Abstract
Background
Controversy exists regarding mechanisms responsible for sex based outcome differences post-injury. X-chromosome linked immune response pathway polymorphisms represent a potential mechanism resulting in sex based outcome differences post-injury. The prevalences of these variants are known to differ across race. We sought to characterize racial differences and the strength of any sex based dimorphism post-injury.
Study Design
A retrospective analysis was performed using data derived from the National Trauma Data Bank (NTDB 7.1, 2002–2006). Blunt injured adult (>15yr) patients, surviving > 24hrs, with an injury severity score (ISS) >16 were analyzed (n=244,371). Patients were stratified by race (Caucasian, Black, Hispanic, Asian) and multivariable regression analysis was used to characterize the risk of mortality and the strength of protection associated with sex (Female vs. Male).
Results
When stratified by race, multivariable models demonstrated Caucasian females had an 8.5% lower adjusted risk of mortality (OR 0.91, 95%CI 0.88–0.95, p < 0.001) relative to Caucasian males, with no significant association found for Hispanics or Blacks. An exaggerated survival benefit was afforded to Asian females relative to Asian males, having over a 40% lower adjusted risk of mortality (OR 0.59, 95%CI 0.44–78, p < 0.001). Asian males had over a 75% higher adjusted risk of mortality relative to non-Asian males (OR 1.77, 95%CI 1.5–2.0, p<0.001), while no significant difference in the mortality risk was found for Asian females relative to non-Asian females.
Conclusions
These results suggest that Asian race is associated with sex based outcome differences which are exaggerated, resulting due to worse outcome for Asian males. These racial disparities suggest a negative male X-chromosome linked effect as the mechanism responsible for these sex based outcome differences.
Introduction
An important and persistent finding has been that males and females respond differently following traumatic injury and hemorrhagic shock, with a relative protection afforded to females.1,2 An increasing body of evidence from animal models has revealed that sex hormones, and or their derivatives, play an intricate role in the pathological response to trauma-hemorrhage. Estrogen and testosterone in disparate ways have been shown to influence the hemodynamic, immunologic, organ system and cellular responses to traumatic insult in animals.1–10 This large body of literature has evolved attempting to elucidate the mechanisms responsible for sex based outcome differences post-injury, however, a significant divide continues to exist between what experimental laboratory investigations have revealed and what has been shown clinically in humans.2,11–22
Males and females also differ genetically, primarily due to genes that reside on the X chromosome.2,23 Females have two X chromosomes by definition, however, only one is functional as the other is inactivated early in development. This process occurs randomly in each cell, thus a female is a mosaic having different proportions of cells in each tissue with X linked genes derived from either of her parents. 24 Females would potentially be less affected by an unfavorable X-linked genetic polymorphism due to this mosaic expression pattern and outcome differences would demonstrate a negative or detrimental male effect.25–29 Importantly, X-chromosome linked polymorphisms in pathways critical to the innate immune response to sterile injury have been shown to be associated with poor outcome in sepsis and represent a plausible mechanism for sex based outcome differences following injury.30–32 Interestingly, the prevalence of these X-chromosome linked polymorphisms are known to vary significantly across different racial groups. 31,33
The main objective of the current analysis was to characterize the strength of any sex based outcome differences across different racial groups using a large national trauma dataset while controlling for differences in injury characteristics and severity. We hypothesized that the strength of any sex based dimorphism following significant injury will vary across different racial groups and demonstrate a negative male X-chromosome linked pattern.
Methods
We performed a retrospective analysis of data from the National Trauma Data Bank (NTDB 7.1) over the 2002–2006 time period. This dataset represents one of the largest national repositories of trauma patients and contains data for over 1.9 million trauma admissions during the study time period. To characterize sex based outcome differences following severe traumatic injury and how the strength of any dimorphism post-injury varies across different racial groups, we first selected injured adult (age > 15 years) patients who suffered significant, blunt, traumatic injury (Injury Severity Score, ISS > 16), who survived beyond the initial 24 hours from the time of injury with a patient’s race documented in the dataset (excluding missing or ‘unknown’ data). Significantly injured patients were selected (ISS > 16), as prior studies have suggested it is in these patients where sex based outcome differences are strongest and most apparent.2,23,34 Patients with penetrating injury were excluded because the risk of mortality correlates with the trajectory of the injury more so than the severity of injuries which occur. Similarly, those patients who did not survive beyond 24 hours were considered unsalvageable, with their ultimate outcome resulting irrespective of their demographics or sex. The injured study cohort of patients was then further stratified by race, as documented in the dataset (Caucasian, Black, Hispanic, Asian). Those patients whose race was classified as ‘Other or Missing’ in the dataset were not included in the race stratified analysis as there was no way to obtain or impute the missing data and the ‘Other’ category represents such a variable group of patients when looking at racial disparities. The primary outcome for the analysis was in-hospital mortality.
