BstEII (0) and electrophoresed. The filter was hybridized with a vh probe (black bar in diagram). Right, depiction of PCR products and their BstEII sites, fragment sizes after BstEII incubation, underlined fragment detected by vh probe. Non-switched G2B (green) has no BstEII sites whereas non-switched G2A (blue) contains one in JH segment and one in Cμ2 (arrows). Digestion with BstEII generates bands of ~322 bp (G2A), ~777 bp (G2B), and ~647 bp (switched or mutated BstEII) as detected by vh probe in the samples.
Panel B. Scoring of G2 sequences from cDNA library clones in Table 1. The relative ratios of G2B to switched Ig/G2A mutant (shark-33, 11:1; shark-GR, 4:22; shark-JS, 6:6) respectively correlate with the 777 bp/647 bp signal intensity detected by phosphorimaging of panel A lanes (lane 4, 3719/396; lane 6, 497/2514; lane 8, 2567/3004).
Neonatal shark-AQ and three other pups show little mutated or switched G2 sequences (Fig. 3A, lanes 1,2; Fig. S1A). Sharks-JS, GR and 33 on the other hand serve as positive controls. The good correlation of the 777/647 bp ratio with G2B/switched-G2A frequencies observed in three cDNA libraries (Fig. 3B) demonstrates how the RT-PCR products accurately reflect the switched Ig content. A marked difference in the 647 bp band can be observed between the immunized and non-immunized samples.
In another experiment the G2A and the G2B C region-carrying populations were separately amplified (Fig. S1B). Switched sequences bearing the G2A C region were observed in the same individuals and in the relative amounts as in Fig. 3A, whereas in the parallel G2B samples there was little reciprocal switching detectable. Because G2B is upstream of G2A (Fig. 1) we investigated whether the biased switched combinations are a result of the relative physical location of the genes.
Switching is not restricted by gene position
Only switching events taking place between G5 and the other Igμ can be scored with confidence by PCR methods because in these cases subfamily-specific markers distinguishing JH from CH are separated by 2 bp (Fig. 2A). The location of G5 with respect to two upstream Igμ has been determined (Fig. 1), so we looked for all possible switches to the G5 C region as well as reciprocal events involving G5 VDJ.
G5 C region-containing Ig were amplified from sharks-AQ, JS, GR and PI and selected for switched sequences by differential digestion (Fig. S1, C–E). None was obtained from neonate shark-AQ. In contrast, the switched G5 C region-containing sequences cloned from shark-JS included G2A, G2B, G4A and G4CG VDJ and those from shark-GR G4A and G4CG VDJ (series I–V, Fig. S2).
Direct amplification was also performed using subfamily specific primers, for example, in the G4 leader (G4L) and in the G5 C region (G5CH1R, Fig. S2 diagram). Diverse sequences were successfully amplified, with several clones sharing the same VDJ as clones obtained in the first, bulk approach (paired, Fig. S2 I, II), showing that the same switched population was detected in both experiments. Virtually every IgH within 340 kb (G2A, G2B) and elsewhere (G1, G4A, G4CG) was found switched to the G5 C region.
We then looked for and found reciprocal combinations for G5 VDJ (Fig. S2, series VIII–XI). For genes of established order (Fig. 1), reciprocal switching of the G2B or G2A VDJ to the G5 C region and the G5 VDJ to G2B or the G2A C region demonstrates that switching combinations are independent of the genes’ relative positions on the chromosome. In particular, switching of VDJ to upstream C regions show that these events may involve the homologous chromosome.
Related clones suggest switching is not transient
The frequent finding of related clones (Figures 2C, S3, Table 1 footnotes c and d) demonstrates that not only did they originate from activated B cells but also that their switched configuration, even with a non-functional VDJ, is a stable feature in dividing cells undergoing SHM.
Since many cloned combinations in shark-GR involved G4 VDJ and G5 C, we sought nonfunctional VDJ in the reciprocal combination (Fig. S2, sets X, XI). In two such related clones the mutations consist of in/del and alter important structural residues (Fig. S3A). In the absence of shark B cell lines we cannot ascertain whether they arose from a recombination process involving exchange for a productive VDJ. The deleterious changes contrast with the conservative nature of the FR changes in in-frame VDJ shown in Fig. S3B and Fig. 2, suggesting that in the latter, translated products were under selection.
