Abstract
Background
Debate on how to manage pediatric cutaneous melanoma patients continues; particularly in those with sentinel lymph node(SLN) metastases who are at higher risk of poor outcomes. Management is often based on adult algorithms, although differences in clinical outcomes between pediatric and adult patients suggests that melanoma in pediatric patients differs biologically. Yet, there are no molecular prognostic studies identifying these differences.
Objectives
We investigated the epigenetic(methylation) regulation of several tumor-related genes(TRGs) known to be significant in adult melanoma progression in histopathology(+) SLN metastases(n=17) and primary tumors(n=20) of pediatric melanoma patients to determine their clinical relevance.
Methods
AJCC Stage I-III(n=37) pediatric cutaneous melanoma patients(≤ 21 years at diagnosis) were analyzed. Gene promoter methylation of TRGs: RASSF1A, RARβ2, WIF1, and APC was evaluated.
Results
Hypermethylation of RASSF1A, RARβ2, WIF1, and APC in histopathology(+) SLNs was 29.4%(5/17), 25%(4/16), 25%(4/16), and 18.8%(3/16), respectively. When matched to adult cutaneous melanomas by Breslow thickness and ulceration, hypermethylation of all four TRGs in SLN(+) pediatric melanoma patients was equivalent to or less than in adults. With a median follow-up of 55 months, SLN(+) pediatric melanoma patients with hypermethylation of >1 TRGs versus ≤1 TRG had worse disease-free(p=0.02) and overall survival(p=0.02).
Conclusions
Differences in the methylation status of these TRGs in the SLN(+) pediatric and adult melanoma patients may account for why SLN(+) pediatric patients have different clinical outcomes. SLN biopsy should continue to be performed; within SLN(+) pediatric melanoma patients, hypermethylation of TRGs can be used to identify a subpopulation at highest risk for poor outcomes who warrant vigilant clinical follow-up.
INTRODUCTION
Although pediatric cutaneous melanoma is rare, accounting for approximately 1-3% of all cutaneous melanomas, the incidence is increasing at an average annual rate of 2.8%, while the overall incidence of melanoma is only increasing at 2.2% annually.1,2 Consequently, less is known regarding melanoma in young patients and therefore, management of pediatric cutaneous melanoma patients is often based on adult algorithms.3 However, melanoma in young patients may differ from adults in etiology, natural history as well as clinical outcome, and extrapolation from treatment of adults may not always be appropriate.
In adult cutaneous melanoma, sentinel lymph node(SLN) biopsy has become standard of care in staging adult melanoma patients and has been shown to be the strongest predictor of disease outcome in patients with intermediate thickness melanomas.4,5 Several studies have shown that at presentation, on average, pediatric cutaneous melanoma patients have thicker lesions than adults and a higher rate of SLN positivity by histopathological analysis.6-8 Surprisingly, pediatric melanoma patients with positive SLNs have a better clinical prognosis than adult patients; however, the molecular differences accounting for this disparity are largely unknown.9,10
Pediatric cutaneous melanoma tumors have been characterized for genomic studies and have shown some differences compared to adult melanomas, but have not been of prognostic utility.11,12 Epigenetic changes have been shown to alter gene expression without changing the DNA sequence and include dysregulated mechanisms such as DNA methylation, histone modification, and microRNA expression. Epigenetic inactivation of tumor suppressor genes has been implicated in tumorigenesis of various malignancies, including cutaneous melanoma.13-15 Our group has shown that epigenetic aberrations, such as hypermethylation of CpG islands in the promoter region of tumor-related genes (TRGs), can significantly silence gene function and promote tumor progression in adult cutaneous melanoma.16-18 Given the possible biological and demonstrated clinical differences in outcomes between adult and pediatric melanoma patients, we hypothesized that these differences may be reflected in the epigenetic profile of these TRGs in our pediatric melanoma patients. In this study, we investigated the epigenetic regulation of several TRGs in pediatric cutaneous melanoma patients by examining the DNA gene promoter methylation status of the primary tumor and histopathology(+) SLN. These TRGs included:RASSF1A (RAS association domain family 1A), RARβ2 (retinoic acid receptor-β2), WIF1 (Wnt inhibitory factor-1), and APC (adenomatous polyposis coli), which were selected because they have been shown to be significant in tumor progression in adult cutaneous melanoma.16 In the same study in adult melanoma patients, an increase in hypermethylation of the TRGs WIF1 and RASSF1A was associated with advancing clinical tumor stage.16 The objective was to determine the clinical relevance of these TRGs in our pediatric cutaneous melanoma patients and to compare the methylation levels of the TRGs in adult versus pediatric patients.