First, the study cohort was compared in a univariate fashion across sex. Logistic regression modeling was then used to determine the independent risks of mortality associated with female sex relative to male sex after controlling for important confounders and differences in the cohort across males and females. In addition to sex (female vs. male), our regression model included and controlled for differences in age, blunt injury mechanism (MVC, pedestrian, assault, fall), presenting systolic blood pressure, injury severity (component AIS scores), presenting GCS, TRISS probability of survival, presence of hypotension upon arrival (SBP < 90mmHg), need for urgent operative intervention (ED to OR, yes/no) and intubation status (yes/no). These covariates in the model were selected for their clinical significance as prognostic factors following injury and those which were statistically different (p< 0.001) in the male and female univariate comparison.
A stratified analysis utilizing the same regression models was then performed to characterize differences in the strength of the sex dimorphism across individual racial groups and across injury severity. Finally, Cox proportional hazard regression analysis was utilized to characterize the timing of outcome differences across sex and race, after controlling similar confounders, as was performed in our logistic regression models.
Data were summarized as mean ± SD, median [inter-quartile range], or percentage (%). Student-t or Mann-Whitney statistical tests were used to compare continuous variables, while Chi-Square or Fisher’s Exact test was used for categorical variables. Due to the large sample size of the analysis for the unadjusted comparisons, we utilized a significance level of p < 0.001 as the value indicating statistical significance. Results of the logistic regression analysis are presented as odds ratios (OR) with 95% confidence intervals (CI), while Cox regression results are presented as hazard ratios (HR) with 95% CI. For our regression modeling, odds and hazard ratios were considered statistically significant at a p-value < 0.05. Statistical analyses were performed using SPSS 18 (SPSS, Chicago, IL).
Results
Of the 1.9 million injured patients from the 2002–2006 time periods, 244,371 patients met all inclusion and exclusion criteria and were selected as the study cohort. Overall, the cohort was 70% male, with a median age of 42 years [IQR 26,58]. As expected due to the inclusion criteria, the cohort was significantly injured as demonstrated by a median ISS of 24 [IQR 18,29], with 18% of patients requiring urgent operative intervention (To OR from ED), and an overall mortality rate of 12.2%. The cohort was predominantly Caucasian, with similar proportions of Blacks and Hispanics and a much smaller proportion of Asians. (Caucasian: n=176,862 −72.4%, Blacks: n= 27,242 −11.1%, Hispanics: n= 22,331 −9.1 %, Asian: n= 4,007 −1.6%, Other/Missing: n= 13,929 −5.7%). Secondary to the characteristics of the data set 1.7% of patients were characterized under the race variable as ‘Other’ while 4.0% had missing data. When males and females were compared across the entire cohort, female injured patients were somewhat older and more commonly hypotensive. Males and females were clinically similar in blunt mechanism of injury, injury severity, presenting GCS, probability of survival (TRISS), intubation status, ICU and ventilator requirements and crude mortality, yet were all statistically different (p<0.001) based upon the p-value selected for the analysis. (Table 1.) When the cohort was compared across race, Blacks and Hispanics were younger with lower presenting GCS scores, while Asians more commonly presented hypotensive (SBP<90mmHg). No significant differences were found in overall injury burden as demonstrated by similar ISS scores. (Table 2.) When unadjusted crude mortality was stratified and compared across race and sex, Asian males and females had a higher mortality, with Blacks having lower overall mortality. (Table 3.)
Table 1.