Switch junctions
The reciprocal switches occurring between G2B, G2A, and G5 suggest that the process could involve transchromosomal recombination and/or gene conversion. We attempted to isolate sequences containing a switchpoint located downstream of JH; the combination of the G4 VDJ switched to the G5 C was selected for the disparity in their J-C intronic sequences and because many G4 to G5 switched sequences were isolated from shark-GR (Fig. S2, I and II).
We had previously found extensive mutation and evidence of DNA breaks within the 500 bp 3′ JH from genomic VDJ [16], an area prone to SHM. The fruitful cloning efforts were directed to this region in cDNA, with first-strand primers targeting points in the G5 J-C intron (Fig. 4, top). Sequences isolated in these experiments are shown in Fig. 4 and Fig. S4. The switch junctions of all cloned sequences are shown in detail in Fig. S4, B-P, with arrows marking the 5′-most site where cleavage could have occurred on the donor sequence. Subsequent experiments involving cDNA primers located further downstream revealed switch junctions at primarily similar locations (Fig. S4 D-II). Most of the switch joints are accompanied by insertions of unknown origin (Fig. 4; Fig. S4, F–I, K–O) that suggest modification of DNA ends during repair processes such as NHEJ [5, 6]. This area did not contain non B-DNA structure or greater levels of RGYW motifs.
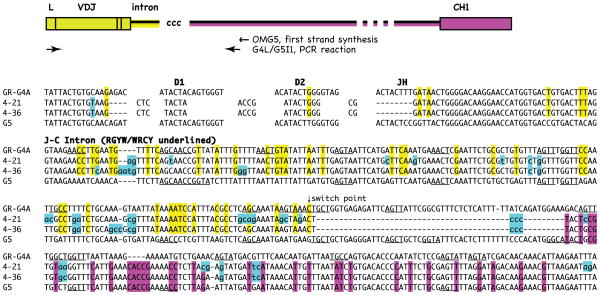
Figure 4.
Two clones share CDR3 and switch junctions. Top, Diagram shows sequences detected by RT-PCR using primers in the J-C intron of G5 and leader of G4. Yellow indicates sequence identity with G4, pink with G5; “ccc” insertion at putative switch point. Not drawn to scale, as indicated by break symbol. Bottom. G4 VDJ joined to G5 intron are compared to G4A and G5 reference sequences. Related clones 4–21 and 4–36 share CDR3 and mutations (blue) through VDJ and JH flank. The flanking intron is labeled. Hotspot motifs RGYW/WRCY are underlined in the reference sequences. Arrow (switch point) indicates where the G4A sequence identity ceases. This is followed nontemplated addition (ccc) and identity with G5 (in pink). Accession numbers JQ272798-99.
What is more, we have discovered that some of these VDJ-switched intron sequences are clonally related to VDJ switched to C region from the G4G5 series (Fig. S2, I). This is schematically depicted in Fig. 5. The isolation of four such related pairs demonstrated that some switched VDJ did derive the novel C region through recombination in the J–C intron (Fig. 5, Fig. S5). Thus, the isolation of related sequences, using successively (1) primers in VH FR1 and CH1 and (2) primers in the leader and J-C intron, demonstrates that the intronic junctions are in vivo gene products. Most importantly, these pairs show that the connection between VDJ and switched C region physically existed prior to VDJ to CH splicing. As such, we concluded that the switched VDJ and C were recombined at the DNA level.
Figure 5.
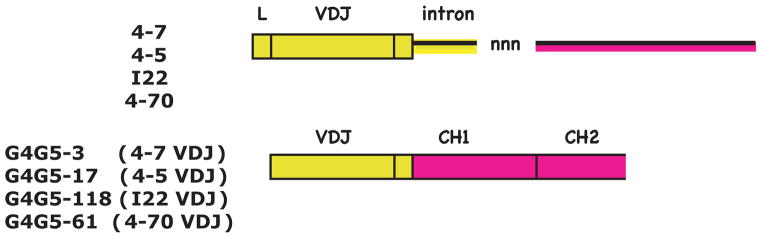
Clonally related VDJ are flanked with switched intron or spliced to switched C region. Top, diagram depicts RT-PCR product generated using primers in the G5 J-C intron and the leader of G4, as in Fig. 4. Bottom, diagram depicts RT-PCR product generated using primers in the G5 C region and universal primers in VH, as in Fig. S2. Yellow highlight indicates G4 sequence, pink G5. Clones 4-7 and G4G5-3 share VDJ and mutations; 4–7 sequence was switched to G5 in the J-C intron whereas G4G5-3 VDJ was spliced to G5 C region. Three other such pairs were found (G4G5-17/4-5, G4G5-118/I22, G4G5-61/4-70). Sequence comparisons are shown in Fig. S5. Full alignments with G5 intron are shown in Fig. S4: D (4–5), E (4–7), F (4–70), O (I22). Accession numbers JQ272797, JQ272804, GQ359826-27.
