Abstract
Peripheral Artery Disease (PAD) represents a burgeoning form of cardiovascular disease associated with significant clinical morbidity and increased 5 year cardiovascular disease mortality. It is characterized by impaired blood flow to the lower extremities, claudication pain and severe exercise intolerance. Pathophysiological factors contributing to PAD include atherosclerosis, endothelial cell dysfunction, and defective nitric oxide metabolite physiology and biochemistry that collectively lead to intermittent or chronic tissue ischemia. Recent work from our laboratories is revealing that nitrite/nitrate anion and nitric oxide metabolism plays an important role in modulating functional and pathophysiological responses during this disease. In this review, we discuss experimental and clinical findings demonstrating that nitrite anion acts to ameliorate numerous pathophysiological events associated with PAD and chronic tissue ischemia. We also highlight future directions for this promising line of therapy.
Keywords: ischemia, angiogenesis, arteriogenesis, vasodilation, exercise, blood flow, claudication
Introduction and Significance of PAD
Peripheral artery disease (PAD) is a form of cardiovascular disease (CVD) caused by atherosclerosis which leads to arterial obstruction and decreased blood flow to the lower extremities. The latest American Heart Association statistics report that approximately eight million patients in the United States are affected with PAD, including 12–20% of individuals older than age 60[1], with an even larger number remaining undiagnosed[2]. Thus the total PAD burden is estimated to be as high as 12–14 million in the US alone. The most frequent clinical symptomatic manifestation of PAD is intermittent claudication (IC) defined as, pain in 1 or both legs during exercise that is relieved with rest[3]. This inability to match oxygen delivery to tissue demands during physical activity severely debilitates subjects with PAD such that approximately 1/3rd have pain ambulating in their home with a similar number having pain after walking one block[4]. These patients suffer a markedly impaired quality of life[5] and a high perception of disability[6].
Although measures of conduit vessel and gross limb blood flow, such as brachial systolic blood pressure index (ABI), are used to diagnose PAD, they show a poor relationship with functional capacity[7–12]. It appears that the key to increasing functionality in IC patients may lie at the resistance arteries, arterioles and capillaries that serve the skeletal muscle tissue distal to the site of stenosis. These are the vessels which are responsible for much of the oxygen delivery[13] and become hypoxic during the increased demands for perfusion accompanying physical exertion. This concept is illustrated by the fact that surgical revascularization does not normalize exercise capacity[14], but exercise training does[15, 16] suggesting the ability to distribute available blood and oxygen to working tissues more efficiently via training maybe a key to improving physical function.
An intervention that could (A) acutely improve oxygenation to areas of ischemia and (B) chronically increase vessel growth to these ischemic areas, would allow for greater exercise tolerance, ease the burden of exercise compliance and facilitate greater improvements in function and quality of life. Such an intervention would be a significant step forward in the treatment of PAD.
PAD and Nitric Oxide Bioavailability
Dysfunction of the endothelium is an early event in the development of atherosclerosis and is associated with the presence of cardiovascular risk factors[17–19], diabetes[20], and cardiovascular diseases, including PAD[21, 22]. A hallmark feature of endothelial dysfunction in these conditions is abnormal vascular reactivity mediated, in part, by reduced levels of endothelium-derived nitric oxide (NO)[23].
Endothelial nitric oxide synthase (eNOS) is the primary endogenous source of NO generation in the vascular system[24] and its expression has been reported as directly proportional to plasma nitrite levels[25, 26], suggesting nitrite may reflect cardiovascular NO bioavailability. Under basal conditions, eNOS catalyzes the oxidation of L-arginine to NO and L-citrulline, with NADPH and oxygen serving as co-substrates[27]. Mammalian NOS are all heme-containing flavoproteins which transfer NADPH-derived electrons to the heme with the aid of tetrahydrobiopterin as a co-factor and is regulated by calmodulin[28]. The vasodilatory activity of NO occurs in the vascular smooth muscle due to its interaction with the iron heme in guanylate cyclase[29], causing its activation as the enzyme to produce guanosine 3’-5’-monophosphate (cGMP) from guanosine 5’-triphosphate (GTP). cGMP activates several protein kinases causing calcium extrusion from the cell, increased uptake by the sarcoplasmic reticulum and subsequent dilation. Nitric oxide also acts as an important signaling molecule regulating vascular inflammation[30], platelet function[31], angiogenesis[32, 33], and cellular respiration [34] and protection from ischemia reperfusion injury[35, 36]. A consistent feature in atherosclerotic vascular disease is dysfunction in NO-dependent signaling processes, occurring either through a deficit in NO synthesis, NO bioavailability, or both.