Group Comparison across Sex
| Variable | Females (n=74,370) | Males (n=170,001) | p Value |
|---|---|---|---|
| Age, y (range) | 46 (27–69) | 40 (25–55) | <0.001 |
| ED SBP, mmHg | 128±37 | 131±37 | <0.001 |
| Hypotensive % (SBP<90mmHg) | 10.5 | 9.0 | <0.001 |
| Mechanism, % | <0.001 | ||
| MVC/MCC | 61 | 64 | |
| Pedestrian struck | 8 | 7 | |
| Assault | 7 | 8 | |
| Fall | 13 | 12 | |
| Other | 11 | 9 | |
| ED GCS (range) | 15 (7–15) | 15 (4–15) | <0.001 |
| Injury Severity Score (range) | 23 (18–28) | 23 (18–29) | <0.001 |
| Head/Neck AIS Score | 1.22±2 | 1.27±2 | <0.001 |
| Face AIS Score | 0.29±0.6 | 0.36±0.7 | <0.001 |
| Chest AIS Score | 0.98±2 | 0.99±2 | 0.094 |
| Abdomen AIS Score | 0.50±1 | 0.42±1 | <0.001 |
| Extremity AIS Score | 0.87±1 | 0.78±1 | <0.001 |
| Skin AIS Score | 0.06±0.2 | 0.07±0.03 | <0.001 |
| ED to OR % | 17.8 | 18.0 | 0.088 |
| Intubation Status (% yes) | 2.7 | 2.9 | 0.017 |
| TRISS probability of survival (range) | 0.94 (0.80–0.98) | 0.95 (0.78–0.98) | <0.001 |
| LOS, d (range) | 7 (4–14) | 7 (3–15) | <0.001 |
| ICU, d (range) | 2 (0–6) | 2 (0–7) | <0.001 |
| Ventilator, d (range) | 0 (0–2) | 0 (0–3 | <0.001 |
| Mortality, % | 12.6 | 12.0 | <0.001 |
p< 0.001 Considered statistically significant.
ED SBP, emergency department systolic blood pressure; MVC, motor vehicle collision; MCC, motorcycle collision; ED GCS, emergency department Glasgow Coma Score; AIS, abbreviated injury score; OR, operating room; TRISS, trauma score-injury severity score; LOS, length of stay; ICU, intensive care unit.
Table 2.
Injury Characteristics of the Study Cohort across Race
| Variable | Caucasian (n=176,862) | Black (n= 27,242) | Hispanic (n= 22,331) | Asian (n= 4,007) | p Value |
|---|---|---|---|---|---|
| Age, y (range) | 44 (26–61) | 39 (25–51) | 31 (23–45) | 45 (27–63) | <0.001 |
| ED GCS (range) | 15 (5–15) | 14 (3–15) | 14 (4–15) | 15 (10–15) | <0.001 |
| ISS (range) | 24 (18–29) | 24 (18–29) | 24 (18–29) | 25 (18–29) | 0.036 |
| TRISS survival probability (range) | 0.94 (0.79–0.98) | 0.96 (0.81–0.98) | 0.94 (0.75–0.98) | 0.94 (0.75–0.98) | <0.001 |
| Hypotensive, % | 8.7 | 8.7 | 8.8 | 10.4 | <0.001 |
| ED to OR, % | 17.6 | 19.3 | 21.3 | 19.6 | <0.001 |
p<0.001 Considered statistically significant. Independent Kruskal-Wallis and Chi-Square statistic testing across the four racial groups was utilized.
ED GCS, emergency department Glasgow Coma Score; ISS, injury severity score; TRISS, Trauma Score – Injury Severity Score; OR, operating room.
Table 3.
Unadjusted Mortality Rates across Sex (Female vs Male) Stratified by Race
| Sex | Caucasian, % | Black, % | Hispanic, % | Asian, % | p Value |
|---|---|---|---|---|---|
| Females | 12.7 | 10.1 | 12.6 | 15.2 | <0.001 |
| Males | 11.9 | 10.8 | 12.1 | 17.7 | <0.001 |
p<0.001 Statistically significant; Chi-Square statistic testing across the four racial groups was utilized.