MATERIALS AND METHODS
Tumor specimens
Approval for the use of human tissues was obtained from the joint IRB of the John Wayne Cancer Institute and Saint John’s Health Center (SJHC), and the Sydney South West Area Health Service Ethics Review Committee. Analysis was conducted on paraffin-embedded archival tissue specimens of pediatric cutaneous melanoma patients diagnosed at SJHC from November 1996-June 2007 and at Royal Prince Alfred Hospital from December 1996-November 2007. Patients were staged using the 2009 AJCC staging system for cutaneous melanoma.19 Pediatric melanoma patients were defined as ≤ 21 years of age at the time of diagnosis, as previously described.20 Primary tumors(n=20) and SLN(n=17) specimens from a total of 37 AJCC Stage I-III patients were studied. Patients undergoing SLN biopsy had either lymphoscintigraphy, injection of isosulfan blue, or a combination of both. The 17 SLNs were histopathology(+) as defined by hematoxylin and eosin (H&E) staining; these patients ultimately underwent complete lymph node dissection (CLND). Review of histopathology records demonstrated that the mean Breslow thickness of primary tumors in the pediatric melanoma patients with histopathology(+) SLNs(n=17) was 4.13 ± 2.96 mm and the mean Breslow thickness of the primary tumor(n=20) was 1.78 ± 1.33 mm. Data from adult melanoma patients used for comparison to our pediatric patients was matched by Breslow thickness (within 1 mm) in both the primary tumor and SLN(+) metastases groups. The mean Breslow thickness of primary tumors in the matched pediatric and adult cutaneous melanoma patients(n=20) was 1.78 ± 1.33 mm and 1.73 ± 1.27 mm, respectively. In the matched SLN(+) metastases group(n=12), the mean Breslow thickness for the pediatric melanoma patients was 3.45 ± 2.04 mm and 3.28 ± 1.69 mm in adults. Clinicopathological characteristics of the pediatric cutaneous melanoma patients are summarized in Table 1. The clinicopathological characteristics of the subset of pediatric primary and SLN(+) metastasis patients that was matched to our adult patients is shown in Table 2.
Table 1. Clinicopathological Characteristics of Pediatric Melanoma Patients.
| Factors | Pediatric melanoma patients (%) (n=37) |
|---|---|
| Gender | |
| Male | 20 (54) |
| Female | 17 (51) |
| Age (years) | |
| ≤ 18 | 18 (49) |
| > 18 but ≤ 21 | 19 (51) |
| Tumor type | |
| Primary | 20 (54) |
| Sentinel lymph node (SLN) (+) | 17 (51) |
| AJCC stage | |
| I | 12 (32) |
| II | 2 (5) |
| III | 23 (62) |
| Histological subtype (primary tumor) | |
| Superficial spreading | 9 (45) |
| Nodular | 5 (25) |
| Atypical nevus | 2 (10) |
| Unclassified | 4 (20) |
| Primary site | |
| Extremities | 15 (41) |
| Head/Neck | 13 (35) |
| Trunk | 9 (24) |
| Breslow thickness (mm) | |
| < 1.00 | 9 (24) |
| 1.01-2.00 | 9 (24) |
| 2.01-4.00 | 11 (30) |
| > 4.00 | 8 (22) |
| Ulceration | |
| Present | 7 (19) |
| Absent | 27 (73) |
| Unknown | 3 (8) |
| Mitotic index | |
| ≥11/mm2 | 8 (22) |
| 5-10/mm2 | 6 (16) |
| 1-4/mm2 | 22 (60) |
| 0/mm2 | 0 (3) |
| Unknown | 1 |
Table 2. Comparison of Clinicopathological Characteristics of a Matched Subset of Pediatric Melanoma Patients to Adult Melanoma Patients.
