INTRODUCTION AND BACKGROUND
Percutaneous image guided interventions have become a widely accepted treatment option for selected patients as they provide an effective, expeditious, cost-effective, and minimally invasive form of management for localized cancer sites (1).While thermal ablation has become the standard of care for some patients, there are several key limiting factors that make improvements in efficacy desirable (2,3). Hori et al. reported that the recurrence rate of liver tumors after RF ablation correlated with tumor size, with a recurrence rate of 14.8 % for lesions <2.5cm and >50% for lesions >2.5cm in a 36 month follow-up period (4). Several problematic areas of RF ablation are often targeted and include increasing the ablative treatment in the periphery of the lesions and enhancing ablations by reducing the “heat sink” effect of tumor blood flow. Mechanical methods, including intravascular embolization (5) or occlusion (6), have been utilized to decrease tumor blood flow prior to ablation. While these studies have demonstrated more effective ablation, they occlude blood flow to both the tumor and the target organ. Further, these methods are more invasive and associated with increased complications.
Another method of decreasing tumor blood flow prior to ablation involves systemic administration of vasoactive pharmacologic agents. Vasopressin (7), epinephrine (8), and arsenic trioxide (9) have all been explored as potential agents. The results suggested that increasing organ blood flow decreased the effectiveness of RF ablation, while decreased flow was associated with enhanced ablation. However, the efforts to modulate blood flow had a systemic effect and were non-specific to the target organ or affected cardiovascular status. Therefore, a more tumor-specific approach to perfusion modulation is advantageous.
Prior research shows that tumor vessels are inherently different when compared with normal organ vessels. They are immature, lack normal smooth muscle and pericyte structure (10,11), or keep maximal vasodilation secondary to low pH produced by hypermetabolism (12). Therefore, tumor vessels do not exhibit the same response to vasoactive agents as do normal blood vessels. However, the flow within tumor blood vessels is indirectly affected via the response of normal blood vessels to vasoactive drugs. Vasoconstrictive agents will constrict normal vessels which will paradoxically increase blood flow into the tumor vessels. Conversely, vasodilating agents will dilate normal vessels and therefore lower resistance within the normal hepatic parenchymal vasculature while the tumor vasculature remains of normal caliber and resistance (13). This will avert blood away from the tumor vessel bed via a “steal” effect. This mechanism of action will be refered to as vasomodulation. Often times this term is used when referring to neuronally mediated vasodilation or vasoconstriction. However, the term is more general and will be used to describe the perfusion changes of the tumor vasculature as it is not the tumor vessels which are changing in caliber. Rather, the blood vessels within the adjacent normal parenchyma are being changed which then, in turn, effects the perfusion of the tumor via a “steal” effect.
Approaching thermal ablation treatment of liver tumors with this vasomodulation framework allows the selection of vasoactive drugs based upon the anatomic and functional differences between normal and neoplastic tissue. This allows development of a safe and controlled method to decrease tumor blood flow prior to thermal ablation and thus increase the ablative treatment in the periphery of the lesion and reduce the “heat sink” effect of tumor blood flow.
In the current study we sought to further investigate and exploit the inherent differences in the vasculature of tumors versus normal parenchyma and the efficacy of RF ablation using Glucagon (GN), Adenosine (AD), and a combination of AD + GN as compared with normal saline (NS) controls using an N1-S1 tumor model implanted in Sprague-Dawley rat livers. We sought specifically to evaluate pharmacologic agents and doses that yielded the greatest amount of decreased tumor perfusion and resulted in a greater percentage of tumor ablation. Contrast-enhanced ultrasound techniques were used to quantify changes in both hepatic and tumor perfusion. Correlation of percentage of ablation and tumor perfusion was performed.
