Abstract
Right ventricular function (RVF) is often selectively declined after coronary artery bypass graft surgery. In adult patients with congenital heart disease (CHD) the incidence and persistence of declined RVF after cardiac surgery is unknown. The current study aimed to describe RVF after cardiac surgery in these patients. Adult CHD patients operated between January 2008 and December 2009 in the Academic Medical Centre in Amsterdam were studied. Clinical characteristics, laboratory tests, surgical data and intensive care unit outcome were obtained from medical records. RVF was measured by trans-thoracic echocardiography (TTE) and expressed by tricuspid annular plane systolic excursion (TAPSE), tissue Doppler imaging (RV S’) and myocardial performance index (MPI) pre-operatively and direct, at intermediate and late follow up. Of a total of 185 operated, 86 patients (mean age 39 ± 13 years, 54% male) had echo data available. There was a significant fall in RVF after cardiac surgery. TAPSE and RV S’ were significantly higher and MPI was significantly lower pre-operatively compared to direct post-operative values (TAPSE 22 ± 5 versus 13 ± 3 mm (P < 0.01), RV S’ 11 ± 4 versus 8 ± 2 cm/s (P < 0.01) and MPI 0.36 ± 0.14 vs 0.62 ± 0.25; P < 0.01). There were no significant differences in left ventricular function pre-operatively compared to post-operative values. Right-sided surgery was performed in 33, left-sided surgery in 37 and both sided surgery in 16 patients. Decline in RVF was equal for those groups. Patients with severe decline in RVF, were patients who underwent tricuspid valve surgery. Decline in RVF was associated with post-operative myocardial creatine kinase level and maximal troponin T level. There was no association between decline in RVF and clinical outcome on the intensive care unit. 18 months post-operatively, most RVF parameters had recovered to pre-operative values, but TAPSE which remained still lower (P < 0.01). CHD patients have a decline in RVF directly after cardiac surgery, regardless the side of surgery. Although a gradual improvement was observed, complete recovery was not seen 18 months post-operatively.
Keywords: Right ventricular function, Cardiac surgery, Congenital heart disease, Echocardiography
Introduction
Cardiac surgery relieves symptoms and increases life expectancy in cardiac patients, with and without congenital heart disease (CHD). However, patients undergoing surgery are at risk of complications, including right ventricular (RV) failure. RV failure is often difficult to diagnose and to treat. A decrease in right ventricular function (RVF) directly after surgery has been demonstrated in patients who underwent coronary artery bypass grafting [1–3]. In some patients, the decline in RVF persisted up to 18 months. Whether the RVF shows a similar decline after cardiac surgery in adult patients with congenital heart disease (CHD) is not known. Especially in these patients, contribution of the RV to cardiac pump function is essential. RVF has been shown a determinant of clinical status and long-term patients with CHD [4, 5]. In addition, CHD patients undergo more often right-sided surgery. Whether CHD patients with right-sided heart lesions and, hence, increased right atrial pressure, have different RVF after cardiac surgery than would patients with left-sided heart lesions is unknown. Transthoracic echocardiography (TTE) is an accessible and robust tool for assessing both right (RVF) and left ventricular function (LVF) prior to and after cardiac surgery. The current study aimes to describe RVF measured by TTE after cardiac surgery in patients with right- and left-sided CHD disease. We hypothesized that patients with right-sided CHD and consequently, an increased right ventricular load, would be at greater risk of postoperative decline in RVF than patients with left-sided disease.
Methods
Data collection
Adult CHD patients operated between January 2008 and December 2009 in the Academic Medical Centre in Amsterdam were studied. Clinical characteristics, laboratory tests and surgical data were obtained from medical records.