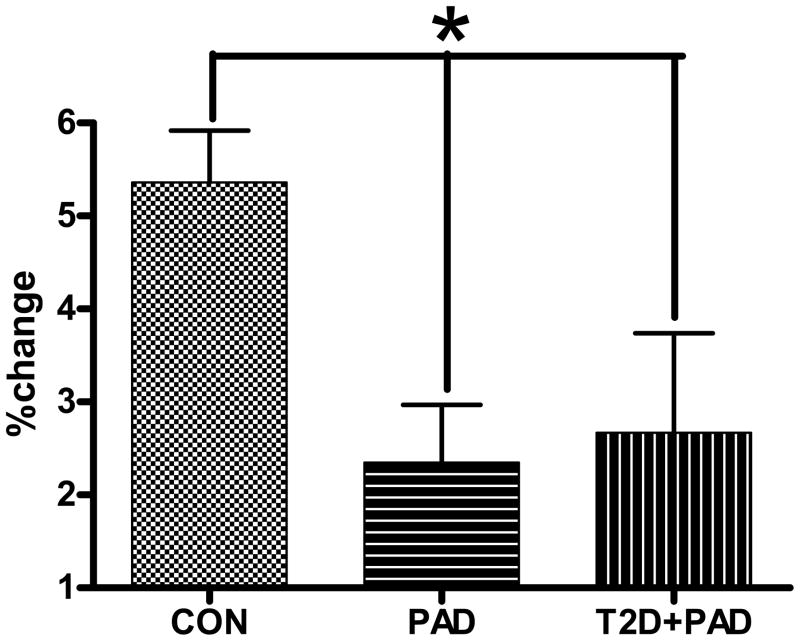
Vascular endothelial production of NO, assessed non-invasively using ultrasound techniques of brachial artery flow-mediated vasodilation (BAFMD), is impaired in PAD[37] (Figure 1), and is an independent predictor of long-term cardiovascular events which adds to the prognostic value of the ABI[21, 22]. Thus, compromised NO-bioactivity due to reduced NO stores or biological activity has been advanced as a significant contributor to the abnormal physiological responses and poor clinical outcomes in these diseased populations.
Figure 1.
Brachial artery flow mediated dilation peak percent changes in diameter from baseline. All values are statically adjusted for differences in age between groups. * = significantly different at the p<0.05 level. CON = subjects with 2 or more risk factors for CVD but no clinical disease, PAD = subjects with diagnosed PAD, T2D+PAD = subjects with diagnosed PAD in the presence of Type II Diabetes Mellitus. Adapted from Allen et al., Nitric Oxide Biology and Chemistry 20: 231–237, 2009.
It was believed, until recently, that the bioactivity of NO was limited both temporally and spatially to the proximity of the vascular endothelium where it was produced, as it is readily inactivated in the vasculature[38]. This concept has been challenged by studies of inhaled and intravenously applied NO[39–41], as well as an animal model with cardiac specific eNOS over-expression[42], or pharmaceutical induction of eNOS activity[43], which indicate established transport mechanisms in the blood, taking NO equivalents from areas of production to be used at critical areas of the circulation, where it can influence macro- and micro-vascular tone and possibly vasculopathy. It appears that in vivo NO bioavailability may be regulated by formation of NO-containing compounds in the blood[44, 45] (S-nitrosothiols[46], N-nitroso proteins and iron-nitrosyl complexes[47], as well as nitrite [48, 49] and potentially nitrated-lipids[50]). These NO-derived species may be transported throughout the vasculature in both the plasma[41] and red blood cells(RBC)[51]. Under normal conditions, this endocrine activity role is precisely controlled: during normoxia NO is conserved but under hypoxic conditions NO is liberated[52] and can initiate vasodilation and vascular remodeling responses, which are particularly pertinent for PAD in the presence of conduit vessel stenosis.
Increased Plasma Nitrite as a Source of Nitric Oxide Signaling in Hypoxia
The discovery of endocrine NO mechanisms highlight the importance of non-enzymatic sources of NO that may be amenable to therapeutic manipulation. For example, the metabolic products of NO metabolism such as nitrite and nitrate, once thought of as NO metabolism end-products, may serve as an alternative source of NO that can be rapidly reduced to NO under certain physiological conditions[53], such as hypoxia and ischemia (Figure 2). Nitrite has recently been advanced as a circulating NO storage depot and delivery source, reacting with oxyhemoglobin to form nitrate and methemoglobin (metHb) or with deoxyhemoglobin to form NO[54, 55], nitrosylhemoglobin [39, 56], and other NO adducts[47, 57, 58]. Since nitrite is found ubiquitously in the systemic circulation, the dual fates of nitrite metabolism position it as a unique physiological source of NO that may target pathophysiological conditions, such as tissue ischemia.