MATERIAL AND METHODS
All procedures were in compliance with the National Institute’s of Health’s Guide for the Care and Use of Laboratory Animals. For all surgical and imaging procedures, the animals were anesthetized with isoflurane 2-5% given by mask to effect. The animals were monitored throughout the procedures and were allowed to recover after each procedure. Heart rate and blood pressure was monitored and recorded throughout each procedure via a non-invasive tail-cuff plethysmography method (CODA 6, Kent Scientific, Torrington, CT) according to manufacturer’s recommendations. Briefly, animals were anesthetized with 1.5% isoflurane with tails exposed and placed on an infrared heating pad to ensure tail temperatures of 34 °C for 15 minutes prior to measurements. An occlusion cuff was placed proximally on the tail, and a volume pressure recording sensor cuff was placed just distally to the occlusion cuff. The blood pressure was measured every 30 seconds. Analgesia after surgery was administered with buprnorphine (0.01-0.05 mg/kg subcutaneously) every 8-12 hours as needed for pain. Euthanasia was performed by overbreathing carbon dioxide.
N1-S1 cells (ATCC, Manassas, Virginia) were maintained in suspension culture flasks at 37°C and 5% CO2 until needed for inoculation. To maintain the culture, cells were allowed to proliferate in Iscove’s modified Dulbecco’s Medium (Catalog No. 30-2005, ATCC) with 5% fetal bovine serum and passed biweekly. To establish liver tumors, 5 × 106 viable cells suspended in 0.2 ml incomplete medium were inoculated under the capsule of the medial aspect of the left lobe of the liver of Sprague-Dawley rats (6 weeks old, Charles River, average weight, 200-250 g) after a mini-laparotomy. Tumors were allowed to grow for 7-10 days before ultrasound (US) evaluation. A total of 17 tumors were inoculated and subsequently evaluated. Tumor perfusion was evaluated when reaching a size of 1-1.5 cm as measured by US.
Preparation of Ultrasound Contrast Agents
The lipid shell of microbubbles was composed of 1, 2-Dipalmitoyl-sn-Glycero-3-Phosphocholine (DPPC), 1, 2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine (DPPE), 1, 2-Dipalmitoyl-sn-Glycero-3-Phosphate (DPPA) (Avanti Polar Lipids, Inc.; Alabaster, AL) with mass ratio of 5:2:1. The lipid mixture was dissolved in chloroform and dried for two hours under a hood. Glycerol (Sigma-Aldrich Corp.; St. Louis, MO) and PBS (PH 7.0, Sigma-Aldrich Corp.; St. Louis, MO) were then added to the dried lipids and incubated at 37°C for 30 min. The solution was aliquoted into gas-tight vials into which perfluoropropane gas (C3F8) was added. Microbubbles were formed by using a VialMix mixing machine (DuPont Pharma Co., Billerica, MA) to mechanically agitate the solution for 45 s. The morphology of the resulting microbubbles was determined using an optical microscope (Carl Zeiss Achroplan 100, NA 1.0; Thornwood, NY) and the diameter was measured with 90plus Particle Size Analyzer (Brookhaven Instruments Corporation, NY). The diameter of the bubbles is approximately 1 micron.
Contrast-Enhanced Ultrasound
After the animal was placed in a supine position and gas anesthesia was established, the upper abdominal region was shaved. Intravenous access was established in a tail vein for administration of medication and US contrast. Preliminary US images were obtained (Toshiba Aplio® SSA-770A) to verify tumor location. The US probe was secured in place with a clamp. Intermittent-bolus technique was used to assess tumor perfusion changes as described by Krix, et al (14). Using a 12 MHz linear-array probe (1204BT), a bolus injection of 0.3 mL of 20:1 diluted microbubble contrast solution was administered via the tail vein IV. Thirty seconds after contrast administration, 30-second-long raw flash-replenish harmonic images were acquired after 100 frames of high energy flash impulses with the following imaging settings: CHI frequency, H8.0 (MHz); MI, 0.08; imaging frame rate, 10 fps; dynamic range, 80 dB; 2D gain, 65 dB. After 15-20 minutes, a vasodilating medication(s) or equivalent volume of normal saline was given and an additional 5 minutes was allowed to elapse. The type of medication given was chosen at random. Medications evaluated were: adenosine (AD, 1.5 mg/kg), glucagon (GN, 500 ug/kg), adenosine + glucagon (AD+GN, 1.5 mg/kg and 500 ug/kg respectively). Optimal dosages were determined based on review of literature (15,16) and preliminary studies. Using the identical imaging plane, the flash-replenish ultrasound perfusion imaging was repeated with a second administration of bolus injection of 0.3 mL of 20:1 diluted microbubble contrast solution via the tail vein IV.