Echocardiography
Pre-operative TTE were collected as well as TTE performed directly (1–4 months), intermediate (5–8 months) and late (10–18 months) after surgery. Echocardiography was performed with a Vivid 7 ultrasound system (General Electric, Milwaukee, United States of America). All echocardiographic images were acquired and recorded digitally. All studies were analyzed by a single observer with more than 10 years experience in echocardiography in adult patients with CHD, who was blinded to clinical information. Parasternal and apical views were obtained and valve regurgitation was quantified according to the guidelines [6, 7]. RVF was evaluated by means of tricuspid annular plane systolic excursion (TAPSE), tissue Doppler imaging (RV S’) and myocardial performance index (MPI). Tricuspid annular plane systolic excursion (TAPSE) was obtained from the apical four-chamber view. The difference in the displacement of the right ventricular base from end-diastole to end-systole at the junction of the tricuspid valvular plane was used to determine TAPSE. Tissue Doppler imaging S (RV S’) was measured using a pulsed wave Doppler sampling gate of 6–6 mm and a sweep of 100 mm/s. The myocardial performance index (MPI) was defined as the sum of the isovolumic contraction time and isovolumic relaxation time divided by ejection time obtained from tissue Doppler imaging. The mean of three TAPSE, RV S’ and MPI measurements were used for analyses. The study population was divided into three groups representing good, moderate, impaired ventricular function.
Statistics
For statistical analysis SPSS 18.0 (SPSS Inc, Chicago, Illinois) was used. Descriptive data are presented as mean ± standard deviation. The difference between pre-operative and post-operative RVF was calculated with paired t-test. Statistical differences of categorical variables were analyzed using Chi square test. Univariate linear regression analysis was performed to determine whether changes in RVF were associated with pre-operative, peri-operative or post-operative factors. Values below 0.05 were considered to be significant.
Results
Pre-operative RVF
For a total of 185 operated CHD patients, 86 patients (mean age 39 ± 13 years, 54% male) echo data was available (Table 1). Diagnosis of patients with right-sided disease included Tetralogy of Fallot (n = 13), atrial septal defect (n = 11), pulmonary valve defect (n = 5), tricuspid valve defect (n = 5) or morbus Ebstein (n = 6). Left-sided disease patients had Marfan syndrome (n = 10), ventricular septal defect (n = 9), aortic coarctation (n = 8) or aortic valve disease (n = 19). Pre-operative moderate or severe tricuspid valve regurgitation was present in 26 patients and 18 patients had moderate or severe pulmonary valve regurgitation. Moderate or severe aortic regurgitation was seen in 16 patients and 14 patients had moderate or severe mitral regurgitation.
Table 1.
Baseline characteristics
| Number | 86 | |
| Age, mean (sd), years | 39 ± 13 | |
| Weight, mean (sd), kg | 71 ± 13 | |
| Male, % | 54 | |
| NYHA, mean (sd) | 1.9 ± 0,9 | |
| Prior cardiac surgery, % | 49 | |
| Pre-operative | ||
| Laboratory | ||
| NT-pro-BNP, median (range), ng/l | 344 (49–2,675) | |
| Creatinine, mean (sd), umol/l | 75 (50 − 108) | |
| Echocardiography | ||
| Pulmonary artery pressure, mean (sd), mmHg | 36 ± 18 | |
| Elevated pulmonary artery pressure | 8 | |
| Moderate/severe | 18 | |
| Pulmonary regurgitation | ||
| Tricuspid reguritation | 26 | |
| Aortic regurgitation | 16 | |
| Mitral regurgitation | 14 | |
| Severe global RV dysfunction | 5 | |
| RA dilatation | 15 | |
| RV dilatation | 26 | |
| RV hypertrophy | 8 | |
| RVSP, mean (sd), mmHg | 39 ± 18 | |
| TAPSE, mean (sd), mm | 22 ± 5.6 | |
| RV S, mean (sd), cm/s | 11 ± 3.6 | |
| MPI, mean (sd), cm/s | 0.36 ± 0.13 | |
| Severe global LV dysfunction | 0 | |
| LA dilatation | 6 | |
| Left ventricular ejection fraction, mean (sd), % | 50 ± 10 | |
| Peri-operative | ||
| Aortic cross clamp time, mean (sd), minutes | 112 ± 44 | |
| Extracorporal perfusion time, mean (sd), minutes | 157 ± 66 | |
| Right-sided cardiac surgery | ||
| Pulmonary valve surgery | 22 | |
| Tricuspid valve surgery | 16 | |
| Closure atrial septal defect | 11 | |
| Left-sided cardiac surgery | ||
| Closure ventricular septal defect | 2 | |
| Aortic valve surgery | 36 | |
| Mitral valve surgery | 9 | |
| Both-sided cardiac surgery | ||
| Combinations | 16 | |
| Post-operative | ||
| Stay on intensive care unit, mean (sd), hours | 29 ± 18 | |
| Stay on intensive care unit >48 h | 5 | |
| Inotropics, % | 36 | |
| Fluid balance in first 24 h, median (range), ml | 782 (−776 to 4783) | |
TAPSE: tricuspid annular plane systolic excursion; kg; kilograms; ml: milliliter
MPI: myocardial performance index; NYHA: New York Heart Association
ng/l: nanagram per liter; umol/l: micromol/l; RV: right ventricle; RA: right atrium
RVSP: right ventricular systolic pressure; LA: left atrium; LV:left ventricle
For TAPSE 86, for RV S’ 66 and for MPI 59 patients had pre-operative measurements. Pre-operative RVF was better in patients who underwent cardiac surgery for the first time (TAPSE 24 mm, RV S’ 13 cm/s and MPI 0.33) compared those who had a re-operation (TAPSE 18 mm, RV S’ 10 cm/s and MPI 0.38).