At this time we cannot conclude if all or even the majority of switched IgH are formed by intra- or interchromosomal recombination in the J-C intron, but of 17 unique G4A VDJ switched to the G5 C region, four were related to the VDJ isolated with switch junctions in the intron (Fig. S2 I, asterisked). This proportion indicates that switching events apparently involving DNA DSB and repair by NHEJ are not infrequent. There is no definitive evidence for gene conversion for switch; any such event must extend from the J-C intron through the C exons (>7 kb). In the cDNA libraries none of the sequences were chimeric within the C region or beginning within VH.
AID
Results presented in Fig. 3A and Fig. S1A show that little Ig switching occurs in four shark pups. This was confirmed in parallel experiments where we tried to clone intronic switch junctions among pup shark-AQ Ig transcripts. In contrast to older sharks, AID is barely detectable in neonatal tissues like the epigonal organ and spleen (unpublished results), correlating with their low levels of SHM [11, 13, 17].
The unique feature of shark SHM is that half of substitutions are adjacent nucleotide changes with a biased occurrence at RGYW/WGCY hotspots [11, 18, 19]. SHM extends into the JH 3′ intronic flank for 1–1.5 kb, with a high frequency of duplications and deletions, sometimes accompanied by nontemplated additions [16]. Although the current work was done on cDNA, all mutational changes other than the switching process are similar in character to previous observations made in shark B cell genomic DNA. Moreover the nature of the switch junctions resembles DNA DSB and repair processes found in the intron of non-switched Ig.
Switching to and from IgW
The epigonal organ in shark is analogous to bone marrow also in the sense that plasma cells tend to home there. After screening the shark-GR library with μ probe we found not only more G2B VDJ switched to the G2A C region than non-switched G2B (Table 1), but we also detected IgW VDJ switched to the G2A C region (Fig. S6A). We then used IgW VH and CH probes to assess the extent of switching with Igμ genes, isolating not only additional IgW VDJ switched to Cμ sequences but also one cDNA that was IgM VDJ switched to the Igω C region (Fig. S6B). As listed in Table 1 (footnotes c and d) the switched clones in the epigonal organ are highly amplified.
Among the 177 μ+ genomic BAC library clones [12, 13] not one contained Igω sequence, and in preliminary studies on 70 BAC clones carrying >8 different Igω genes not one had IgM sequence (unpublished results). The shark IgW H chain contains 2–6 Cω domains, is expressed only in classical monomeric form, and is believed to be related to Igδ [9]; its function is not established. The cDNA that are chimeric for IgM and IgW resemble class-switched sequences as defined in mammals – but interestingly the process is not unidirectional, as it is in tetrapods.
DISCUSSION
We have identified a somatic recombination process that occurs between IgH clusters in shark B lymphocytes, whose transcribed products are primarily productive VDJ switched to a novel C region. Our data are consistent with a SHM-mediated switching mechanism whose differences from tetrapod CSR may reflect an early process that existed in the ancestral vertebrate. The process is concomitant with AID/SHM activity: hardly detectable in neonates but vigorous after deliberate immunization in older animals. Furthermore, SHM occurs throughout the switching process since it is present both before and after the switching event. Not only is switching between IgM and IgW bidirectional, but every gene inspected can undergo switching, regardless of the relative chromosomal location of the partner. The discussion focuses on those differences from the well-studied mammalian systems in order to extract the elemental features of CSR as it evolved in the ancestral vertebrate Ig gene system.
Relationship of SHM and isotype switching
The S regions in mammals contain long stretches of repetitive motifs that are 60% GC-rich and specialized features that promote switching through R-loop formation [20, 21]. Although the amphibian Xenopus S regions have normal (40%) GC content and do not form R-loops, they contain palindromes and RGYW/WRCY motifs, and the region enables switch recombination when it replaces the mouse counterpart [22–24]. However, the shark J-C intron does not possess such structures. The location and nature of the shark switch junctions show that the DNA breaks leading to isotype switching arise from AID-mediated lesions that occur in proximity to the VH promoter. We have found no evidence for other promoters in the J-C intron. Both SHM and switching originate from AID lesions, and it may be that intrinsic properties of the J-C intron sequence render it a recombination hotspot. As such, in the earliest version of Ig switching SHM and CSR are contiguous. We speculate that it was only after amphibian divergence, with the introduction of S regions, that SHM and CSR became independent, spatially and temporally, as they are in mammals [25–27].