Perfusion Analysis
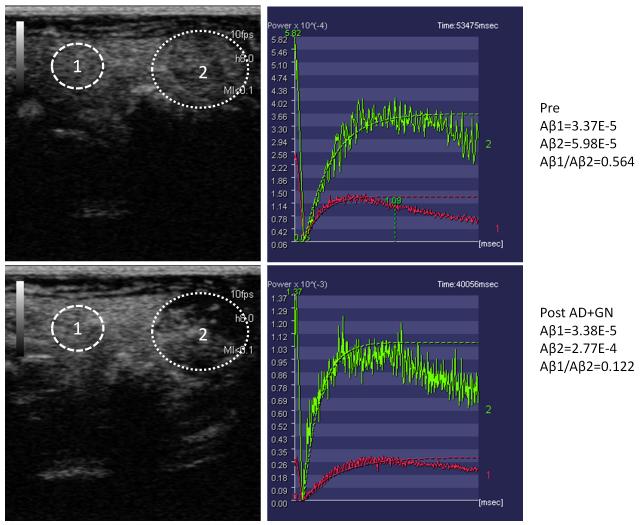
The raw perfusion images were loaded onto CHI-Q software available on the Toshiba Aplio® system to measure the acoustic intensity (AI) in regions of interest at identical depth both within the tumor and in adjacent normal liver parenchyma. All regions of interest were of identical size in both pre and post vasomodulation. The time-intensity-curve was fit with a mono-exponential function: y=A(1-exp(-β*t)), where A is the plateau intensity or microvascular blood volume and β is the rate constant reflecting microvascular red blood cell (RBC) velocity. Blood flow was determined by the product of A and β (14). A ratio of tumor perfusion to normal hepatic parenchymal perfusion was calculated. These normalized relative perfusion ratios were then compared both prior to and following administration of either NS or a vasodilating medication as listed above. An example of the above described technique is illustrated in Figure 1.
Figure 1.
Representative method of assessing relative tumor perfusion prior to (above) and following Adenosine + Glucagon (AD+GN) (below). Circle 1 within tumor and cicle 2 within normal liver. Graphs show decrease in ratio of A β in tumor versus A β in liver reflecting relative tumor hypoperfusion following vasomodulation with AD+GN.
Vasomodulation and Radiofrequency Ablation
Approximately 24-48 hours after analyzing the tumors with CE-US, the animals were brought back for ablation. After anesthesia was established, the upper abdomen was re-shaved and cleaned with iodine solution. Sterile towels were applied and intravenous access established. A 2-cm midline laparotomy was performed and the liver exposed. After securing access of the tumor, the animal was given AD (1.5 mg/kg), GN (500 ug/kg), AD+GN (1.5 mg/kg and 500 ug/kg respectively), or an equivalent volume of normal saline as detailed above. Each animal was given the same medication that they had received while being evaluated under CE-US. After a 5 minute waiting period, a 22-gauge RF probe was advanced into the tumor. All tumors were ablated for 2 minutes at 80°C using a 1 cm exposed tip electrode connected with a 500 kHz RF generator (Radionics, Cool-tip RF system, Radionics Inc., Burlington, MA) with a grounding pad placed underneath the animal. The power was approximately 1-2 W.
Following ablation, the incision was temporarily closed and the animals were kept under anesthesia. 120 minutes after treatment, the animals were euthanized. The ablated tumors were dissected out and measured. The tissue was cut perpendicular to the needle tract. To assess for mitochondrial enzyme activity, staining was performed by incubating representative tissue slices for 30 minutes in 2% TTC (Fisher Scientific). As has been previously described in the literature, the absence of mitochondrial enzyme activity accurately reveals irreversible cell injury induced by percutaneous tumor ablation (7, 17). Using this method, viable tissue with intact mitochondrial enzyme activity will stain red while ablated and nonviable tissue will not turn red. The area of ablated tumor was calculated on the measurements of the diameter of non-stained tissue and based on the consensus interpretations of two of the investigators who were blinded to the presence or absence of pharmacologic intervention.