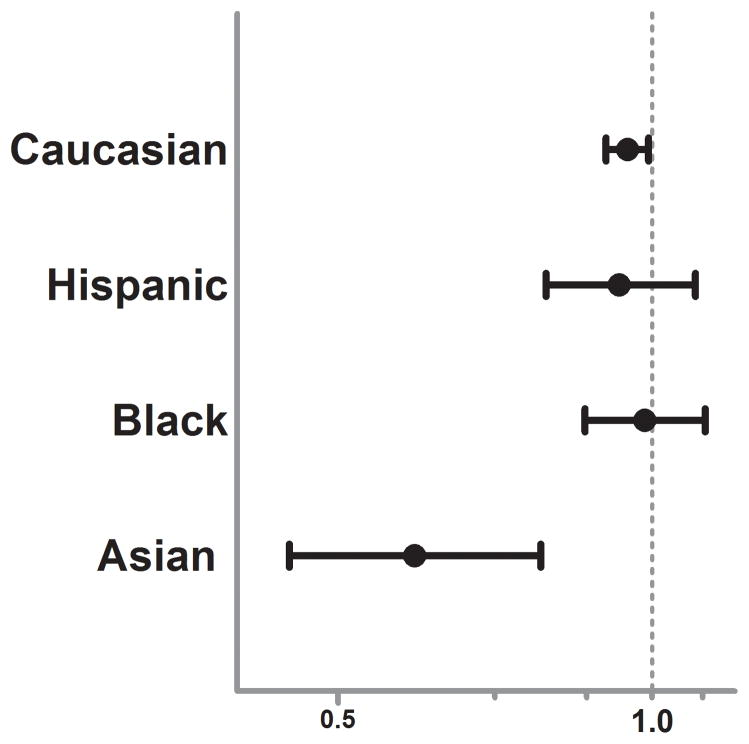
With mortality as the dependent variable and sex (female versus male) as the primary independent variable, our regression model was a good predictor of mortality with an area under the curve (AUC) of 0.852 via receiver operating characteristic (ROC) curve analysis and showed adequate calibration and fit for the data (Hosmer-Lemeshow Goodness-of -Fit x2 of 6.54, p = 0.59) For the entire study cohort, in multivariable logistic regression analysis female sex was associated with an 8% lower independent risk of mortality (OR 0.92, 95%CI 0.88–0.95, p < 0.001) after controlling for important differences and confounders across sex. When stratified by race (Caucasian, Black, Hispanic, Asian), the mortality protection associated with female sex in Caucasians remained similar and statistically significant (OR 0.94, 95%CI 0.90–0.98, p<0.001) while no significant sex dimorphism was found for Black or Hispanics. (Figure 1.) Interestingly, as compared to these other racial groups, Asian females were found to have over a 40% lower independent risk of mortality as compared to Asian males (OR 0.59, 95%CI 0.44–78, p < 0.001). This exaggerated sex based outcome difference was robust and significant despite the smaller sample size and the relative paucity of Asians in the overall study cohort.
Figure 1.
Forest plot depicting the adjusted mortality Odds Ratio for female versus male sex post-injury. Odds Ratio and 95% CI plotted after controlling for age, blunt injury mechanism (MVC, pedestrian, assault, fall), presenting systolic blood pressure, injury severity (component AIS scores), presenting GCS, TRISS probability of survival, presence of hypotension upon arrival (SBP < 90mmHg), need for urgent operative intervention (ED to OR, yes/no) and intubation status (yes/no).
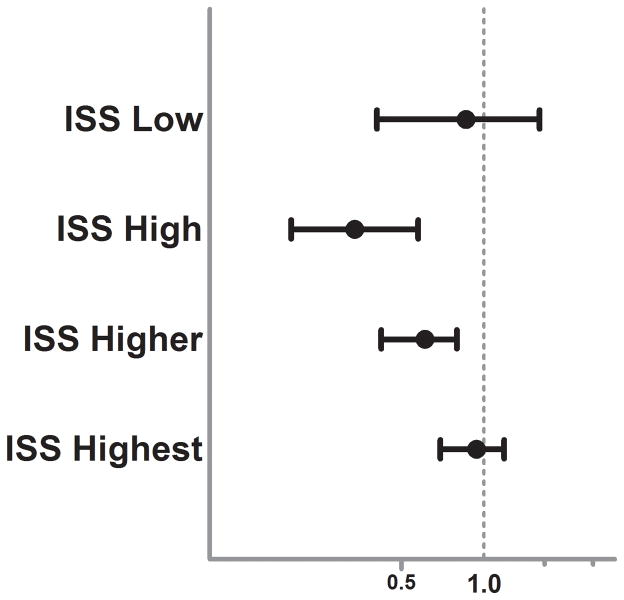
To further analyze the strength of this sex dimorphism in Asians, we stratified the Asian subgroup regression analysis by injury severity (ISS, Figure 2.). This attempted to decipher whether specific injury patterns were responsible for the sex based outcome racial disparities or whether these findings were proportional to the overall injury burden. The results demonstrated that the strength of the female protection in Asians was strongest in those with moderate to severe injury and the associated lower mortality risk was lost as injury severity became more extreme. Due to the known sex hormone changes which occur over age in females due to menopause, we tested for any interaction between sex and age in the Asian subgroup regression model. Importantly, the interaction was not significant (p=0.167) suggesting that the relationship may hold for all ages across the Asian cohort. To determine whether these outcome differences were secondary to male or female driven outcome differences we stratified our regression models across sex and compared Asian females to non-Asian females and Asian males to non-Asian males. In these models, Asian females had no significantly different mortality risk as compared to non-Asian females (OR 1.16, 95%CI 0.94–1.4, p=0.16). Interestingly, Asian males as compared to non-Asian males had over a 75% higher independent mortality risk (OR 1.77 95%CI 1.5–2.0, p<0.001). There were no significant relationships found for any of the other racial groups in our regression models. In Cox hazard regression modeling, this exaggerated Asian male mortality risk (Asian vs non-Asian females-HR 1.08, 95%CI 0.92–1.2, p=0.382, Asian vs. non-Asian males-HR 1.45, 95%CI 1.3–1.6, p<0.001) remained and further revealed that these differential outcome risks diverge early after injury, as demonstrated by the separation of the survival curves in the Asian and non-Asian male comparison (Figure 3A and B.)