CSR in mammals usually takes place intrachromosomally but can also occur between alleles. In rabbits, trans-recombination occurs with a frequency of 3–8% [28] and interallelic CSR in mice engineered to monitor this phenomenon takes place at similar and higher frequencies [29]. A rate-determining synapsis step for two IgH may render shark CSR less frequent, just as trans-recombination constitutes a fraction of total isotype switching in rabbits. There is very little information on chromosomal distribution of IgH genes in cartilaginous fishes. In one study the many Igω and Igμ genes in clearnose skate appear to be in multiple chromosomal sites, as observed by fluorescence in situ hybridization [30]. As it seems unlikely that all 9–12 Igμ and >8 Igω genes would be linked in nurse shark, switching could involve breakage and joining between non-homologous chromosomes, like a balanced translocation.
Parallels with aberrant chromosomal recombination
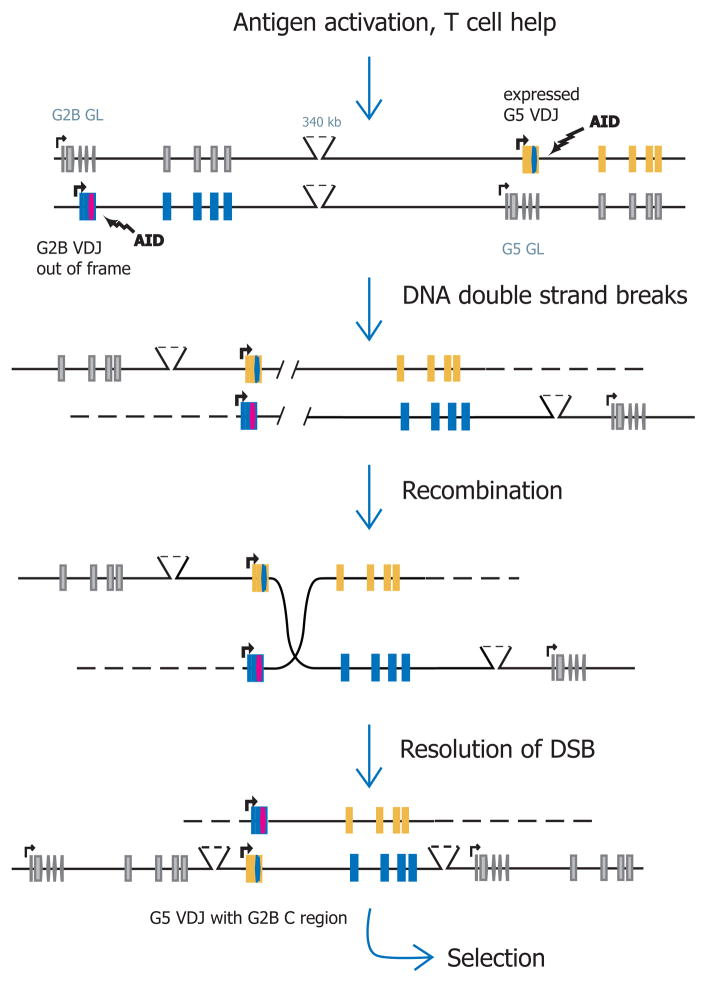
What factors might predispose recombination between the shark IgH? We suggest a comparison can be made with AID-generated translocation in mammalian B cells. The broken DNA ends generated during SHM and CSR at Ig loci can participate in translocations where their non-Ig partners tend to be AID “off”-targets [31, 32]. As found after genome-wide mapping of translocations in B cells [33, 34], those factors determining the joining partners include chromosomal proximity, proximity of the DSB, and transcriptional activity. Because AID is nonetheless preferentially recruited to Ig genes through unknown mechanisms that involve cis elements [35, 36], chromatin modifications [37–39], and AID-cofactors [reviewed in 40], the available broken DNA ends in an activated shark B cell will primarily be from several IgH genes. We propose that the circumstances that congregate by chance to promote aberrant recombination in mammalian B cells are naturally converged in the case of shark B cells undergoing programmed DNA breaks at multiple locations.