Statistical Analysis
Tumor perfusion changes and the diameter of RF-induced coagulation were compared between the treatment groups. Unpaired Student t tests were used for comparisons between the treatment groups and the no treatment group (Microsoft Excel 2007, Redmond, WA). A p value of < 0.05 was considered significant. One-way analysis of variance, with paired comparisons of group means, was used for group comparisons (Minitab 15, State College, PA).
RESULTS
Pharmacologic Modulation of Tumor Perfusion
Changes in tumor perfusion ratios from baseline are provided in Table 1. As expected, the normalized tumor perfusion ratio did not significantly change with administration of NS (1.38% +/− 3.93%). Administration of GN resulted in a significant decrease in normalized tumor perfusion ratio by 66.22% +/− 24.57% (p < 0.01). Following AD injection, there again was a decrease in normalized tumor perfusion ratio by 71.45% +/− 22.72% (p < 0.01). While the combination of AD+GN resulted in the largest decrease in normalized tumor perfusion ratio by 74.98% +/− 16.58% (p < 0.01), the differences between the treatment groups were not significant. There was no significant change in heart rate or blood pressure in any of the rats in any of the groups.
Table 1.
Change in tumor perfusion ratio following vasomodulation
| Agent | Pre-vasomodulation Tumor perfusion Ratio |
Post-Vasomodulation Tumor Perfusion Ratio |
% Change (p < 0.01) |
|---|---|---|---|
| Normal Saline (N=4) | 1.238 +/− 1.387 | 1.285 +/− 1.466 | 1.38 +/− 3.93 |
| Glucagon (N=5) | 0.752 +/− 0.458 | 0.334 +/− 0.299 | -66.22 +/− 24.57 |
| Adenosine (N=4) | 3.124 +/− 2.366 | 0.790 +/− 1.025 | -71.45 +/− 22.72 |
| Adenosine+Glucagon (N=4) |
1.349 +/− 1.321 | 0.405 +/− 0.492 | -74.98 +/− 16.58 |
N= number of treated rats, %= percent
Correlation of Tumor Perfusion with Post-Ablation Necrosis
The cross-sectional areas of both the tumor and zone of ablation as well as the percentage of tumor ablation are summarized in Table 2 and illustrative examples of the 4 groups may be seen in Figure 2. There was no significant difference in pre-ablation tumor size between the 4 groups. The mean zone of ablation in the control group (NS) was calculated at 0.31 +/− 0.13 cm2 which corresponds to 27.45% +/− 14.70% ablation. There was an increase in both the size of the ablated area and the percentage of ablated tumor in all 3 treatment groups. The differences between the treatment groups and the control were significant with a p value < 0.01. The largest area of ablation is seen in the AD+GN treatment group with a mean zone of ablation calculated at 0.82 +/− 0.21 cm2 which corresponds to 64.97% +/− 15.26 % of tumor ablated. When compared to the NS control, this represents a 165% increase in mean tumor ablation area. While the combination group (AD+GN) resulted in the largest area of ablation, the differences between the treatment groups were not statistically significant.
Table 2.
Change in ablation area following vasomodulation.
| Agent | Tumor CS area (cm2) | Ablation CS area (cm2) | % tumor ablation (p< 0.01) |
|---|---|---|---|
| Normal Saline (N=4) | 1.16 +/− 0.16 | 0.31 +/− 0.13 | 27.45 +/− 14.70 |
| Glucagon (N=5) | 1.10 +/− 0.26 | 0.70 +/− 0.15 | 64.09 +/− 7.30 |
| Adenosine (N=4) | 1.11 +/− 0.16 | 0.62 +/− 0.03 | 56.57 +/− 9.04 |
| Adenosine+Glucagon (N=4) |
1.20 +/− 0.05 | 0.82 +/− 0.21 | 64.97 +/− 15.26 |
N= number of treated rats, cm2= centimeters squared, %= percent
Figure 2.