Figure 2.
Forest plot depicting the adjusted mortality Odds Ratio for female versus male sex in the Asian subgroup stratified by injury severity score (ISS). Odds Ratio and 95% CI plotted after controlling for age, blunt injury mechanism (MVC, pedestrian, assault, fall), presenting systolic blood pressure, injury severity (component AIS scores), presenting GCS, TRISS probability of survival, presence of hypotension upon arrival (SBP < 90mmHg), need for urgent operative intervention (ED to OR, yes/no) and intubation status (yes/no).
Figure 3.
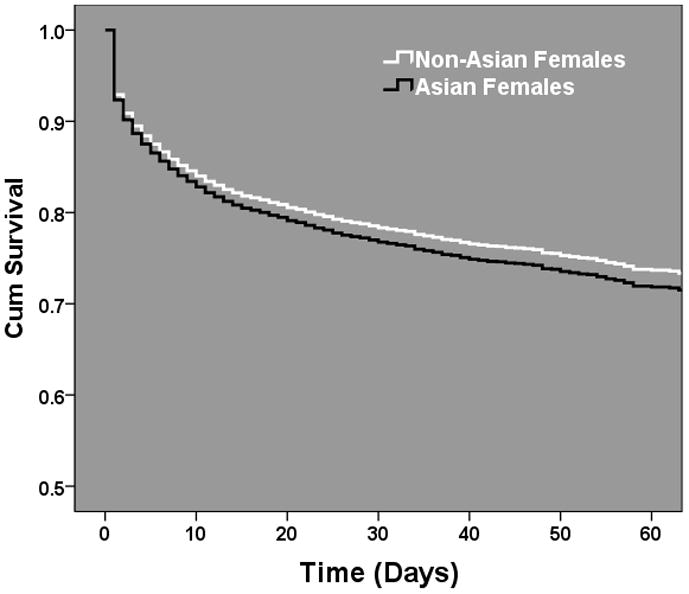
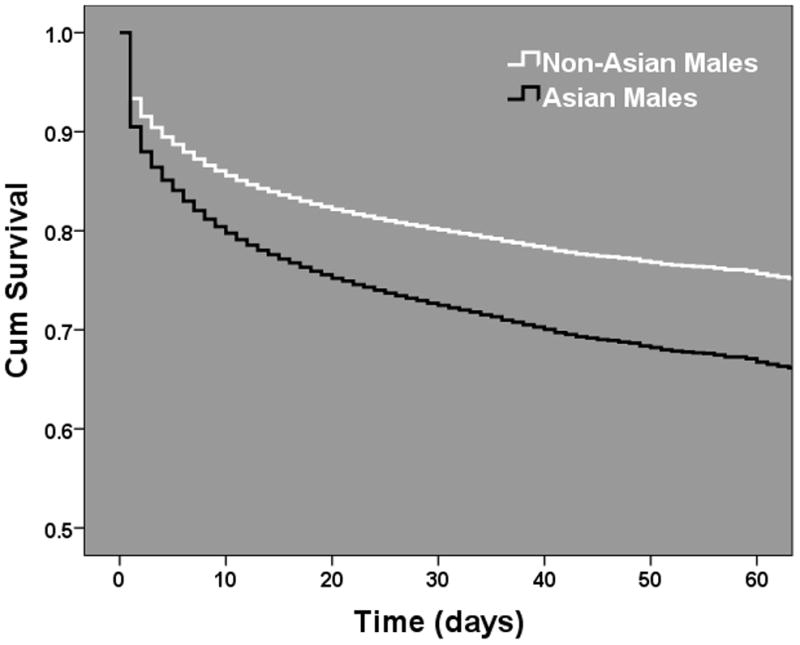
Male and female Cox proportional hazard regression survival functions comparing adjusted mortality risks across Asian and Non-Asian injured patients. (A) Female comparison, p=0.38. (B) Male comparison, p < 0.001. Odds Ratio and 95% CI plotted after controlling for age, blunt injury mechanism (MVC, pedestrian, assault, fall), presenting systolic blood pressure, injury severity (component AIS scores), presenting GCS, TRISS probability of survival, presence of hypotension upon arrival (SBP < 90mmHg), need for urgent operative intervention (ED to OR, yes/no) and intubation status (yes/no).