| Factors | Pediatric melanoma patients (%) (n=32) |
Adult melanoma patients (%) (n=32) |
|---|---|---|
| Gender | ||
| Male | 16 (50) | 18 (56) |
| Female | 16 (50) | 14 (44) |
| Age (years) | ||
| ≤ 18 | 15 (47) | 0 |
| > 18 but ≤ 21 | 17 (53) | 0 |
| >21 | 0 | 32 (100) |
| Tumor type | ||
| Primary | 20 (63) | 20 (63) |
| Sentinel lymph node (SLN) (+) | 12 (38) | 12 (38) |
| AJCC stage | ||
| I | 8 (25) | 9 (28) |
| II | 6 (19) | 7 (22) |
| III | 18 (56) | 16 (50) |
|
Histological subtype (primary tumor) |
||
| Superficial spreading | 12 (38) | 13 (41) |
| Nodular | 12 (38) | 7 (22) |
| Atypical nevus | 2 (6) | 0 |
| Acrolentiginous | 0 | 7 (22) |
| Unclassified | 6 (19) | 5 (16) |
| Primary site | ||
| Extremities | 11 (34) | 18 (56) |
| Head/Neck | 12 (38) | 9 (28) |
| Trunk | 9 (28) | 5 (16) |
| Breslow thickness (mm) | ||
| < 1.00 | 9 (28) | 10 (31) |
| 1.01-2.00 | 9 (28) | 7 (22) |
| 2.01-4.00 | 8 (25) | 10 (31) |
| > 4.00 | 6 (19) | 5 (16) |
| Ulceration | ||
| Present | 7 (22) | 7 (22) |
| Absent | 22 (68.8) | 22 (69) |
| Unknown | 3 (9.4) | 3 (9) |
| Mitotic index | ||
| ≥11/mm2 | 7 (22) | 5 (16) |
| 5-10/mm2 | 4 (13) | 3 (9) |
| 1-4/mm2 | 20 (63) | 19 (59) |
| Unknown | 1 (3) | 5 (16) |
DNA isolation
Seven μM sections of paraffin-embedded archival tissue from primary tumors and histopathology(+) SLNs were cut for DNA isolation. An H&E slide was prepared from each specimen to confirm tumor location for laser capture microdissection. Tumor cells were captured and microdissected onto laser capture microdissection caps using the Applied Biosystems Arcturus Laser Capture Microdissection System (Life Technologies, CA). On cap sodium bisulfite modification was performed by incubating the cap in 0.2 mol/L NaOH at 37°C for 15 min and then in a 4.5 mol/L sodium bisulfite solution containing sodium metabisulfite, hydroquinone, and NaOH (pH 5) at 60°C for 8hrs, as previously described.21 After incubation, the cap was rinsed with distilled H2O and soaked in 0.3 mol/L NaOH for 15min. The film was then removed from the cap and immersed in lysis buffer containing proteinase K and Tween 20 at 50°C for 8 hrs followed by proteinase K denaturing at 95°C for 15 min.
Methylation-specific PCR and capillary array electrophoresis
The methylation status of the specimens was assessed for RASSF1A, RARβ2, WIF1, and APC by methylation-specific PCR using two sets of fluorescent-labeled primers for each gene, designed to amplify methylated or unmethylated DNA sequences. The sequences for methylated and unmethylated forward and reverse primers were as previously described.16 Bisulfite-modified DNA was subjected to PCR amplification in a final reaction volume of 10μl containing PCR buffer, MgCl2, dNTP, primers, and AccuStart Taq DNA polymerase (Quanta Biosystems, MD) as previously described.16 PCR amplification was performed with an initial 10min incubation at 95°C followed by 40 cycles of denaturation at 95°C for 30sec, annealing for 30sec at 61°C for all methylated primers and 59°C for unmethylated primers, extension at 72°C for 30sec, and a final hold at 72°C for 7min. Each assay included universal unmethylated and methylated controls.
Methylation PCR products were assessed using capillary array electrophoresis (CAE)(CEQ 8000XL; Beckman Coulter, CA) as previously described using Beckman Coulter WellRED dye-labeled phosphoramidites (Sigma-Aldrich, CA).16 Forward methylated sequence-specific primers were labeled with D4 dye, and forward unmethylated sequence-specific primers were labeled with D3 dye. PCR products were mixed with loading buffer and a dye-labeled size standard (Beckman Coulter) and loaded in a 96-well plate for CAE peak ratio analysis. Specimens showing a peak for unmethylated DNA were recorded as unmethylated and those showing a peak for methylated DNA or peaks for both methylated and unmethylated DNA were considered methylated.22
Biostatistics analysis
Categorical data were analyzed using the χ2 test. Fisher’s exact test was used when analyzing small sample sizes and the Pearson’s chi-square test was used when analyzing ≥3 categorical variables. P values <0.05 were considered to be statistically significant. Cox proportional hazards regression models were created for overall survival and disease-free survival calculations incorporating multiple variables. Statistical calculations were performed using JMP software version 7.0 (SAS Institute, NC). Studies were developed and reported according to the REMARK guidelines.23
RESULTS
Methylation promoter status of the TRGs in pediatric melanoma patients
The frequency of hypermethylation of RASSF1A, RARβ2, WIF1, and APC from pediatric cutaneous melanoma primary tumors was 35%, 45%, 20%, and 25%, respectively; and 29.4%, 25%, 25%, and 18.8%, respectively in histopathology(+) SLNs (Table 3). Of all the TRGs analyzed, only WIF1 hypermethylation in the SLN(+) metastases was slightly higher than in the primary tumor (25% vs. 20%; Table 3), but this was not statistically significant. In the primary tumors and histopathology(+) SLNs, 60% (12/20) and 64.7% (11/17) were hypermethylated for ≥ 1 TRG, 45% (9/20) and 23.5% (4/17) for ≥ 2 TRGs, and 20% (4/20) and 5.9% (1/17) for ≥ 3 TRGs, respectively. None of the patients demonstrated hypermethylation of ≥ 4 TRGs.