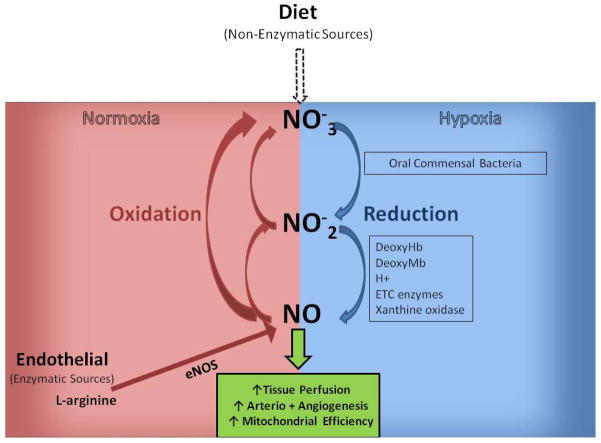
Figure 2.
Nitrate-Nitrite-Nitric Oxide Formation/Recycle Pathways.
In the presence of oxygen endothelial nitric oxide synthase (eNOS) catalyzes the oxidation L-arginine to NO. NO can exhibit biological effects and has been shown to increase tissue perfusion along with angio- and arteriogenesis in PAD models. NO may also be rapidly oxidized to nitrite (NO2−) and nitrate (NO3−). A secondary source of vascular NO is via diet. Consumption of food stuffs high in inorganic nitrate (green leafy vegetables, beetroot) have been shown to increase plasma nitrate which can be secreted in saliva and reduced to nitrite by commensal bacteria in the mouth. Nitrite can then be further reduced to NO (and other biologically active nitrogen oxides) via several mechanisms which are expedited under hypoxic conditions. Hence, although some of the circulating nitrate and nitrite are excreted in the kidneys they are also able to be recycled back to NO.
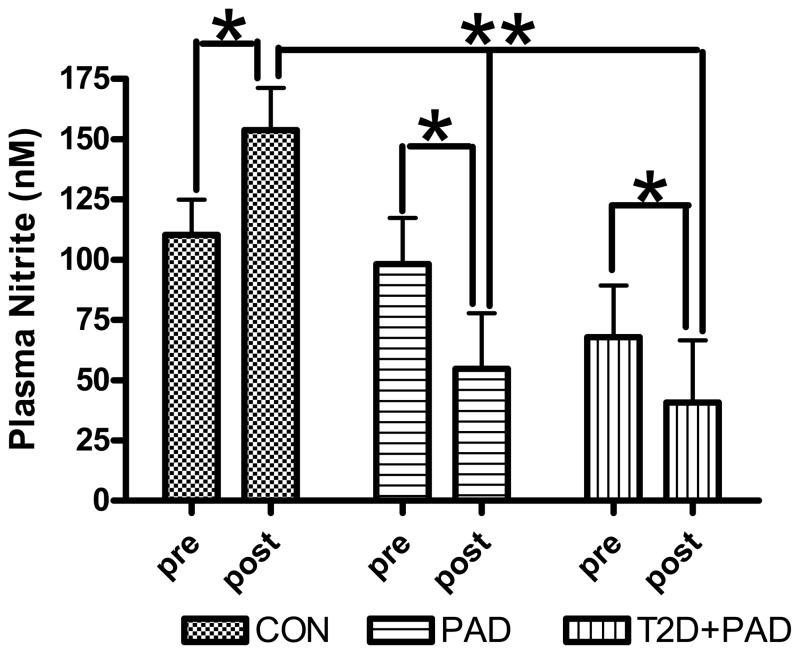
In 2000 Gladwin et al., reported artery to venous nitrite gradients in the forearm of healthy subjects during L-NMMA infusion coupled with handgrip exercise suggesting intravascular nitrite is consumed during physiological stress[59]. Similarly, our recent data[37, 60] describe a net loss of nitrite stores following maximal exercise stress in both diabetic PAD and PAD-only patients (who also displayed endothelial dysfunction) compared with healthier individuals (Figure 3). These results suggest a significant decrease in the NO pool during periods of exercise-induced ischemia in affected patients, most likely, in an attempt to normalize blood flow and oxygen delivery. Over time and given the intermittent periods of ischemia in these patients, it is conceivable that NO stores may become depleted[42], contributing to reduced function and the atherosclerotic disease process. Consequently, therapies that would effectively deliver NO to the peripheral tissues that are under-perfused due to occlusive vascular disease may represent a critical therapeutic goal.
Figure 3.