Discussion
Few effective interventions exist which alter the morbidity and mortality that inherently follows traumatic injury.35–40 Determining the mechanisms responsible for the sex dimorphism following traumatic injury may allow poorly characterized or novel risk factors and therapeutic targets to be determined with the potential to improve outcomes, irrespective of sex, following injury.
The hormonal milieu of the pro-estrus female rodent has been found to be protective following trauma and hemorrhage.7,8 17β-estradiol is the primary circulating estrogen in female animals, and studies have demonstrated that both cardiovascular and immunologic function are preserved in female rodents when they are in the pro-estrus phase (when 17β-estradiol levels are highest).7,8,41 A similar large body of evidence has accumulated concerning the detrimental effects of testosterone and its derivatives following trauma-hemorrhage in animals.3–6,42 Interestingly, recent prospective evidence in humans, where sex-hormone levels were measured 48-hours following injury, demonstrated that estrogen levels were positively associated with a greater risk of mortality in both males and females, a conclusion which contradicts the majority of the experimental animal literature.11 Similar findings for non-injured but critically ill patients have also been found by the same group.12,13 There continues to be this divide between the ‘bench and bedside’ in regards to our understanding of sex based outcome differences following injury with a paucity of clinical investigations into other alternative, yet plausible mechanisms.2,11–22
Results from the current analysis provide evidence for a robust racial disparity in the strength of the sex dimorphism with Asian females having over a 40% lower independent risk of mortality as compared to Asian males which was significantly exaggerated relative to the proportionally larger Caucasian, Black and Hispanic racial groups. This exaggerated Asian sex dimorphism resulted from an elevated independent risk of mortality in Asian males as compared to non-Asian males while Asian females had similar independent mortality risks as compared to non-Asian females. The most important question remaining is the mechanism that is responsible for this negative male exaggerated sex dimorphism in the Asian subgroup.
Females would potentially be less affected by an unfavorable X-chromosome linked genetic polymorphism due to the mosaic expression pattern of X-chromosome and it has been shown in other disease processes that this mosaic pattern can be protective for females.25,27–29 It is also known that the Toll-like receptor (TLR) signaling cascade plays an essential role in the early activation of innate immune response following injury resulting in induced activation of nuclear factor (NF)-kappaB, a critical event in the transcriptional regulation of many sepsis-associated proinflammatory mediators.31,43–48 These two facts are provocative as several important members of the TLR signaling cascade reside on the X chromosome.26,49,50 Recent evidence has demonstrated that the IL-1 receptor-associated kinase (IRAK-1) gene in humans can exist as a variant haplotype which contains a single nucleotide polymorphism. IRAK-1 plays a central role in the regulation and expression of sepsis-associated pro-inflammatory mediators.31,51 This gene resides on the X-chromosome and this variant haplotype has been found to be common in Caucasians (20%) and significantly associated with more severe organ dysfunction and higher mortality in patients with sepsis.31,32 Even more intriguing is the fact that the prevalence of this IRAK-1 polymorphism has been demonstrated to vary across racial groups with over a 70% prevalence in Asian populations.33 (http://hapmap.ncbi.nlm.nih.gov/)
This analysis has several important implications. First, the analysis demonstrates a negative male outcome pattern for mortality in the Asian cohort. Although females have a lower risk of mortality, the sex stratified analysis suggests this is due to worse outcome for Asian males relative to their male counterparts in the other racial groups. This would be the outcome pattern that would be predicted due to an X-chromosome linked negative polymorphism mechanism. Equally plausible, this exaggerated pattern could stem from sex hormone differences across race, secondary to the detrimental affects known to be associated with testosterone derivatives found in the experimental animal literature. Importantly, the results in the Asian subgroup were found not to significantly interact with age suggesting that age of the female and her sex hormone status may not be the primary driving mechanism for these results. Due to the retrospective nature of this analysis, however, the sex hormonal environment around the time of injury and the prevalence of X-chromosome polymorphisms in innate immune pathways are unknown for these injured patients. However, these results suggest and provide justification for further investigation into alternative mechanisms in addition to sex hormones which may be responsible for the sex dimorphism following injury, irrespective of race. The current results are plausible and further strengthened by the relationship between the sex dimorphism in Asians and injury severity. As injury severity increases the strength of the sex dimorphism increases to a point, despite the small sample size of the Asian subgroup in the analysis. As injury severity continues to increase, the sex dimorphism no longer is demonstrated, suggesting that the mechanism for these sex based outcome differences is strong and robust but with such high injury severity, mortality increases irrespective of sex beyond certain injury thresholds. If not more important, these sex based mortality risk differences across the Asian subgroup occur early post injury. This suggests that rather than differences in infectious outcomes or sepsis, these outcome risk differences may result from differences in hemorrhage tolerance or an excessive early immune response.