Surgery
Right-sided surgery was performed in 33 patients, involving pulmonary valve (n = 18), tricuspid valve (n = 10) and closure of an atrial septal defect (n = 5). Left-sided surgery was performed in 37 patients involving aortic valve (n = 30), mitral valve (n = 5) and closure of a ventricular septal defect (n = 2). Combination of right- and left sided surgery was performed in 16 patients (tricuspid valve + aortic valve n = 1, tricuspid valve + mitral valve n = 1, pulmonary valve + aortic valve n = 6, tricuspid valve + aortic valve + mitral valve n = 1, tricuspid valve + atrial septal defect + mitral valve n = 2, atrial septal defect + mitral valve n = 1, tricuspid valve + mitral valve n = 2, pulmonary + tricuspid valve + aortic valve n = 1). Mean extracorporal perfusion time was 157 min and aortic cross clamp time was 112 min.
Directly after surgery
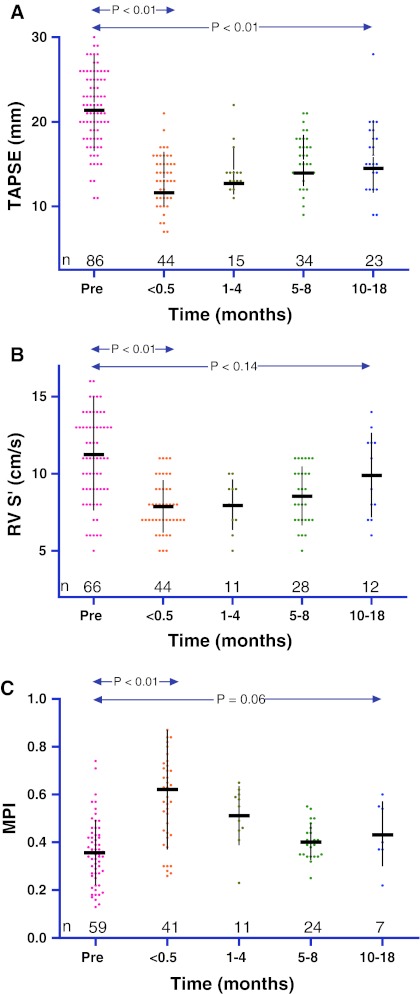
There was a significant fall in RVF after cardiac surgery (Fig. 1). For TAPSE 44, for RV S’ 44 and for MPI 41 patients had measurements within the first two weeks post-operatively. TAPSE and RV S’ were higher and MPI was lower pre-operatively compared to direct post-operative values (TAPSE 22 ± 5 vs. 13 ± 3 mm (P < 0.01), RV S’ 11 ± 4 vs. 8 ± 2 cm/s (P < 0.01) and MPI 0.36 ± 0.14 vs. 0.62 ± 0.25; P < 0.01). There were no significant differences in left ventricular function (LVF) pre-operatively compared to post-operative values.
Fig. 1.