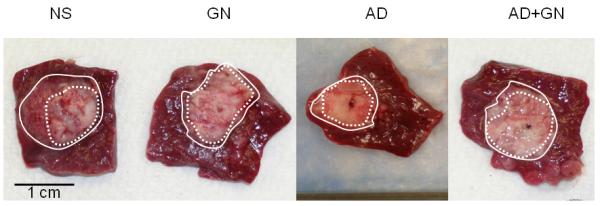
Post-ablation Tumors stained with 2% TTC. Dash line reflects border of ablated tumor. Solid line indicates border of viable tumor. NS = normal saline. AD = adenosine. GN = glucagon.
DISCUSSION
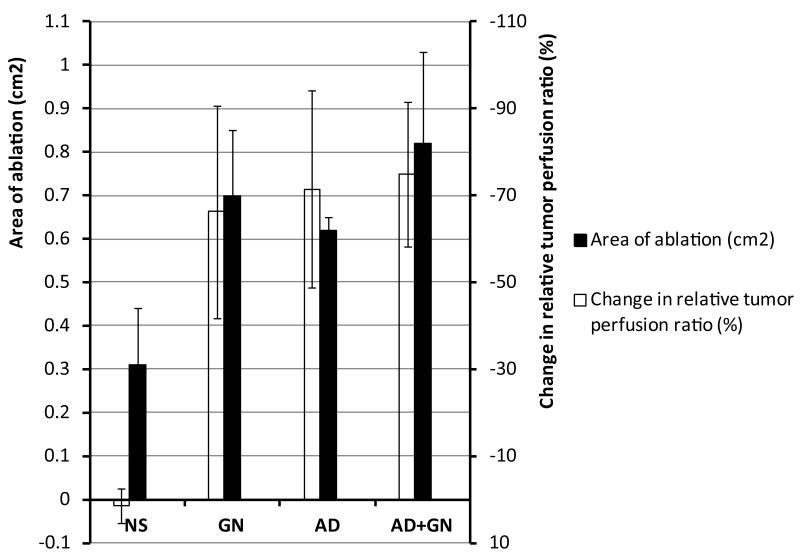
We have been able to demonstrate that, in an animal model, systemic administration of an organ-specific vasodilating agent is an effective method to cause a relative decrease in tumor perfusion. This relative decrease yields increased efficacy in radiofrequency ablation with larger zone of tumor necrosis and lower cell viability (Figure 3). When compared with alternative methods to decrease tumor blood flow such as endovascular embolization, this technique is noninvasive and reversible. Additionally, as these medications are routinely given during other imaging studies (e.g. stress cardiac nuclear scintigraphy examination and magnetic resonance imaging of the abdomen) they are used frequently and readily transferable to clinical practice.
Figure 3.
Graph simultaneously comparing vasomodulation agents and normal saline (NS) control compared with area of ablation (cm2) (left y-axis) and change in relative tumor perfusion ratio (%) (right y-axis). AD = adenosine. GN = glucagon.
Adenosine was chosen as a vasodilating agent as it has been shown to increase hepatic arterial flow (16) and has a beneficial safety profile (18). Further, given that hepatocellular carcinoma is largely supplied by the arterial vasculature (19), this agent was felt to have the greatest potential for effective tumor vasomodulation. We have shown that adenosine is able to cause a relative decrease in tumor perfusion by 71.45 +/− 22.72% (p < 0.01) when compared with surrounding liver parenchyma. This corresponds to an increase in tumor necrosis following RFA (0.62 +/− 0.03 cm2) when compared with NS control (0.31 +/− 0.13 cm2). This represents a 100% increase in ablation size (p <0.01). As per our hypothesis, hepatic parenchymal vasodilation with lowered arterial resistance will result in a secondary “steal” effect whereby blood flow is paradoxically decreased in the tumor secondary to the immature nature of tumor vasculature with an inherent inability to respond to normal vasodilating stimuli. Therefore, vasodilating medications cause relative tumor hypoperfusion (when compared with baseline measurements) that results in more efficacious ablation.