This analysis does have several important limitations. First, this study is a retrospective analysis utilizing data which was not recorded to answer the specific hypothesis asked in this study. As with similar large national datasets, the possibility of missing data, inaccurate data and misclassification exist. The racial demographic data collected in the trauma data set lacks specificity as ‘Asian’ race may represent a multitude of different racial groups all with significantly different genetic make-up and backgrounds. Similarly, how race was documented and whether it was self reported is not known or able to be surmised from the dataset. Missing race data represents an important limitation, however, the relatively small proportion of missing date (4.0%) limits its significance. It may be that specific injury mechanisms and patterns are different across racial groups despite attempting to control for any differences in the regression modeling despite our attempts to control for injury differences. Potential confounding variables, which were not recorded in the data set and were unable to be controlled for in the multivariable regression models, may be responsible for the associations described and the conclusions formulated. The Asian subgroup represented a small proportion of the patients in the analysis. Although our regression analysis revealed an impressively strong and robust sex dimorphism in the Asian group, the proportionally smaller size remains a potential limitation for the analysis. There were significant differences across male and female groups and across race subgroups and although we attempted to either adjust for these differences or stratify the regression analysis to reduce the confounding associated with these differences, the possibility exists that the sex or racial subgroups were not similar enough to appropriately compare.
The results of the current analysis suggest an exaggerated sex dimorphism exists for the Asian race resulting secondary to poor outcome for Asian males, following significant traumatic injury. This sex based racial disparity corresponds to prevalence rates for known X-chromosome immune pathway polymorphisms and suggests a negative male X-chromosome linked effect. Further research is needed to investigate the mechanisms responsible for these sex based outcome differences post-injury, allowing a bridging or closure of the ‘bench to bedside’ gap in our understanding of these clinical findings. This acquired understanding will allow the ability to better prognosticate and predict those patients at highest risk for poor outcome and promote novel interventions that will improve outcomes for both males and females following injury.
Acknowledgments
Supported by: NIH NIGMS K23GM093032
Footnotes
Presented at the annual meeting of the Shock Society, Norfolk, VA, June 2011.
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 2.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008;83:499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Knoferl MW, Ayala A, et al. Testosterone and estrogen differently effect Th1 and Th2 cytokine release following trauma-haemorrhage. Cytokine. 2001;16:22–30. doi: 10.1006/cyto.2001.0945. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Angele MK, Ayala A, Cioffi WG, et al. Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol. 1998;274:C1530–1536. doi: 10.1152/ajpcell.1998.274.6.C1530. [DOI] [PubMed] [Google Scholar]
- 6.Angele MK, Ayala A, Monfils BA, et al. Testosterone and/or low estradiol: normally required but harmful immunologically for males after trauma-hemorrhage. J Trauma. 1998;44:78–85. doi: 10.1097/00005373-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Jarrar D, Wang P, Cioffi WG, et al. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 8.Knoferl MW, Jarrar D, Angele MK, et al. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2001;281:C1131–1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 9.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Hu S, Chen J, et al. Mechanism of hepatoprotection in proestrus female rats following trauma-hemorrhage: heme oxygenase-1-derived normalization of hepatic inflammatory responses. J Leukocyte Biol. 2009;85:1015–26. doi: 10.1189/jlb.0508288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dossett LA, Swenson BR, Evans HL, et al. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surgical Inf. 2008;9:41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Critical Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 15.Bowles BJ, Roth B, Demetriades D. Sexual dimorphism in trauma? A retrospective evaluation of outcome. Injury. 2003;34:27–31. doi: 10.1016/s0020-1383(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 16.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Rappold JF, Coimbra R, Hoyt DB, et al. Female gender does not protect blunt trauma patients from complications and mortality. J Trauma. 2002;53:436–441. doi: 10.1097/00005373-200209000-00007. discussion 441. [DOI] [PubMed] [Google Scholar]