Changes in circulating plasma NO2− prior to and following a maximal CPX. Samples were collected prior to (pre), and 10 min following (post) GXT. Data is represented as actual NO2− yield (nM) for each group. Values are statistically adjusted for differences between groups in age and VO2peak. * = significantly different within groups at the p<0.05 level. ** = significantly different between groups at the p<0.01 level. CON = subjects with 2 or more risk factors for CVD but no clinical disease, PAD = subjects with diagnosed peripheral arterial disease, T2D+PAD = subjects with diagnosed PAD in the presence of Type II Diabetes Mellitus. Adapted from Allen et al., Nitric Oxide Biology and Chemistry 20: 231–237, 2009.
Although many structurally and chemically diverse NO-donor compounds have been synthesized and used widely in experimental studies, no NO-donor has been approved for use in the clinic. This limitation stems largely from the inability to achieve site specific delivery to discrete locations in vivo, with consequences being that non-specific NO-donors elicit systemic vascular effects resulting in hypotension. Moreover, NO substrate therapy using L-arginine have also been unsuccessful in part due to transport issues, asymmetric dimethlyarginine competition, arginase activity, and dysfunctional eNOS activity (i.e. uncoupled)[61]. In fact, numerous experimental therapeutic approaches have involved augmenting NO bioavailability through an eNOS dependent pathway, which may not be ideal under pathophysiological conditions due to factors such as oxidative stress, insufficient substrate availability, and enzyme dysfunction. Therefore, an alternative source for NO generation is desirable and likely to be most effective during various vasculopathies.
It has long been recognized that nitrite can serve as a vasodilator[62]. However the concentrations of nitrite and the pH of these initial experiments were beyond physiological levels. Subsequently, numerous studies in mice, rats, sheep, dogs and man now confirm nitrite to be a potent vasodilator through its one electron reduction back to NO [31, 54, 63–66] under various conditions that also potently inhibits I/R injury[35, 36]. Exact mechanisms for this reduction of nitrite to NO in hypoxia can involve several pathways including, chemical acidification[52] xanthine oxidase[36], deoxyhemoglobin[54], deoxymyoglobin[67] and mitochondrial enzymes[68]; however, it is not clear which mechanism is most important for various vascular pathologies. Likewise, several studies provide evidence of increases in RBC-nitrite along with S-nitrosthiols in both the plasma (SNO-albumin and SNO-glutathione) and the red cells (SNO-Hb) following supplementation of plasma nitrite.
Increasing Plasma Nitrite as a Treatment for PAD
(a) Endogenous increases
It is estimated that ≈70% of resting plasma nitrite is derived from eNOS activity in humans and other mammalian species[25]. Given that supervised exercise training has been shown to increase both endothelial function and is the most efficacious non-invasive option for patients with claudication[15, 16]. It is logical to assume that exercise training may be a viable method to increase vascular NO bioavailability and plasma nitrite stores during exercise induced ischemia.
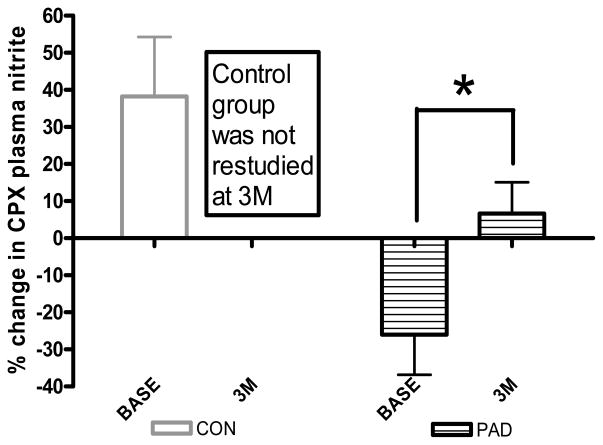
We recently demonstrated increases in time to claudication onset pain (66%), peak walking time (52%) and endothelial function (2.42±0.8% to 4.33±0.7%) in PAD subjects following 3 months of supervised exercise training (30–40min treadmill walking at the claudication pain threshold 3* per week)[60]. Figure 4 shows the corresponding changes in plasma nitrite flux (%change concentration) during cardiopulmonary exercise testing before (−26±11%) and following the supervised exercise training (+6.6±8%). The change from a deficit to an increase suggests the production of NO may outstrip the exercise induced hypoxic consumption following training and the data is more in accordance with the control group response (+49%). There were no changes in resting ankle-brachial index values for any of the treatment groups, suggesting the degree of limb stenosis/occlusion at rest remained similar. Interestingly the absolute (nM) change in plasma nitrite flux pre to post 3 months training was significantly related to the change in time to claudication onset pain (r2=0.61, p≤0.05)[60] suggesting the balance point for nitrite bioavailability is linked to the point at which subjects experience symptoms of tissue ischemia and approach the limit of their physical function.
Figure 4.