Peri-operative right ventricular function in CHD patients a TAPSE: tricuspid annular plane systolic excursion; mm: milimeter b RV S’: right ventricular tissue Doppler imaging; cm/s: centimeter per second c MPI: myocardial performance index
Decline in RVF was equal in right-, left- and both sided surgeries (Table 2). The decline in RVF was larger in patients who underwent their first cardiac surgery (TAPSE-11 mm, RV S’ -5 cm/s and MPI 0.19) compared to patients who underwent re-operation (TAPSE -5, RV S’ -1 and MPI 0.25). Decline in RVF was significantly associated with tricuspid valve surgery, TAPSE (β = −0.33 P = 0.03) and RV S’ (β = −0.44 P < 0.01). No other types of surgery were individually associated with a decline in RVF. Extracorporal perfusion time and aortic cross clamp time were not associated with RVF decline.
Table 2.
Changes in right ventricular function and intensive care outcome
| Δ Tapse (mm) | Δ RV S’ (cm/s) | Δ MPI | ICU stay (hours) | Inotropics (%) | FB 24 h (ml) | Max CKMB | Max Trop T | |
|---|---|---|---|---|---|---|---|---|
| Side surgery | ||||||||
| Right-sided surgery | −7.2 | −2.9 | 0.27 | 25 | 30 | 678 | 51 | 1.43 |
| Left-sided surgery | −9.3 | −3.1 | 0.17 | 32 | 52 | 727 | 33 | 1.0 |
| Both-sided surgery | −7.3 | −2.8 | 0.2 | 38 | 64 | 1,700 | 57 | 1.95 |
| Cardiac surgery | ||||||||
| First | −10 | −4.7 | 0.19 | 28 | 44 | 1,180 | 52 | 1.76 |
| Re-operation | −5.6 | −1.2 | 0.25 | 33 | 46 | 633 | 38 | 1.03 |
TAPSE, Tricuspid annular plane systolic excursion; RV S’, Right ventricular systolic; MPI, Myocardial performance index; ICU, Intensive care unit; FB, Fluid balance; max CKMB, Maximal myocardial creatine kinase; max Trop T, Maximal troponin T level; mm, Millimeter; cm/s: centimeter per second; Ml, Milliliter; Δ, Delta
Maximal myocardial creatine kinase level was associated with decline in RVF (TAPSE β = −0.34 P = 0.03, RV S’ β = −0.45 P < 0.01, β = −0.26 P = 0.09) and maximal troponin T level (TAPSE β = −0,41 P = 0.03, RV S’ β = −0.51 P < 0.01, β = −0.36 P = 0.06). On the intensive care unit no association was seen between decline in RVF and stay in hours, use of inotropes and fluid balance in the first 24 h.
Intermediate and late follow-up
For TAPSE 34, for RV S’ 28 and for MPI 24 patients had measurements 5−8 months post-operatively. For TAPSE 34, for RV S’ 23 and for MPI 7 patients had measurements 5–8 months post-operatively. Compared to the first post-operative measurement, except for TAPSE which remained lower (P < 0.01), RVF increased at 18 months post-operatively (TAPSE 15 ± 3 mm; RV S’ 10 ± 3 cm/s and MPI 0.45 ± 0.14). Of the patients who had a TAPSE measurement available late follow-up, six (26%) underwent tricuspid valve surgery. Recovery of RVF was not different for patients who underwent their first cardiac surgery compared to patients who underwent re-operation.
Discussion
In this study, we found a significant and selective decline in RVF after cardiac surgery in all adult CHD patients. No differences were observed between patients who underwent surgery on right, left or both sides of the heart. Although a gradual improvement was observed, complete recovery was not seen even 18 months after surgery. This suggests that the effects of cardiac surgery on the right ventricle may be long lasting.
Trans-thoracic echocardiography and ventricular function
Non-invasive assessment of RVF is routinely performed with trans-thoracic echocardiography (TTE), before and after cardiac surgery [8–10]. Unfortunately, no single measurement of anatomy or function can adequately describe the form or performance of the right ventricle [11]. It is concluded in the past that the measurement of TAPSE offers a simple echocardiographic parameter which reflects right ventricular ejection fraction [12]. Furthermore, tissue Doppler is highly reproducible.
Decline in right ventricular function after cardiac surgery
Decline in RVF after cardiac surgery has been reported over two decades ago. Qualitative analyses demonstrated an impairment of RV filling, contraction and overall function during surgery itself, which persisted immediately after cardiac surgery. This decline was seen irrespective of the use of cardiopulmonary bypass. In patients with atrial septal defects, RVF after open-heart surgery has been compared with percutaneous closure [13]. This study showed a decline in RVF after surgery which was not seen after percutaneous closure.