- 19.Coimbra R, Hoyt DB, Potenza BM, et al. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma. 2003;54:689–700. doi: 10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- 20.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. discussion 938-940. [DOI] [PubMed] [Google Scholar]
- 21.George RL, McGwin G, Jr, Metzger J, et al. The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 22.George RL, McGwin G, Jr, Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Sperry JL, Nathens AB, Frankel HL, et al. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 24.Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- 25.Migeon BR. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 26.Gunter C. Genome biology: she moves in mysterious ways. Nature. 2005;434:279–280. doi: 10.1038/434279a. [DOI] [PubMed] [Google Scholar]
- 27.Migeon BR. X-chromosome inactivation: molecular mechanisms and genetic consequences. Trends Genet. 1994;10:230–235. doi: 10.1016/0168-9525(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 28.Migeon BR. X chromosome inactivation: theme and variations. Cytogenetic Genome Res. 2002;99:8–16. doi: 10.1159/000071568. [DOI] [PubMed] [Google Scholar]
- 29.Migeon BR. X inactivation, female mosaicism, and sex differences in renal diseases. J Am Soc Nephrol. 2008;19:2052–2059. doi: 10.1681/ASN.2008020198. [DOI] [PubMed] [Google Scholar]
- 30.Liu G, Tsuruta Y, Gao Z, et al. Variant IL-1 receptor-associated kinase-1 mediates increased NF-kappa B activity. J Immunol. 2007;179:4125–4134. doi: 10.4049/jimmunol.179.6.4125. [DOI] [PubMed] [Google Scholar]
- 31.Arcaroli J, Silva E, Maloney JP, et al. Variant IRAK-1 haplotype is associated with increased nuclear factor-kappaB activation and worse outcomes in sepsis. Am J Respir Crit Care Med. 2006;173:1335–1341. doi: 10.1164/rccm.200603-341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toubiana J, Courtine E, Pene F, et al. IRAK1 functional genetic variant affects severity of septic shock. Crit Care Med. 2010;38:2287–2294. doi: 10.1097/CCM.0b013e3181f9f9c7. [DOI] [PubMed] [Google Scholar]
- 33.Ishida R, Emi M, Ezura Y, et al. Association of a haplotype (196Phe/532Ser) in the interleukin-1-receptor-associated kinase (IRAK1) gene with low radial bone mineral density in two independent populations. J Bone Miner Res. 2003;18:419–423. doi: 10.1359/jbmr.2003.18.3.419. [DOI] [PubMed] [Google Scholar]
- 34.Sperry JL, Friese RS, Frankel HL, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. discussion 578-579. [DOI] [PubMed] [Google Scholar]
- 35.Manship L, McMillin RD, Brown JJ. The influence of sepsis and multisystem and organ failure on mortality in the surgical intensive care unit. Am Surg. 1984;50:94–101. [PubMed] [Google Scholar]
- 36.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Nathens AB, Marshall JC. Sepsis, SIRS, and MODS: what’s in a name? World J Surg. 1996;20:386–391. doi: 10.1007/s002689900061. [DOI] [PubMed] [Google Scholar]
- 39.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 40.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995;23:474–480. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Mizushima Y, Wang P, Jarrar D, et al. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg. 2000;232:673–679. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remmers DE, Wang P, Cioffi WG, et al. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–2925. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 43.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukocyte Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 44.Kaczorowski DJ, Nakao A, Mollen KP, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 45.Levy RM, Prince JM, Yang R, et al. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 46.Moeinpour F, Choudhry MA, Kawasaki T, et al. 17 Beta-estradiol normalizes Toll receptor 4, mitogen activated protein kinases and inflammatory response in epidermal keratinocytes following trauma-hemorrhage. Molecular Immunol. 2007;44:3317–3323. doi: 10.1016/j.molimm.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh YC, Frink M, Kawasaki T, et al. Downregulation of TLR4-dependent ATP production is critical for estrogen-mediated immunoprotection in Kupffer cells following trauma-hemorrhage. J Cellular Physiol. 2007;211:364–370. doi: 10.1002/jcp.20943. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh YC, Frink M, Thobe BM, et al. 17Beta-estradiol downregulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Molecular Immunol. 2007;44:2165–2172. doi: 10.1016/j.molimm.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross MT, Grafham DV, Coffey AJ, et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27:597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 51.Thomas JA, Allen JL, Tsen M, et al. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol. 1999;163:978–984. [PubMed] [Google Scholar]