Changes in Plasma Nitrite Flux (%change in plasma nitrite concentration) pre to post Cardiopulmonary Exercise Testing (CPX) both baseline (Base) and 3 month (3M-post exercise training) visits. Samples were collected prior to (Rest), and 10 min following CPX. * = significantly different within groups at the p<0.05 level. CON = subjects with 2 or more risk factors for CVD but no clinical disease, PAD = subjects with diagnosed peripheral arterial disease. Adapted from Allen et al., Free Radical Biology and Medicine 49: 1138–1144, 2010.
The initiation of an exercise training regimen causes changes in many physiological processes which may influence the production and consumption of nitric oxide species such that it is difficult to precisely determine the effects of plasma nitrite levels on PAD outcome measures. Accordingly, to assess the effects of increased plasma nitrite in the absence of exercise training adaptations via exogenous supplementation is a useful approach.
(bi) Exogenous Increases (Humans)
Substantial elevations in plasma nitrite can also occur through increasing dietary nitrate. Dietary nitrate is rapidly absorbed in the stomach and duodenum and has a plasma half-life of approximately 5–6 hours[69]. Although much of it is excreted in the urine, up to 25% of plasma nitrate is taken up by salivary glands[70]. Commensal bacteria in the mouth then reduces some of the nitrate to plasma nitrite[71]. The acidic environment of the stomach causes some nitrite to be converted to NO then absorbed as bioactive nitrogen oxides such as nitrous acid. The remaining nitrite is then absorbed in the intestines and enters the plasma. As shown in our preliminary results, and by others[31, 72–76] ingesting high nitrate dietary sources leads to substantial increases in plasma nitrite in as few as 1 to 2hrs after consumption and moderate elevations may last for up to 24 hrs. Recently studies have shown the ingestion of organic nitrate (beetroot juice) as a single dose and over several days for 4 weeks, in healthy subjects caused increases in plasma nitrite from ≈125 nM[72, 73] to ≈600 nM[74] and were accompanied by a decrease in SBP of ≈6 mmHg[72–74] and an increase in time to exhaustion during cycle exercise[72, 73]. These changes involved an increase in cGMP concentration, suggesting the mechanism is NO mediated[74].
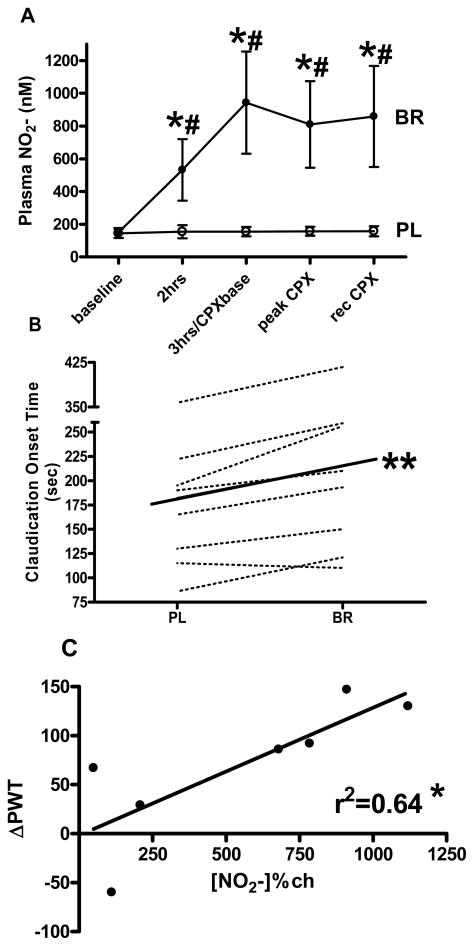
We recently showed a six-fold increase in plasma nitrite 3 hours following consumption of 500ml of nitrate rich beetroot juice (compared to an orange juice placebo) in 8 subjects (4 male, 4 female) age 67±13y with intermittent claudication (ABI in the incident leg of 0.64±0.2) (Figure 5a) [77]. This was accompanied by an 18% (32sec) increase in average exercise time before the subject reported the onset of claudication pain (COT) (Figure 5b), and a 17% (65sec) increase in time to exhaustion (not shown). The mechanism for this improvement is not totally clear but given there were also reductions in blood pressures and fractional oxygen extraction in the working tissue (measured by near-infra red spectroscopy in the gastrocnemius muscle in the incident limb) during CPX testing, suggests increased tissue perfusion is the most likely explanation. Interestingly there were no changes in ABI measures or arterial endothelial function following supplementation (not shown). Figure 5c shows there is a relationship between Δplasma[NO2−] from pre to 3hours post beverage during the beetroot visit and the ΔPWT between placebo and beetroot visits, suggesting plasma [NO2−] may directly influence exercise tolerance in subjects with PAD. Taken together these findings support the hypothesis that nitrite related NO-signaling increases peripheral blood flow in areas of tissue hypoxia and increases exercise tolerance in subjects with PAD.