Table 3. TRG Promoter Hypermethylation in Primary Tumors and Histopathology(+) SLNs of Pediatric Melanoma Patients.
| TRG | Specimen | TRG hypermethylation frequency (%) (total n=37) |
|---|---|---|
| RASSF1A | Primary | 7/20 (35) |
| SLN | 5/17 (29) | |
| RARβ2 | Primary | 9/20 (45) |
| SLN | 4/16a(25) | |
| WIF1 | Primary | 4/20 (20) |
| SLN | 4/16a (25) | |
| APC | Primary | 5/20 (25) |
| SLN | 3/16a (19) |
One specimen from methylation analysis of these TRGs that provided non-informative results by CAE was excluded from analysis.
Patient clinicopathological parameters were compared with the frequency of hypermethylation of the TRGs in primary tumors and histopathology(+) SLNs. As a single TRG, only hypermethylation of RASSF1A was significantly associated with primary tumors located in the head and neck (61.5% (8/13), p=0.018; Table 4).
Table 4. Clinicopathological parameters vs. TRG hypermethylation frequency in primary tumors and histopathology(+) SLNs of pediatric cutaneous melanoma patients.
| Factors | Patients (n=37) |
RASSF1A(%) (n=37) |
RARβ2(%) (n=36*) |
APC(%) (n=36*) |
WIF1(%) (n=36*) |
|
|---|---|---|---|---|---|---|
| Gender* | ||||||
| Male | 20 | 6/20 (30) | 9/20 (45) | 4/19 (21.1) | 4/19 (21.1) | |
| Female | 17 | 6/20 (30) | 4/16 (25) | 4/17 (23.5) | 4/17 (23.5) | |
|
Age(years) (≤21)* |
||||||
| ≤ 18 | 18 | 5/18 (27.8) | 4/18 (22.2) | 4/17 (23.5) | 4/17 (23.5) | |
| > 18, ≤ 21 | 19 | 7/19 (36.8) | 9/18 (50) | 4/19 (21.1) | 4/19 (21.1) | |
|
Primary Site** |
||||||
| Extremities | 15 | 2/15 (13.3) | 5/14 (35.7) | 4/14 (28.6) | 3/15 (20) | |
| Head/Neck | 13 | 8/13 (61.5) | 5/13 (38.5) | 3/13 (23.1) | 2/13 (15.4) | |
| Trunk | 9 | 2/9 (22.2) | 3/9 (33.3) | 1/9 (11.1) | 3/8 (37.5) | |
|
Breslow Thickness* |
||||||
| <1.00mm | 9 | 3/9 (33.3) | 4/9 (44.4) | 3/9 (33.3) | 2/9 (22.2) | |
| 1.01-2.00mm | 9 | 3/9 (33.3) | 4/9 (44.4) | 1/9 (11.1) | 3/8 (37.5) | |
| 2.01-4.00mm | 11 | 5/11 (45.5) | 3/10 (30) | 3/10 (30) | 2/11 (18.2) | |
| >4.00mm | 8 | 1/8 (12.5) | 2/8 (25) | 1/8 (12.5) | 1/8 (12.5) | |
| Ulceration* | ||||||
| Present | 7 | 2/7 (28.6) | 2/7 (28.6) | 1/7 (14.3) | 2/7 (28.6) | |
| Absent | 27 | 9/27 (33.3) | 11/26 (42.3) | 6/26 (23.1) | 6/26 (23.1) | |
| Unknown | 3 | 1/3 (33.3) | 0/3 | 1/3 (33.3) | 0/3 | |
|
Mitotic Index* |
||||||
|
High ≥ 11/mm2 |
8 | 2/8 (25) | 2/8 (25) | 0/8 | 1/8 (12.5) | |
|
Intermediate 5-10/mm2 |
6 | 2/8 (25) | 3/8 (37.5) | 2/8 (25) | 1/8 (12.5) | |
| Low ≤ 4/mm2 | 22 | 8/22 (36.4) | 8/22 (36.4) | 6/22 (27.3) | 6/22 (27.3) | |
| Unknown | 1 | 0/1 | 0/1 | 0/1 | 0/1 |
NS
all NS except RASSF1A (p=0.018)
Comparison of TRG methylation in pediatric matched to adult melanoma patients
When matched by Breslow thickness and ulceration to adult melanoma patients, hypermethylation of these TRGs in primary tumors from the pediatric melanoma patients did not significantly differ from adult primary melanomas: RARβ2 (45% vs. 50%); WIF1 (20% vs. 11%); or APC (25% vs. 36%) (Fig. 1). However, RASSF1A where the frequency of hypermethylation was significantly higher in pediatric vs. adult primary tumors (35% vs. 0%; p=0.009; Fig. 1). In the SLN(+) metastases group, hypermethylation of the RASSF1A in the pediatric melanoma patients was the same as in adults, however, lower for RARβ2 (17% vs. 36%), WIF1 (18% vs. 42%), and APC (17% vs. 60%), although none were statistically significant (Fig. 1).