Figure 6 shows our model for isotype switching at shark IgH. In single B cells we have found 1–3 VDJ rearrangements among 9–10 autonomous Igμ genes and their alleles; primarily one VDJ is functional [15, 13]. We speculate that activation by antigen and T cell help upregulates IgH transcription. AID is recruited to transcribing Ig genes, mostly these are rearranged VDJ, but mutated cDNA sequence from partially rearranged and germline genes have also been isolated [15, 13]. In shark plentiful evidence of DNA DSB and repair has been found in the J-C intron of non-switched genes [16]. The shark IgH are gene duplications and may share nuclear space, as must rabbit IgH alleles where transchromosomal recombination occurs. There could be a clustering of similar cis elements and shared transcription factors, and the proximity of ensuing DSB presents the potential for intra- or interchromosomal recombination. What is more, AID from bony fish (zebrafish, catfish, Japanese puffer fish) has been shown to induce CSR in mouse cells [41, 42], demonstrating that early vertebrate AID already possessed the property of catalyzing recombination between two locations.
Figure 6. Proposed switching events at shark IgH.
A. IgH genes in B lymphocyte. G2B and G5 genes shown in relative order and orientation, as in Fig. 1. Rearrangement has occurred two genes, a productive VDJ at G2B and nonproductive VDJ at G5. Both are transcribed (bent arrows).
B. AID action in B cell. The B cell is activated by antigen and T cell help, transcription is upregulated in G2B and G5 as well as in some non-rearranged genes. AID is preferentially recruited to the highly transcribed genes.
C. DSB at IgH genes. AID-mediated DNA lesions sometimes result in double-strand breaks.
D. Recombination at IgH genes. During DNA repair recombination occurs between the two IgH. An example of the G5 VDJ switching to the upstream G2B C region is clone G5G2-21, Figure S2. E. Switched IgH. The B cells with novel antigen receptors undergo selection.
CONCLUSIONS
Based on the established IgH germline gene complement in nurse shark, we have discovered that there exist cDNA sequences composed of VDJ from one gene cluster and C region from a different one: the classical feature of switched Ig H chains. The extent of switching correlated with increasing age, deliberate immunization, and AID expression as well as being concomitant with SHM. Every Igμ gene inspected can undergo switching, and switching sites show evidence of DSB and recombination. There are no S regions; sharks possess the earliest version of Ig switching where SHM and CSR are contiguous activities that became independent only after amphibian divergence.
We hypothesize that programmed DNA lesions initiated by AID at multiple, functionally independent, and distant Ig genes prompt recombination in a shark B lymphocyte. Thus, determining factors that are predicted to lead to chromosomal aberrations and oncogenesis in other systems were co-opted by the evolving immune system in the ancestral vertebrate. Our findings demonstrate evolutionary pressure for flexible handling of pathogen during an antibody response.
EXPERIMENTAL PROCEDURES
Animals
Nurse sharks (Ginglymostoma cirratum) were captured off the coast of the Florida Keys. Some animals were sacrificed on arrival (shark-33, shark-626) and others had been immunized with DNP-LPS (shark-PI) or hen egg lysozyme (shark-JS, shark-J) or Ebola virus (shark-GR) [11–13, 16]. These sharks range from two years (shark-626) to seven years (shark-GR) and are referred to as “adult” with respect to state of their immune systems. The pups were <1 week (AQ, EC, TH) to <2 months (LA) [13]. The cDNA libraries from shark-33 and shark-GR epigonal organs, and shark-JS spleen were cloned into λZAP Express XR (Loftstrand Labs Ltd.) [11, 12].
Library screening
To distinguish the IgM subfamilies G1-G5 during cDNA library screening, all the IgM CH-positive phage lysates were subjected to PCR using a primer combination (two forward primers 50% G2CH1: 5′-GGACTACTCCCCTGACA-3′ and 50% G4CH1: 5′-GAAAGGTGGGAAGCCCT-3′ with reverse primer PANCH2-3: 5′-GGAACTCAAAGTTAGGAG-3′) that produced a 253 bp G2 CH fragment or a 217 bp G4 CH fragment. The remaining “negative” lysates would contain G1, G3 or G5 sequences and were identified by restriction endonuclease sites as described previously [11]. For direct sequencing partial sequences were amplified from phage lysates using T3 and CH2-3′ (5′-ACCTGGCAKGTATARAC-3′).