Figure 5.
Changes in (A) plasma nitrite (NO2−) concentration over time and (B) Claudication Onset Time (COT) prior to (baseline) and following consumption of a high nitrate (BR) or placebo (PL) beverage. (C) Shows the relationship between change in plasma nitrite [NO2−] from baseline to 3 hours following BR beverage and change in peak walk time (ΔPWT). 3hrs/CPXbase indicates the time point 3 hours following beverage consumption which was also just prior to commencement of the cardiopulmonary exercise test (CPX). Peak indicates immediately at time to exhaustion. Rec indicates 10 minutes after time to exhaustion. Values are group mean ± standard error. * = significantly different from placebo group p≤0.05, ** = significantly different from PL group p≤0.01, # = significantly different from baseline p≤0.05. Figures 4a-c adapted from Kenjale et al., Journal of Applied Physiology 110: 1582–1591, 2011
(bii) Exogenous Increases (Animals)
In order to evaluate the effect of sodium nitrite on tissue angiogenesis in the setting of chronic ischemia, we have previously documented the effects of chronic sodium nitrite therapy in a murine model with permanent femoral artery ligation of the hind limb[33]. Artery ligation significantly reduces limb blood flow, lowers tissue perfusion, and causes profound tissue ischemia distal to the occlusion. Ascending doses of sodium nitrite were administered via intraperitoneal injection twice daily for 7 days starting within 2 hours post-femoral artery ligation. We observed a dose dependent relationship between nitrite dose and improved tissue perfusion, with 165 μg/kg nitrite appearing to be the optimal dose. Observable improvements in tissue reperfusion occurred as early as 3 days post-occlusion. However, over the same time course, sodium nitrate therapy was unable to significantly augment these parameters. Importantly, when the NO scavenger carboxy-PTIO (1mg/kg) was co-administered with 165 μg/kg nitrite the increases in perfusion were completely abrogated suggesting the mechanism of effect is NO mediated. We also observed that tissue nitrite levels were significantly elevated at early time points compared with the non-ischaemic limb, and that tissue nitrosothiol and nitrosohaem were elevated later as levels of nitrite decreased. These observations suggest that nitrite anion might possibly undergo differential tissue metabolism over time in ischaemic vs. non-ischaemic tissue. Lastly, nitrite therapy diminished eNOS protein expression over time, reinforcing the notion of a nitrite/NO endocrine system and possibly a negative feedback system to the endothelium.
A follow up study was performed to identify potential molecular mechanisms of nitrite mediated ischemic vascular remodeling and tissue reperfusion. Whole genome array analysis was performed on ischemic and non-ischemic muscle from PBS or nitrite treated mice at early and late time points before and after restoration of ischemic tissue blood flow. Numerous unique gene targets were identified that were either up or down regulated across a wide range of biological and physiological responses [59]. Network analysis of early gene expression changes at day 3 revealed significant changes in expression of genes regulating immunological processes and organismal survival such as various chemokines, matrix metalloproteinases, and transcriptional regulators (e.g. SOCS3) that indicated down regulation of innate immune response pathways. Conversely, by day 7 network analysis revealed prominent changes in gene expression regulating protein post-translational modifications and tissue morphology including molecules such as intergrins, matrix metalloproteases, and metabolic signal mediators (e.g. adiponectin). These findings provide some initial insight into potential cellular and molecular mechanisms of nitrite beneficial effects, yet additional direct studies are needed.
Nitrite based therapeutic approaches and future directions
Therapeutic trails employing nitrite approaches are underway for several cardiovascular disease states[78]. While it is premature to speculate on outcomes or effects, it is clear that nitrite based therapy appears promising for peripheral artery disease and other conditions involving ischemic vascular disease. Finally, additional basic science studies are also underway to determine important molecular mediators conveying beneficial effects of nitrite therapy during specific disease states.
Highlights.
Nitrite anion is important in the functional and pathophysiological responses in PAD
We discuss NO bioavailability in PAD and plasma nitrite as a source of NO in hypoxia
We discuss endogenous and exogenous sources of plasma nitrite
We review the effects on vascular structure, function and physical performance
We highlight basic findings translated to animal and human models
Acknowledgments
JDA is the principal investigator for an NIH funded trial investigating the use of inorganic nitrates in PAD and a PI of a clinical site for for a phase II clinical trial (Theravasc, Inc.).
This work was supported in part by the NIH grant 5R01 HL-755752 and by grants from Wake Forest University Translational Science Center and Duke University Claude D. Pepper Older American Independence Center (AG0287 from the NIA) grants to J.D.A and HL80482 and DK43785 to C.G.K.