Our second agent to be evaluated was glucagon which was selected as prior studies demonstrated a 50% increase in portal venous velocity and a 120% increase in portal venous flow (20). Further, glucagon has a large safety profile and is routinely used during imaging examinations. Administration of glucagon caused a 66.22 +/− 24.57% (p < 0.01) decrease in tumor blood flow relative to surrounding normal liver parenchyma. The exact reasons for this decrease are less evident when compared with adenosine. It is possible that there is a component of arteriovenous shunting involved as has been reported to occur in hepatocellular carcinoma (21). If this were the case, dilation of the portal venous system would cause a larger component of AV shunting within the tumor and possibly result in decrease tumor perfusion. As would be expected, the relative tumor hypoperfusion caused by GN results in a larger area of necrosis (0.70 +/− 0.15 cm2) when compared with NS control (0.31 +/− 0.13 cm2). This represents a 126% increase in ablation size (p <0.01).
Lastly, we evaluated a combination of adenosine and glucagon with the hypothesis that maximally dilated arterial and portal venous systems may result in even greater degrees of tumor hypoperfusion. However, while the percentage decrease of normalized tumor perfusion was the largest of the treatment groups (74.98 +/− 16.58%), the differences between the treatment groups was not statistically significant. We postulate that we had reached a maximum in terms of normal hepatic perfusion while using the agents alone. This is most likely due to the reciprocal changes in blood flow between the hepatic artery and portal vein which occurs physiologically and it is likely that no further increases in hepatic perfusion could be tolerated by systemic administration. Therefore, the combination of two agents was unable to cause any further increase in normal hepatic parenchymal perfusion and the degree of “steal” effect was unable to be significantly increased. This explains why the relative changes in perfusion were similar and corresponding changes in tumor necrosis area were not significantly different (Tables 1 and 2).
It is counterintuitive to think that increasing perfusion of an organ will result in better ablation of a tumor within that organ. It could be argued that the amount of increased heat sink from the surrounding hyperperfused normal hepatic parenchyma would counteract any perceived enhanced ablation effects from relative tumor hypoperfusion secondary to steal effect. As we have shown, the effect of tumor hypoperfusion on ablation outcome outweighs the effect of increased heat sink from hyperperfused target organ. By this logic, it would be not unreasonable to argue that organ hypoperfusion would result in decreased ablation efficacy when compared with controls. This is not the case as shown by Goldberg, et al (7). While it may be conjectured that the tumor did not experience any significant perfusion changes within it and therefore there was enhanced ablation secondary primarily to a lowered heat sink effect, these questions remain unanswered by the current project and may be interesting topics of research for the future.
Potential limitations of this study include the nature of the tumor model itself. Given that we evaluated a tumor that is subcapsular in location, the tumor is not completely surrounded by normal hepatic parenchyma. Intuitively, one could argue that if a tumor model was used that was at a greater depth within the target organ, then the “steal” effects would be even greater. Additionally, these tumors were not followed over the course of days to weeks, but were euthanized 2 hours after the procedure. Therefore, progression of tumor growth or survival of the animals was unable to be evaluated. However, this method has been used throughout the literature (22, 23) as a reliable means of therapeutic assessment. Another potential limitation of this study was the size of the treatment groups. As previously discussed, the data was evaluated with one-way analysis of variance with paired comparisons of group means. While ANOVA showed that the differences between the treatment groups and the control was significant with p < 0.01, differences between the treatment groups themselves was not significant. If larger numbers of animals were used, it is possible that small differences between treatment groups would have been elucidated. Lastly, as this procedure was done under direct visualization via a midline laparotomy, it did not completely simulate clinical practice. However, given the size of the tumors and the inability to control breathing within the animal, direct visualization of the tumor was felt to be most accurate and reproducible. Further, the ablations were performed by a single operator (H.W.) using identical technique including depth within the tumor and angle of entry.