Fig. 1. TRG promoter hypermethylation in primary tumors and histopathology(+) SLNs of pediatric melanoma patients matched to adult patients.
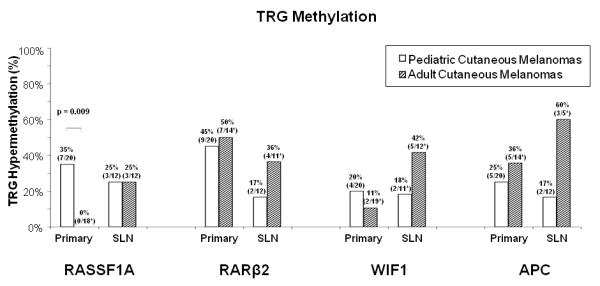
The frequency of hypermethylation of the TRGs in primary tumors and histopathology(+) SLNs of pediatric cutaneous melanoma patients matched to adult patients by Breslow thickness and ulceration is represented for RASSF1A, RARβ2, WIF1, and APC. Only RASSF1A hypermethylation in the primary tumors of the pediatric melanoma patients differed significantly from that in adult patients (p=0.009). *Specimens from methylation analysis of these TRGs that provided non-informative results by CAE were excluded from analysis. **Specimens from methylation analysis of APC with insufficient tissue or DNA for analysis were excluded. The previous adult cutaneous melanoma study16 was used to match to pediatric melanoma patients.
Correlation of TRG methylation with clinical outcomes in the pediatric melanoma patients
Hypermethylation of TRGs in the primary tumor of the pediatric melanoma patients did not correlate to disease outcome. However, hypermethylation of TRGs in patients with histopathology(+) SLNs demonstrated an inverse relationship to survival. Overall, in the histopathology(+) SLN pediatric patients, there were 4 deaths (4/17; 23.5%) and 5 recurrences (5/17; 29.4%). Of these SLN(+) patients, those demonstrating >1(+) hypermethylated TRG in the SLN (n=4) versus those with ≤1 hypermethylated TRG (n=13) had a poorer OS and DFS (p=0.02; p=0.02, respectively, log-rank test). This followed a trend similar to adult SLN patients.16
DISCUSSION
In adult melanoma patients, SLN biopsy has become standard of care for staging early stage adult cutaneous melanoma patients, as data strongly supports that the SLN status is a major prognostic factor for predicting tumor recurrence and survival.4,5 Recently, studies of SLN biopsy in patients with pediatric cutaneous melanoma have shown that there is a higher incidence of SLN(+) in pediatric patients as compared to adults, but for unknown reasons, pediatric melanoma patients generally have a lower incidence of recurrence and improved DFS.7-10
Our group and others have shown that epigenetic inactivation of TRGs via hypermethylation of CpG island promoter regions is significant in adult melanoma progression.16,24-26 To our knowledge, this is the first study to evaluate the epigenetic changes of these TRGs in pediatric cutaneous melanoma patients. Promoter hypermethylation of the TRGs in pediatric patients’ primary tumors overall was not significantly greater than SLN(Table 3). When the pediatric cutaneous melanoma patients were matched to our adult melanoma patients16 by Breslow thickness and ulceration in order to try to delineate similarities or differences between methylation patterns of the TRGs between the two groups, TRGs hypermethylation in primary tumors from the pediatric patients was comparable or lower than in adults with the exception of RASSF1A(Fig. 1). Hypermethylation of RASSF1A in the primary tumor of pediatric melanoma patients was statistically significantly higher (p=0.009) than in adult primaries. TRG methylation increased significantly with advancing clinical stage in adult melanoma patients,16 suggesting that TRG inactivation is associated with tumor progression. A similar trend was not present of pediatric patients in comparing primary to SLN. The overall lower rates of hypermethylation of the TRGs in the SLN of pediatric patients as compared to in adults may help explain why SLN(+) pediatric patients have better outcomes than their adult counterparts. The lower methylation levels of these TRGs in pediatric melanoma patients in conjunction with immunity controlling metastatic disease progression. Stronger immunity may contribute to the overall improved survival of pediatric melanoma patients with nodal metastases as compared to adults. Pediatric patients have been known to have a stronger immune response than adults in general. Validation studies are needed to investigate these interesting findings in a multicenter study where patient sample size can be increased.