Footnotes
Conflicts of Interest: C.G.K. and T.G. have commercial and intellectual property interests in TheraVasc Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, et al. Heart Disease and Stroke Statistics—2012 Update. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendermacher BL, et al. Symptomatic peripheral arterial disease: the value of a validated questionnaire and a clinical decision rule. British Journal of General Practice. 2006;56(533):932–937. [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch AT, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt WR. Medical Treatment of Peripheral Arterial Disease and Claudication. N Engl J Med. 2001;344(21):1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 5.Regensteiner JG, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13(1):15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 6.Olsen P, et al. Long-term results after arterial surgery for arteriosclerosis of the lower limbs in young adults. Eur J Vasc Surg. 1988;2:15–18. doi: 10.1016/s0950-821x(88)80101-4. [DOI] [PubMed] [Google Scholar]
- 7.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion. Vasc Med. 2004;9(4):293–301. doi: 10.1191/1358863x04vm572ra. [DOI] [PubMed] [Google Scholar]
- 8.Hiatt WR, et al. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81(2):602–9. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt WR, et al. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol. 1996;81(2):780–8. doi: 10.1152/jappl.1996.81.2.780. [DOI] [PubMed] [Google Scholar]
- 10.Mannarino E, et al. Effects of physical training on peripheral vascular disease: a controlled study. Angiology. 1989;40(1):5–10. doi: 10.1177/000331978904000102. [DOI] [PubMed] [Google Scholar]
- 11.Pernow B, Zetterquist S. Metabolic evaluation of the leg blood flow in claudicating patients with arterial obstructions at different levels. Scand J Clin Lab Invest. 1968;21(3):277–87. doi: 10.3109/00365516809076995. [DOI] [PubMed] [Google Scholar]
- 12.Zetterquist S. The effect of active training on the nutritive blood flow in exercising ischemic legs. Scand J Clin Lab Invest. 1970;25(1):101–11. doi: 10.3109/00365517009046196. [DOI] [PubMed] [Google Scholar]
- 13.Tsai AG, JPC, Intaglietta M. Oxygen Gradients in the Microcirculation. Physiological Reviews. 2003;83(3):933–963. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- 14.Regensteiner JG, et al. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44(1):1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 15.Patterson RB, et al. Value of a supervised exercise program for the therapy of arterial claudication. Journal of Vascular Surgery. 1997;25(2):312–319. doi: 10.1016/s0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 16.Regensteiner JG, Hiatt WR. Current medical therapies for patients with peripheral arterial disease: a critical review. The American Journal of Medicine. 2002;112(1):49–57. doi: 10.1016/s0002-9343(01)01034-8. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, et al. Cigarette smoking is associated with dose-related and potentially reversable impairment of endothelium dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, et al. Aging is assocoated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 19.Taddei S, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RA. Dysfunction of vascular endothelium in diabetes mellitus. Circulation. 1993;87(Suppl V):V67–V76. [Google Scholar]
- 21.Brevetti G, et al. Endothelial Dysfunction and cardiovascular Risk Protection in Periperal Arterial Disease: Additive Value of Flow-Mediated Dilation to Ankle-Brachial Pressure Index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 22.Gokce N, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 23.Thorne S, et al. Early endothelial dysfunction in adults at risk from atherosclerosis: different responses to L-arginine. Journal of the American College of Cardiology. 1998;32(1):110–116. doi: 10.1016/s0735-1097(98)00211-3. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes PM, et al. The L-Arginine: Nitric Oxide Pathway Is the Major Source of Plasma Nitrite in Fasted Humans. Biochemical and Biophysical Research Communications. 1995;209(2):590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 25.Kleinbongard P, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology and Medicine. 2003;35(7):790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 26.Lauer T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. PNAS. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuehr DJ. Enzymes of the L-Arginine to Nitric Oxide Pathway. The Journal of Nutrition. 2004;134(10):2748S–2751S. doi: 10.1093/jn/134.10.2748S. [DOI] [PubMed] [Google Scholar]
- 29.Griffith T. The nature of endothelium-derived relaxant factor. Nature. 1984;308:645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- 30.Guzik T, Korbut R, Adamek-Guzik T. Nitric Oxide and Superoxide in Inflammation and Immune Regulation. Journal of Physiology and Pharmacology. 2003;54(4):469–487. [PubMed] [Google Scholar]
- 31.Webb AJ, et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arellano Mendoza MG, et al. Nitric Oxide-Dependent Neovascularization Role in the Lower Extremity Disease. Current Pharmaceutical Design. 2007;13(35):3591–3596. doi: 10.2174/138161207782794103. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proceedings of the National Academy of Sciences. 2008;105(21):7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown G, Cooper C. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. Febs Letters. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 35.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101(37):13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen J, et al. Plasma Nitrite Response and Arterial Reactivity Differentiate Cardiovascular Health Status and Performance. Nitric Oxide Biology and Chemistry. 2009;20:231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao JC, et al. Intravascular flow decreases erythrocyte consumption of nitric oxide. PNAS. 1999;96(15):8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamler JS, et al. Blood Flow Regulation by S-Nitrosohemoglobin in the Physiological Oxygen Gradient. Science. 