Screening for IgW-containing sequences from the cDNA library involved double lifts hybridized to IgW VH and IgW CH. The selected phage lysates were separated into three categories: VH-positive/CH-positive, VH-positive/CH-negative, VH-negative/CH-positive. Complete IgW sequences were verified by PCR with universal primers targeting IgW leader (JWF1: 5′-GATTGCTCCWAATCTCKG-3′) and IgW CH1 (two reverse primers 50% IgWCHa: 5′-AGAGACTGTTTCAAATGT-3′ and 50% IgWCHb: 5′-GATTGTTTCGAAGGTATT-3′), followed by sequencing. The phage lysates containing only IgW CH but not VH were amplified with T3 and IgWCHa/b and sequenced; those containing IgW VH but not CH were amplified with JWF1 and IgM primer CH2-3′.
PCR
Oligonucleotides were synthesized by Invitrogen and the areas targeted Ig H chain transcripts are illustrated in Fig. S2, top. First-strand synthesis of cDNA was performed using SuperScript III reverse transcriptase (Invitrogen) and oligo dT or specific primers targeting Cμ: universal C region CH2-3′, or G2 (G2CH2RR: 5′-GATGTCAGAATGCACAC-3′), G2A-specific (G2A-C2a: 5′-CTGAGAAGCTTCCATTCA-3′), G2B-specific (G2B-C2a: 5′-ACACACGGGAGAAGTTAG-3′), G4 (G4CH1A: 5′-TGTAAGTTCCTTTCTTGC-3′), G5 (G5C2: 5′-ACTTTGAGTGGAAGTCAC-3′). This was followed by 30 cycles of PCR. Forward primers include universal primers in FR1 (V1-5 is a mix of 20% V1: 5′-TGACTCAAAAAGTGGCAG-3′, 80% V2-5: 5′-TGAYTCAACCAGAGGCA-3′), V1 being G1-specific and V2-5 for G2-G5. Other forward primers targeted the leader: G2-specific G2V18 (5′-ACCAGAATGACGACGATG-3′), G4-specific G4L (5′-TTCTGACTTTCTTATCCC-3′), and G5-specific G5L2 (5′-TGTTGCTGGCTTTATTAC-3′). Reverse primers targeted Cμ1 or Cμ2 and included: G2 (G2CH2R: 5′-CCGTTCTTCAACCAATTG-3′), G2A-specific (G2A-C2b: 5′-GATGTCAGAATGCACACT-3′), G2B-specific (G2B-C2b: 5′-GATGTCAGAATGCACACG-3′), G4 (G4CH1B: 5′-ATAAATCCAGTCGTGAAG-3′), G5 (G5CH1R: 5′-TCACAGGATATTTGGTCA-3′).
First strand primers targeting the G5 J-C intron include: OMG (at 651 bp, 5′-GAATCCAATCAACTCAATTC-3′), G5I2 (at 557 bp, 5′-ACTCAAATCAACAGATTGAGA-3′), OMG5 (at 551 bp, 5′-AAACTCAACGACTCAAATCA-3′); PCR performed with G4L and G5I1 (5′-AGAAAATTTGAAGACAGAAGT-3′) produced products of about 900 bp (see Fig. S4, top). The I3-series was obtained using first strand primer G5I4 (5′-CAGCCTAATTGGTCAAAT-3′) at 1202 bp in the G5 J-C intron; PCR with G4L and G5I3 (at 1098 bp, 5′-AGCAAATGAATGCACGA-3′) generated fragments of ~1498 bp.
Probes and blotting
Probes to IgM H chain were derived from G2 cDNA (vh, cμ1, cμ2, cμ3-cμ4) had been described elsewhere [11]. Other probes were derived from an IgW cDNA sequence; the primers targeting V region (vhw, 285 bp; W5′: 5′-CAGGTCAAACCTTCAGTT-3′; W3′: 5′-AAACCAGGAAACCTGTAC-3′) and CH1/CH2 (chw, 560 bp, WCH1F1: 5′-AAGATGAGATCAGCCTCC-3′; W543R: 5′-GATCCTGGTACTGAAGCT-3′) were generated by PCR, purified, and radiolabeled (Random Primed DNA Labeling Kit, Roche).
After hybridization, blots were subjected to autoradiography, and signal intensities of bands were quantified using a Storm 860 phosphorimaging system with ImageQuant software (GE Healthcare).
Accession Numbers
DNA sequences listed in Table 1, Figure legends, and in Supplemental Figures were deposited at GenBank (http,//www.ncbi.nlm.nih.gov/Genbank/index.html).
Supplementary Material
Highlights.