In comparing the methylation status of the TRGs to clinicopathological parameters, hypermethylation of RASSF1A was found to be significantly associated with primary melanomas of the head and neck. Several studies in the melanoma literature have demonstrated that primary melanomas of the scalp and neck region portend worse survival for patients than tumors originating from other primary sites.27,28A recent study by Tcheung et al. of 39 cases of pediatric cutaneous head and neck melanomas reported a trend toward decreased survival for patients with scalp and neck melanoma compared with other sites.29 Another issue in head and neck melanomas that compounds the problem is the variability of lymph node drainage in the head and neck region as compared to extremity sites which makes identification of the SLN more technically challenging.
Assessment of the methylation status of these TRGs may help identify a subpopulation of pediatric cutaneous melanoma patients within the histopathology(+) SLN group that is at higher risk for disease relapse and death, since those with >1 hypermethylated TRG in the SLN had the worst outcome. Given that our study was limited by sample size, these findings need to be validated in larger, multicenter studies. However, despite the limited number of patients analyzed, the trends we found were statistically significant and therefore these epigenetic biomarkers could potentially be used in conjunction with standard clinical and histopathological features to determine which histopathology(+) SLN patients may benefit from adjuvant therapy and closer clinical follow-up. In contrast, hypermethylation in the primary tumors did not correlate to disease outcome; however, most of these patients had thin melanomas; and as a result, SLN biopsy was not indicated. In addition, overall, those patients with thin melanomas versus thick tumors have improved disease outcomes.
In adult cutaneous melanomas, the rate of SLN positivity by histopathological analysis is 15% to 20%,30,31 whereas the rate in pediatric cutaneous melanomas 25% to 60%.32,33 In this study, the rate of histopathology(+) SLN was 29% and these patients had a 77% OS and 71% DFS with median 55 month follow-up time, comparable to that reported in the pediatric melanoma literature.7,8 Contrastingly, in adult melanoma patients, the 10 year OS rate was 56% and DFS rate was 48% for patients with histopathology(+) SLNs.34 The mean tumor thickness of the primary tumor in pediatric cutaneous melanoma patients with histopathology(+) SLNs was 4.13 mm; tumor thickness has been shown to be an independent predictor of SLN(+) in pediatric and adult cutaneous melanoma.30,31,35
Our group has shown that lymphatic function, assessed by radiocolloid transit time to and uptake by the SLN, declines with age,36 which may explain the inverse relationship between patient age and SLN(+).10 Changes associated with aging such as damage to elastic tissues, fatty replacement of LNs and a reduction in skin blood and lymphatic flow can impact detection of metastases in the SLN.36 Another possible explanation for the improved survival of histopathology(+) SLN pediatric cutaneous melanoma patients is that systemic host immune responses, such as innate immunity, may be more effective in children than adults.37,38 Draining LNs in pediatric patients may be more effectively immunocompetent against occult metastasis than in adult patients. Recently, Moore-Olufemi et al. demonstrated in a study of prepubescent (<10 years) versus adolescent (≥10-18 years of age) pediatric melanoma patients that younger ages showed increased risk of LN metastasis and thicker tumors; however, OS and event-free survival did not differ by age groups.39 Although it would be interesting to determine if there is a difference in survival among SLN(+) patients that are pre- and post-pubescent, our study was limited by sample size, as only 8 patients were ≤ 14 years old and therefore a sub-analysis of very young patients was not feasible.