Studies on the recovery of declined RVF are contradictory. The notion has been put forward that the decline of RVF is due to recovery of stunned myocardium implying RVF decline may be a relatively short-lived phenomenon [14]. However, a retrospective study, in which the median follow-up after coronary artery bypass surgery was 10 years, suggested that the effects of surgery on the RV may be long-lasting [3].
Potential mechanisms
Various hypotheses regarding the pathogenesis of the selective decline in RVF after cardiac surgery have been put forward. However, no clear cause is known. We based our hypotheses on best data available. We performed an observational study, so we were unable to determine the underlying mechanism. Prospective studies are needed to elucidate this phenomenon. Potentially, acute ischemia or air emboli cause the decline in RVF. In our study decline in RVF was significantly associated with post-operative maximal myocardial creatine kinase level and maximal troponin T level, indicating ischemia. It is possible that the thin-walled RV may be more susceptible to dysfunction secondary to inflammation or effusions post-operatively [3]. These effusion may result from local tissue damage or from a systemic inflammatory response.
Another theory evolves around cytokines. In a state of cardiopulmonary bypass the body releases cytokines which initiate inflammation and pulmonary vasoconstriction [15, 16]. One of these cytokines is endothelin-1 [15, 16]. Endothelin-1 has a vasoconstrictive effect on the pulmonary arterioles and might consequently influence RV afterload [17]. Endothelin-1 appears to play a modest role in healthy organisms, but probably plays a major role in many pathophysiological states [18]. Bond et al. demonstrated that patients with elevated post-operative endothelin-1 levels stayed longer on the intensive care unit and had longer overall hospitalization [15]. In addition, elevated endothelin-1 levels were associated higher pulmonary artery pressures post-operatively in these patients.
Other hypothesis, such as peri-operative temperature variations and the deleterious effects of pericardial disruption on RV filling and function are plausible, but require further investigation. Another theory suggested pericardial adhesions that impair ventricular filling. However, the decline in RVF seen after cardiac surgery is very rapid [19] and interventions to prevent adhesion formation fails to preserve RV function [20]. Changes in RV geometrics in association with changes in inter-ventricular septal paradoxical motion might contribute to the effect of decline in RVF [21]. The RV is poorly protected during cardiopulmonary bypass as perfusion with cardioplegic solution is not optimal [22]. Nevertheless, decline in RVF exists even when off-pump coronary artery bypass grafting surgery is used and seems, therefore, not associated with cardioplegia techniques [23].
Clinical impact
Acute RV failure after cardiac surgery leading to major complications with high in-hospital mortality rates has been demonstrated before [8]. In our study, no relation was observed between the decline in RVF and frequency of right ventricular failure, stay in hours, inotropes dependence and fluid balance in the first 24 h on the intensive care unit. However, this does not mean that this decline in RVF is harmless. The relationship between poor RVF and adverse clinical outcomes has been shown before. Low tricuspid annulus acceleration and low RV systolic velocities are correlated with an increased risk of cardiac death or hospitalisation [24]. Studies in non surgery CHD patients have reported negative effects of declined RVF and consequently enlarged RV [4]. Declined RVF and enlarged RV are associated with exercise limitation, arrhythmias and sudden death [4]. The present study was not designed as an event study, but instead aimed to document the effects of cardiac surgery on RVF. Clinical impact of RVF decline may be difficult to demonstrate in patients who undergo cardiac surgery, as the beneficial effects of the procedure may mask any adverse effects.
We should not assume that RVF decline is unavoidable. Peri-operative myocardial protection and the reduction of RV afterload might minimize RVF decline. The RV is very sensitive to any increases in RV afterload [25]. Pulmonary vascular resistance (PVR), representing RV afterload, can peri-operatively increase up to nearly 4-fold [26]. Reducing RV afterload has been shown to improve RVF, in patients with chronic thrombo-embolic pulmonary hypertension before and after pulmonary thromboendarterectomy [27].
In a small study, peri-operative intervention on the endothelin-1 pathway with a selective endothelin-1 receptor antagonist (ERA) led to a significant decrease in PVR compared to the control group [26]. Another randomized study using peri-operative infusion of selective ERA showed significantly lower plasma levels of inflammatory cytokines (tumor necrosis factor and soluble tumor necrosis factor receptors) in the post-operative period compared to a control group [16].