While this technique may easily be translated into clinical use, the principles that may be gleaned from this study are of potentially greater applicability. If tumor blood flow may be significantly altered via a safe and effective systemic method, this may be applied to different types of tumors in different organs. With selection of alternative, organ-specific vasodilating medications, this technique may potentially be applied to cryoablation of renal cell carcinoma to achieve greater efficiency of tumor ablation. Further investigation is warranted to determine appropriate avenues whereby systemic modulation of tumor blood flow may be utilized to enhance current therapeutic options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiol. 2004;233:729–739. doi: 10.1148/radiol.2333032052. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand J, Sofocleous C, Hanna N, et al. Development of a Research Agenda for the Management of Metastatic Colorectal Cancer: Proceedings from a Multidisciplinary Research Consensus Panel. J Vasc Interv Radiol. 2012;23:153–163. doi: 10.1016/j.jvir.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McWilliams JP, Yamamoto S, Raman SS, et al. Percutaneous Ablation of Hepatocellular Carcinoma: Current Status. J Vasc Interv Radiol. 2010;21:S204–S213. doi: 10.1016/j.jvir.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamakado K, Nakatsuka A, Kobayashi S, et al. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol. 2006;29:389–394. doi: 10.1007/s00270-004-0090-9. [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki T, Kimura T, Kurokawa F, et al. Percutaneous radiofrequency ablation with cooled electrodes combined with hepatic arterial balloon occlusion in hepatocellular carcinoma. J Gastroenterol. 2005;40:171–178. doi: 10.1007/s00535-004-1516-5. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiol. 1998;209:761–767. doi: 10.1148/radiology.209.3.9844671. [DOI] [PubMed] [Google Scholar]
- 8.Horkan C, Ahmed M, Liu Z, et al. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol. 2004;15:269–274. doi: 10.1097/01.rvi.0000109396.74740.c4. [DOI] [PubMed] [Google Scholar]
- 9.Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiol. 2006;240:82–89. doi: 10.1148/radiol.2401050788. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991;138:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson J, Lilja J, Peterson HI. Influence of vasoactive drugs on local tumor blood flow. Eur J Cancer Clin Oncol. 1982;18:677–684. doi: 10.1016/0277-5379(82)90214-0. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Exner AA, Krupka TM, Weinberg BD, Haaga JR. Vasomodulation of tumor blood flow: effect on perfusion and thermal ablation size. Ann of Biomed Eng. 2009;37:552–564. doi: 10.1007/s10439-008-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krix M, Kiessling F, Vosseler S, Kiessling I, et al. Comparison of Intermittent-Bolus Contrast Imaging with conventional Power Doppler Sonography: Quantification of Tumor Perfusion in Small Animals. Ultrasound Med and Biol. 2003;29:1093–1103. doi: 10.1016/s0301-5629(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 15.Kock NG, Roding B, Hahnloser P, Tibblin S, Schenk WG. The effect of glucagons on hepatic blood flow: an experimental study in the dog. AMA Arch Surg. 1970;100:147–149. doi: 10.1001/archsurg.1970.01340200035008. [DOI] [PubMed] [Google Scholar]
- 16.Lautt WW, Legare DJ, d’Almeida MS. Adenosine as putative regulator of hepatic arterial flow (the buffer response) Am J Physiol Heart. 1985;248:H331–H338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- 17.Hakime A, Hines-Peralta A, Peddi H. Combination of Radiofrequency Ablation with Antiangiogenic Therapy for Tumor ablation Efficacy: Study in Mice. Radiol. 2007;244:464–470. doi: 10.1148/radiol.2442061005. [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry. J Am Coll of Cardiol. 1994;23:384–389. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Matsui O, Ueda K, et al. Correlation between the blood supply and grade of malignancy of hepaticellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. Am J Roentgenol. 1999;172:969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki K, Miyazaki M, Onishi S, Ito K. Effects of food intake and various extrinsic hormones on portal blood flow in patients with liver cirrhosis demonstrated by pulsed Doppler with the octoson. Scand J Gastroenterol. 1986;21:1029–1038. doi: 10.3109/00365528608996416. [DOI] [PubMed] [Google Scholar]
- 21.Ngan H, Peh WCG. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36–40. doi: 10.1016/s0009-9260(97)80303-0. [DOI] [PubMed] [Google Scholar]
- 22.Novikoff AB. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957;17:1010–1027. [PubMed] [Google Scholar]
- 23.Ju S, McLennan G, Bennett SL, et al. Technical aspects of imaging and transfemoral arterial treatment of N1-S1 tumors in rats: an appropriate model to test the biology and therapeutic response to transarterial treatments of liver cancers. J Vasc Interv Radiol. 2009;20:140–414. doi: 10.1016/j.jvir.2008.12.408. [DOI] [PubMed] [Google Scholar]