Our study demonstrates that SLN biopsy should continue to be performed in pediatric cutaneous melanoma patients for staging and also because overall, those that are histopathology(+) have worse outcomes. MSLT-II is an ongoing randomized clinical trial in adult melanoma patients to determine if it is necessary for SLN histopathology(+) patients to undergo CLND.5 Since pediatric patients have higher rates of SLN(+) than adults, avoiding CLND may help reduce the morbidity associated with CLND for pediatric patients who have more time to manifest complications associated with the procedure. The caveat however is that these SLN(+) pediatric cutaneous melanoma patients will still need to be closely followed for regional recurrence. Although larger studies are needed to validate our findings, stratification of pediatric patients into those at highest risk for poor outcomes by the addition of epigenetic analysis with SLN biopsy may prove to be a valuable prognostic tool.
What’s already known about this topic?
Given its rarity, management of pediatric cutaneous melanoma is often based on adult algorithms; however, debate continues on how to best manage these patients.
Despite higher rates of sentinel lymph node(SLN) metastasis than adults, stage III pediatric melanoma patient have better clinical outcomes.
Studies in pediatric cutaneous melanoma patients investigating the epigenetic differences of primary and sentinel lymph nodes are absent.
What does this study add?
We demonstrate that epigenetic regulation of tumor-related genes(TRGs) in histopathology(+) SLNs of pediatric melanoma patients has clinical relevance.
Differences in the methylation status of these TRGs in SLN(+) pediatric and adult melanoma patients may account for the clinical differences between the two types of patients.
Particularly, pediatric melanoma patients that are SLN(+) and demonstrate hypermethylation of >1 TRG have the worst outcomes and warrant close clinical follow-up.
ACKNOWLEDGMENTS
This work was supported by Award Number P0 CA029605 and P0 CA012582 from the NIH, NCI and the Sheldon G. Adelson Medical Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NCI or NIH.
Footnotes
CONFLICT OF INTEREST: All authors disclose no conflict of interest.
REFERENCES
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004) Cancer. 2008;112:416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; [accessed January 10, 2011]. 1975-2007. Available: http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 3.Pappo AS. Melanoma in children and adolescents. Eur J Cancer. 2003;39:2651–61. doi: 10.1016/j.ejca.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–11. doi: 10.1097/01.sla.0000181092.50141.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari A, Bono A, Baldi M, et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649–54. doi: 10.1542/peds.2004-0471. [DOI] [PubMed] [Google Scholar]
- 7.Livestro DP, Kaine EM, Michaelson JS, et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer. 2007;110:614–24. doi: 10.1002/cncr.22818. [DOI] [PubMed] [Google Scholar]
- 8.Howman-Giles R, Shaw HM, Scolyer RA, et al. Sentinel lymph node biopsy in pediatric and adolescent cutaneous melanoma patients. Ann Surg Oncol. 2010;17:138–43. doi: 10.1245/s10434-009-0657-4. [DOI] [PubMed] [Google Scholar]
- 9.Sassen S, Shaw HM, Colman MH, et al. The complex relationships between sentinel node positivity, patient age, and primary tumor desmoplasia: analysis of 2303 melanoma patients treated at a single center. Ann Surg Oncol. 2008;15:630–7. doi: 10.1245/s10434-007-9684-1. [DOI] [PubMed] [Google Scholar]
- 10.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11:247–58. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Daniotti M, Ferrari A, Frigerio S, et al. Cutaneous melanoma in childhood and adolescence shows frequent loss of INK4a and gain of KIT. J Invest Dermatol. 2009;129:1759–68. doi: 10.1038/jid.2008.422. [DOI] [PubMed] [Google Scholar]
- 12.Uribe P, Wistuba II, Solar A, et al. Comparative analysis of loss of heterozygosity and microsatellite instability in adult and pediatric melanoma. Am J Dermatopathol. 2005;27:279–85. doi: 10.1097/01.dad.0000171599.40562.7c. [DOI] [PubMed] [Google Scholar]
- 13.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 14.de Maat MF, Umetani N, Sunami E, et al. Assessment of methylation events during colorectal tumor progression by absolute quantitative analysis of methylated alleles. Mol Cancer Res. 2007;5:461–71. doi: 10.1158/1541-7786.MCR-06-0358. [DOI] [PubMed] [Google Scholar]