Limitations
Our study demonstrates a long lasting decline in RVF after cardiac surgery in adult patients with CHD. However, observational studies always carry risks. As there is a significant background variation with time, it can be difficult to be as certain that an intervention is the cause of any changes. However, the decline in RVF after cardiac surgery is so large and so consistent that it seems convincingly it is caused by the cardiac surgery. Furthermore, a similar effect was shown in patients who underwent coronary artery bypass grafting surgery. This suggests that the relationship with cardiac surgery is not an accidental one, but causative and long lasting. Another limitation is TAPSE as an indicator of RVF. In patients who underwent tricuspid valve surgery, TAPSE might be reduced when anuloplasties were performed due to the rigid ring. However, RV S’ decreased and MPI increased in these patients. Moreover, of all patients who had a TAPSE measurement available at late follow-up, a relative big group of six (26%) patients underwent tricuspid valve surgery. However, we note the concomitant RV S’ decrease and MPI increase observed. In addition, MPI might have pseudonormalized in the presence of high RV pressure, which might have occurred in patients with a pulmonary valve stenosis. Finally, of a substantial group of patients echocardiography data was missing. This may have introduced bias. However, missing data is inevitable in a retrospective study and the observed decline of RVF in patients who had data available was large and consistent.
Conclusion
CHD patients have a decline in RVF directly after cardiac surgery, regardless the side of surgery. Although a gradual improvement, complete recovery was not seen 18 months post-operatively.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Boldt J, Zickmann B, Thiel A, Dapper F, Hempelmann G. Age and right ventricular function during cardiac surgery. J Cardiothorac Vasc Anesth. 1992;6:29–32. doi: 10.1016/1053-0770(91)90041-Q. [DOI] [PubMed] [Google Scholar]
- 2.Mishra M, Swaminathan M, Malhotra R, Mishra A, Trehan N. Evaluation of right ventricular function during CABG: transesophageal echocardiographic assessment of hepatic venous flow versus conventional right ventricular performance indices. Echocardiography. 1998;15:51–58. doi: 10.1111/j.1540-8175.1998.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 3.Yadav H, Unsworth B, Fontana M, Diller GP, Kyriacou A, Baruah R, Mayet J, Francis DP. Selective right ventricular impairment following coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 2010;37:393–398. doi: 10.1016/j.ejcts.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Warnes CA. Adult congenital heart disease importance of the right ventricle. J Am Coll Cardiol. 2009;54:1903–1910. doi: 10.1016/j.jacc.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Davlouros PA, Niwa K, Webb G, Gatzoulis MA. The right ventricle in congenital heart disease. Heart. 2006;92:27–38. doi: 10.1136/hrt.2005.077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, Monin JL, Pierard LA, Badano L, Zamorano JL. European association of echocardiography recommendations for the assessment of valvular regurgitation. part 1: aortic and pulmonary regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:223–244. doi: 10.1093/ejechocard/jeq030. [DOI] [PubMed] [Google Scholar]
- 7.Lai WW, Gauvreau K, Rivera ES. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24:691–698. doi: 10.1007/s10554-008-9314-4. [DOI] [PubMed] [Google Scholar]
- 8.Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: i Anatomy, physiology, and assessment. Anesth Analg. 2009;108:407–421. doi: 10.1213/ane.0b013e31818f8623. [DOI] [PubMed] [Google Scholar]
- 9.Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr. 2006;7:430–438. doi: 10.1016/j.euje.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Tulevski II, Dodge-Khatami A, Groenink M, van der Wall EE, Romkes H, Mulder BJ. Quantitative assessment of the pressure and volume overloaded right ventricle: imaging is a real challenge. Int J Cardiovasc Imaging. 2002;18:41–51. doi: 10.1023/A:1014315329767. [DOI] [PubMed] [Google Scholar]