1997;276(5321):2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 40.Cannon RO, III, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108(2):279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rassaf T, et al. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest. 2002;109(9):1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elrod JW, et al. Nitric oxide promotes distant organ protection: Evidence for an endocrine role of nitric oxide. Proceedings of the National Academy of Sciences. 2008;105(32):11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh PK, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovascular Research. 2010;85(4):661–670. doi: 10.1093/cvr/cvq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. New England Journal of Medicine. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 45.The CAFE Investigators, et al. Differential Impact of Blood Pressure-Lowering Drugs on Central Aortic Pressure and Clinical Outcomes. Principal Results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 46.Stamler J, et al. Nitric Oxide Circulates in Mammalian Plasma Primarily as an S-Nitroso Adduct of Serum Albumin. PNAS. 1992;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rassaf T, et al. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radical Biology and Medicine. 2002;33(11):1590–1596. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 48.Dejam A, et al. Emerging role of nitrite in human biology. Blood Cells, Molecules, and Diseases, Proceedings of The Red Cell Club. 2003, 2004 Oct;32(3):423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Lundberg JO, Weitzberg E. NO Generation From Nitrite and Its Role in Vascular Control. Arterioscler Thromb Vasc Biol. 2005;25(5):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 50.Baker PRS, et al. Red cell membrane and plasma linoleic acid nitration products: Synthesis, clinical identification, and quantitation. PNAS. 2004;101(32):11577–11582. doi: 10.1073/pnas.0402587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nature Medicine. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 52.Zweier J, et al. Enzyme-independent formation of nitric oxide in biological tissues. Nature Medicine. 1995;8:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 53.Modin A, et al. Nitrite-derived nitric oxide: a possible mediator of acidic-metabolic vasodilation. Acta Physiologica Scandinavica. 2001;171(1):9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 54.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 55.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia L, et al. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 57.Baker PRS, et al. Fatty Acid Transduction of Nitric Oxide Signaling. Journal of Biological Chemistry. 2005;280(51):42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, et al. Biological activity of nitric oxide in the plasmatic compartment. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gladwin MT, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences. 2000;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen JD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease following 3 months of exercise training. Free Radical Biology and Medicine. 2010;49:1138–1144. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson AM, et al. L-Arginine Supplementation in Peripheral Arterial Disease: No Benefit and Possible Harm. Circulation. 2007;116(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 62.Furchgott R, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953;108(2):129–143. [PubMed] [Google Scholar]
- 63.Dejam A, et al. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 64.Hunter CJ, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. 2004;10(10):1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 65.Larsen FJ, et al. Effects of dietary nitrate on blood pressure in healthy volunteers. New England Journal of Medicine. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 66.Maher AR, et al. Hypoxic Modulation of Exogenous Nitrite-Induced Vasodilation in Humans. Circulation. 2008;117(5):670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 67.Shiva S, et al. Deoxymyoglobin Is a Nitrite Reductase That Generates Nitric Oxide and Regulates Mitochondrial Respiration. Circ Res. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 68.Nohl H, et al. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim Pol. 2000;47(4):913–921. [PubMed] [Google Scholar]
- 69.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 70.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radical Biology and Medicine. 2004;37(3):395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 71.Lundberg JO, et al. Opinion - Nitrate, bacteria and human health. Nature Reviews Microbiology. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 72.Bailey SJ, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109(1):135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 73.Bailey SJ, et al. Dietary nitrate supplementation reduces the O2 cost of low- intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009:00722.2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 74.Kapil V, et al. Inorganic Nitrate Supplementation Lowers Blood Pressure in Humans: Role for Nitrite-Derived NO. Hypertension. 2010;56(2):274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 75.Lansley KE, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. Journal of Applied Physiology. 2011 doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 76.Vanhatalo A, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010:ajpregu.00206.2010. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 77.Kenjale AA, et al. Dietary Nitrate Supplementation Enhances Exercise Performance in Peripheral Arterial Disease. Journal of Applied Physiology. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kevil CG, et al. Inorganic nitrite therapy: historical perspective and future directions. Free Radical Biology and Medicine. 2011;51(3):576–593. doi: 10.1016/j.freeradbiomed.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]