- 15.Hoon DS, Spugnardi M, Kuo C, et al. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–22. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanemura A, Terando AM, Sim MS, et al. CpG island methylator phenotype predicts progression of malignant melanoma. Clin Cancer Res. 2009;15:1801–7. doi: 10.1158/1078-0432.CCR-08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori T, Martinez SR, O’Day SJ, et al. Estrogen receptor-α methylation predicts melanoma progression. Cancer Res. 2006;66:6692–8. doi: 10.1158/0008-5472.CAN-06-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunami E, Shinozaki M, Sim M-S, et al. Estrogen receptor and HER2/neu status affects epigenetic differences of tumor-related genes in primary breast tumors. Breast Cancer Res. 2008;10:R46. doi: 10.1186/bcr2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills O, Messina JL. Pediatric melanoma: a review. Cancer Control. 2009;16:225–33. doi: 10.1177/107327480901600304. [DOI] [PubMed] [Google Scholar]
- 21.de Maat M, Narita N, Bernard A, et al. Development of sporadic microsatellite instability in colorectal tumors involves hypermethylation at methylated-in-tumor loci in adenoma. Am J Pathol. 2010;177:2347–56. doi: 10.2353/ajpath.2010.091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunami E, Shinozaki M, Higano CS, et al. Multimarker circulating DNA assay for assessing blood of prostate cancer patients. Clin Chem. 2009;55:559–67. doi: 10.1373/clinchem.2008.108498. [DOI] [PubMed] [Google Scholar]
- 23.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. J Clin Oncol. 2005;23:9067–72. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 24.Mori T, O’Day SJ, Umetani N, et al. Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy. J Clin Oncol. 2005;23:9351–8. doi: 10.1200/JCO.2005.02.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marini A, Mirmohammadsadegh A, Nambiar S, et al. Epigenetic inactivation of tumor suppressor genes in serum of patients with cutaneous melanoma. J Invest Dermatol. 2006;126:422–31. doi: 10.1038/sj.jid.5700073. [DOI] [PubMed] [Google Scholar]
- 26.Lin YC, You L, Xu Z, et al. Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum Gene Ther. 2007;18:379–86. doi: 10.1089/hum.2006.005. [DOI] [PubMed] [Google Scholar]
- 27.Law MM, Wong JH. Evaluation of the prognostic significance of the site of origin of cutaneous melanoma. Am Surg. 1994;60:362–6. [PubMed] [Google Scholar]
- 28.Lachiewicz AM, Berwick M, Wiggins CL, et al. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144:515–21. doi: 10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 29.Tcheung WJ, Marcello JE, Puja KP, et al. Evaluation of 39 cases of pediatric cutaneous head and neck melanoma. J Am Acad Dermatol. 2011;65:e37–42. doi: 10.1016/j.jaad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–9. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad FF, Stall A, Messina J, et al. The progression of melanoma nodal metastasis is dependent on tumor thickness of the primary lesion. Ann Surg Oncol. 1999;6:144–9. doi: 10.1007/s10434-999-0144-y. [DOI] [PubMed] [Google Scholar]
- 32.Shah NC, Gerstle JT, Stuart M, et al. Use of sentinel lymph node biopsy and high-dose interferon in pediatric patients with high-risk melanoma: The Hospital for Sick Children experience. J Pediatr Hematol Oncol. 2006;28:496–500. doi: 10.1097/01.mph.0000212973.28996.e4. [DOI] [PubMed] [Google Scholar]
- 33.Toro J, Ranieri JM, Havlik RJ, et al. Sentinel lymph node biopsy in children and adolescents with malignant melanoma. J Pediatr Surg. 2003;38:1063–5. doi: 10.1016/s0022-3468(03)00193-3. [DOI] [PubMed] [Google Scholar]
- 34.Nicholl MB, Elashoff D, Takeuchi H, et al. Molecular upstaging based on paraffin-embedded sentinel lymph nodes: ten-year follow-up confirms prognostic utility in melanoma patients. Ann Surg. 2011;253:1–7. doi: 10.1097/SLA.0b013e3181fca894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roaten JB, Partrick DA, Bensard D, et al. Survival in sentinel lymph node-positive pediatric melanoma. J Pediatr Surg. 2005;40:988–92. doi: 10.1016/j.jpedsurg.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Conway WC, Faries MB, Nicholl MB, et al. Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol. 2009;16:1548–52. doi: 10.1245/s10434-009-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez CR, Nomellini V, Faunce DE, et al. Innate immunity and aging. Exp Gerontol. 2008;43:718–28. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegde UP, Chakraborty N, Kerr P, et al. Melanoma in the elderly patient: relevance of the aging immune system. Clin Dermatol. 2009;27:537–44. doi: 10.1016/j.clindermatol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Moore-Olufemi S, Herzog C, Warneke C, et al. Outcomes in pediatric melanoma: comparing prepubertal to adolescent pediatric patients. Ann Surg. 2011;253:1211–15. doi: 10.1097/SLA.0b013e318217e852. [DOI] [PubMed] [Google Scholar]