Switching in sharks consistent with immunization and cytidine deaminase expression
Isotype switching can be reciprocal between genes of established positions
Switch joints show DNA double strand breakage repaired by nonhomologous end-joining
Unlike in tetrapods, switching in shark is bi-direction between IgM and IgW
Acknowledgments
We thank Karen Vasquez for analyzing our sequences for non-B DNA structure, Martin Flajnik for gifts of animal tissue, Jason A. Hackney and Karolina Malecek for sequence analyses, and Michael Lieber for discussion. This work has been supported in part by funding from the National Institutions of Health GM068095 (E.H.).
Footnotes
Supplemental information include six figures (S1-S6) and legends.
None of the authors have a financial interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 3.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri J, Basu U, Zarrin A, Yan C, Franco S, Perlot T, Vuong B, Wang J, Phan RT, Datta A, Manis J, Alt FW. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 5.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stavnezer J, Björkman A, Du L, Cagigi A, Pan-Hammarström Q. Mapping of switch recombination junctions, a tool for studying DNA repair pathways during immunoglobulin class switching. Adv Immunol. 2010;108:45–109. doi: 10.1016/B978-0-12-380995-7.00003-3. [DOI] [PubMed] [Google Scholar]
- 7.Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–13. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- 8.Stavnezer J, Amemiya CT. Evolution of isotype switching. Sem Immunol. 2004;16:257–275. doi: 10.1016/j.smim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Flajnik MF. Comparative analyses of immunoglobulin genes, surprises and portents. Nat Rev Immunol. 2002;2:688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 10.Litman GW, Anderson MK, Rast JP. Evolution of antigen receptors. Ann Rev Immunol. 1999;17:109–47. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- 11.Malecek K, Brandman J, Brodsky JE, Ohta Y, Flajnik MF, Hsu E. Somatic hypermutation and junctional diversification at Ig heavy chain loci in the nurse shark. J Immunol. 2005;175:8105–8115. doi: 10.4049/jimmunol.175.12.8105. [DOI] [PubMed] [Google Scholar]
- 12.Lee V, Huang JL, Lui MF, Malecek K, Ohta Y, Mooers A, Hsu E. The evolution of multiple isotypic IgM heavy chains in the shark. J Immunol. 2008;180:7461–7470. doi: 10.4049/jimmunol.180.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu C, Feng W, Weedon J, Hua P, Stepanov D, Ohta Y, Flajnik MF, Hsu E. The multiple shark immunoglobulin heavy chain genes rearrange and hypermutate autonomously. J Immunol. 2011;187:2492–2501. doi: 10.4049/jimmunol.1101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rumfelt LL, Lohr RL, Dooley H, Flajnik MF. Diversity and repertoire of IgW and IgM VH families in the newborn nurse shark. BMC Immunol. 2004;5:8. doi: 10.1186/1471-2172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malecek K, Lee V, Feng W, Huang JL, Flajnik MF, Ohta Y, Hsu E. Immunoglobulin heavy chain exclusion in the shark. PLoS Biol. 2008;6:e157. doi: 10.1371/journal.pbio.0060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Hsu E. Error-prone DNA repair activity during somatic hypermutation in shark B lymphocytes. J Immunol. 2010;185:5336–5347. doi: 10.4049/jimmunol.1000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz M, Stanfield RL, Greenberg AS, Flajnik MF. Structural analysis, selection, and ontogeny of the shark new antigen receptor (IgNAR), identification of a new locus preferentially expressed in early development. Immunogenetics. 2002;54:501–512. doi: 10.1007/s00251-002-0479-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Tranchina D, Ohta Y, Flajnik MF, Hsu E. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity. 2002;16:571–582. doi: 10.1016/s1074-7613(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 19.Diaz M, Velez J, Singh M, Cerny J, Flajnik MF. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution, the translesion synthesis model of somatic hypermutation. Int Immunol. 1999;11:825–833. doi: 10.1093/intimm/11.5.825. [DOI] [PubMed] [Google Scholar]
- 20.Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 21.Shinkura R, Tian M, Smith M, Chua K, Fujiwara Y, Alt FW. The influence of transcriptional orientation on endogenous switch region function. Nat Immunol. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- 22.Mussmann R, Courtet M, Schwager J, Du Pasquier L. Microsites for immunoglobulin switch recombination breakpoints from Xenopus to mammals. Eur J Immunol. 1997;27:2610–2619. doi: 10.1002/eji.1830271021. [DOI] [PubMed] [Google Scholar]