- 11.Tulevski II, Romkes H, Dodge-Khatami A, van der Wall EE, Groenink M, van Veldhuisen DJ, Mulder BJ. Right ventricular function in congenital cardiac disease: noninvasive quantitative parameters for clinical follow-up. Cardiol Young. 2003;13:397–403. [PubMed] [Google Scholar]
- 12.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 13.Hanséus KC, Björkhem GE, Brodin LA, Pesonen E. Analysis of atrioventricular plane movements by Doppler tissue imaging and M-mode in children with atrial septal defects before and after surgical and device closure. Pediatr Cardiol. 2002;23:152–159. doi: 10.1007/s00246-001-0039-6. [DOI] [PubMed] [Google Scholar]
- 14.Alam M, Hedman A, Nordlander R, Samad B. Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J. 2003;146:520–526. doi: 10.1016/S0002-8703(03)00313-2. [DOI] [PubMed] [Google Scholar]
- 15.Bond BR, Dorman BH, Clair MJ, Walker CA, Pinosky ML, Reeves ST, Walton S, Kratz JM, Zellner JL, Crumbley AJ, Multani MM, Spinale FG. Endothelin-1 during and after cardiopulmonary bypass: association to graft sensitivity and postoperative recovery. J Thorac Cardiovasc Surg. 2001;122:358–364. doi: 10.1067/mtc.2001.114936. [DOI] [PubMed] [Google Scholar]
- 16.Ford RL, Mains IM, Hilton EJ, Reeves ST, Stroud RE, Crawford FA, Jr, Ikonomidis JS, Spinale FG. Endothelin-A receptor inhibition after cardiopulmonary bypass: cytokines and receptor activation. Ann Thorac Surg. 2008;86:1576–1583. doi: 10.1016/j.athoracsur.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valerio JGC, Coghlan JG. Bosentan in the treatment of pulmonary arterial hypertension with the focus on the mildly symptomatic patient. Vasc Health Risk Manag. 2009;5:607–619. doi: 10.2147/VHRM.S4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeill JR. Role of endothelin in regulation of resistance, fluid-exchange, and capacitance functions of the systemic circulation. Can J Physiol Pharmacol. 2003;81:522–532. doi: 10.1139/y03-016. [DOI] [PubMed] [Google Scholar]
- 19.Wranne B, Pinto FJ, Hammarström E, St Goar FG, Puryear J, Popp RL. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. 1991;66:435–442. doi: 10.1136/hrt.66.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindström L, Wigström L, Dahlin LG, Arén C, Wranne B. Lack of effect of synthetic pericardial substitute on right ventricular function after coronary artery bypass surgery. an echocardiographic and magnetic resonance imaging study. Scand. Cardiovasc J. 2000;34:331–338. doi: 10.1080/713783121. [DOI] [PubMed] [Google Scholar]
- 21.Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, Alamanni F, Pepi M. Is right ventricular systolic function reduced after cardiac surgery? a two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 22.Christakis GT, Buth KJ, Weisel RD, Rao V, Joy L, Fremes SE, Goldman BS. Randomized study of right ventricular function with intermittent warm or cold cardioplegia. Ann Thorac Surg. 1996;61:128–134. doi: 10.1016/0003-4975(95)00933-7. [DOI] [PubMed] [Google Scholar]
- 23.Diller GP, Wasan BS, Kyriacou A, Patel N, Casula RP, Athanasiou T, Francis DP, Mayet J. Effect of coronary artery bypass surgery on myocardial function as assessed by tissue Doppler echocardiography. Eur J Cardiothorac Surg. 2008;34:995–999. doi: 10.1016/j.ejcts.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Meluzin J, Spinarová L, Hude P, Krejcí J, Kincl V, Panovský R, Dusek L. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr. 2005;18:435–444. doi: 10.1016/j.echo.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Hines R. Right ventricular function and failure: a review. Yale J Biol Med. 1991;64:295–307. [PMC free article] [PubMed] [Google Scholar]
- 26.Joffs C, Walker CA, Hendrick JW, Fary DJ, Almany DK, Davis JN, Goldberg AT, Crawford FA, Jr, Spinale FG. Endothelin receptor subtype A blockade selectively reduces pulmonary pressure after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;122:365–370. doi: 10.1067/mtc.2001.114938. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard DG, Malouf PJ, Gurudevan SV, Auger WR, Madani MM, Thistlethwaite P, Waltman TJ, Daniels LB, Raisinghani AB, DeMaria AN. Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. JACC Cardiovasc Imaging. 2009;2:143–149. doi: 10.1016/j.jcmg.2008.10.012. [DOI] [PubMed] [Google Scholar]