- 23.Zarrin AA, Alt FW, Chaudhuri J, Stokes N, Kaushal D, Du Pasquier L, Tian M. An evolutionarily conserved target motif for immunoglobulin class-switch recombination. Nat Immunol. 2004;5:1275–1281. doi: 10.1038/ni1137. [DOI] [PubMed] [Google Scholar]
- 24.Zarrin AA, Tian M, Wang J, Borieson T, Alt FW. Influence of switch region length on immunoglobulin class switch recombination. Proc Natl Acad Sci USA. 2005;102:2466–2470. doi: 10.1073/pnas.0409847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebeque SG, Gearhart PJ. Boundaries of somatic mutation in rearranged immunoglobulin genes, 5′ boundary is near the promoter, and 3′ boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990;172:1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue K, Rada C, Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. J Exp Med. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reina-San-Martin B, Difilippantonio S, Hanitsch L, Masilamani RF, Nussenzweig A, Nussenzweig MC. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J Exp Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingzette M, Spieker-Polet H, Yam PC, Zhai SK, Knight KL. Trans-chromosomal recombination within the Ig heavy chain switch region in B lymphocytes. PNAS. 1998;95:11840–11845. doi: 10.1073/pnas.95.20.11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynaud S, Delpy L, Fleury L, Dougier H-L, Sirac C, Cogné M. Interallelic class switch recombination contributes significantly to class switching in mouse B cells. J Immunol. 2005;174:6176–6183. doi: 10.4049/jimmunol.174.10.6176. [DOI] [PubMed] [Google Scholar]
- 30.Anderson M, Amemiya C, Luer C, Litman R, Rast J, Niimura Y, Litman G. Complete genomic sequence and patterns of transcription of a member of an unusual family of closely related, chromosomally dispersed Ig gene clusters in Raja. Int Immunol. 1994;6:1661–1670. doi: 10.1093/intimm/6.11.1661. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–846. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 32.Yamane A, Resch W, Kuo N, Kuchen S, Li Z, Sun HW, Robbiani DF, McBride K, Nussenzweig MC, Casellas R. Deep-sequencing identification of the genomic targets of the cytidine deaminase AID and its cofactor RPA in B lymphocytes. Nat Immunol. 2011;12:62–70. doi: 10.1038/ni.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiarle R, Zhang Y, Frock RL, Lewis SM, Molinie B, Ho YJ, Myers DR, Choi VW, Compagno M, Malkin DJ, et al. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell. 2011;147:107–119. doi: 10.1016/j.cell.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein IA, Resch W, Jankovic M, Oliveira T, Yamane A, Nakahashi H, Di Virgilio M, Bothmer A, Nussenzweig A, Robbiani DF, et al. Translocation-capture sequencing reveals the extent and nature of chromosomal rearrangements in B lymphocytes. Cell. 2011;147:95–106. doi: 10.1016/j.cell.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunnick WA, Collins JT, Shi J, Westfield G, Fontaine C, Hakimpour P, Papavasiliou FN. Switch recombination and somatic hypermutation are controlled by the heavy chain 3′ enhancer region. J Exp Med. 2009;206:2613–2623. doi: 10.1084/jem.20091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kothapalli NR, Collura KM, Norton DD, Fugmann SD. Separation of mutational and transcriptional enhancers in immunoglobulin genes. J Immunol. 2011;187:3247–3255. doi: 10.4049/jimmunol.1101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang FL, Luo Z, Scharff MD. H3 trimethyl K9 and H3 acetyl K9 chromatin modifications are associated with class switch recombination. Proc Natl Acad Sci USA. 2009;106:5288–5293. doi: 10.1073/pnas.0901368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med. 2009;206:1817–1830. doi: 10.1084/jem.20081678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniel JA, Santos MA, Wang Z, Zang C, Schwab KR, Jankovic M, Filsuf D, Chen HT, Gazumyan A, Yamane A, et al. PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science. 2010;329:917–923. doi: 10.1126/science.1187942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storck S, Aoufouchi S, Weill J-C, Reynaud C-A. AID and partners, for better and (not) for worse. Curr Opin Immunol. 2011;23:337–344. doi: 10.1016/j.coi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Barreto VM, Pan-Hammarstrom Q, Zhao Y, Hammarstrom L, Misulovin Z, Nussenzweig MC. AID from bony fishes catalyzes class switch recombination. J Exp Med. 2005;202:733–738. doi: 10.1084/jem.20051378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakae K, Magor BG, Saunders H, Nagaoka H, Kawamura A, Kinoshita K, Honjo T, Muramatsu M. Evolution of class switch recombination function in fish activation-induced cytidine deaminase, AID. Int Immunol. 2006;18:41–47. doi: 10.1093/intimm/dxh347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.