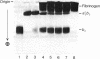
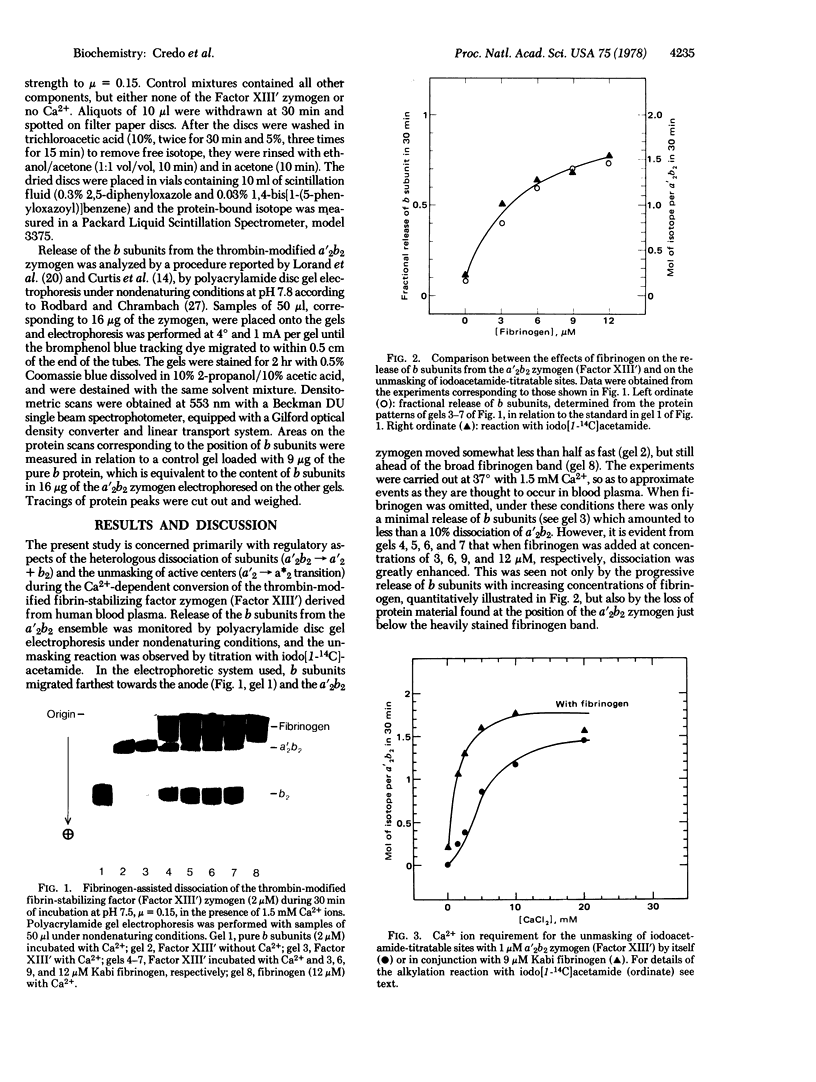
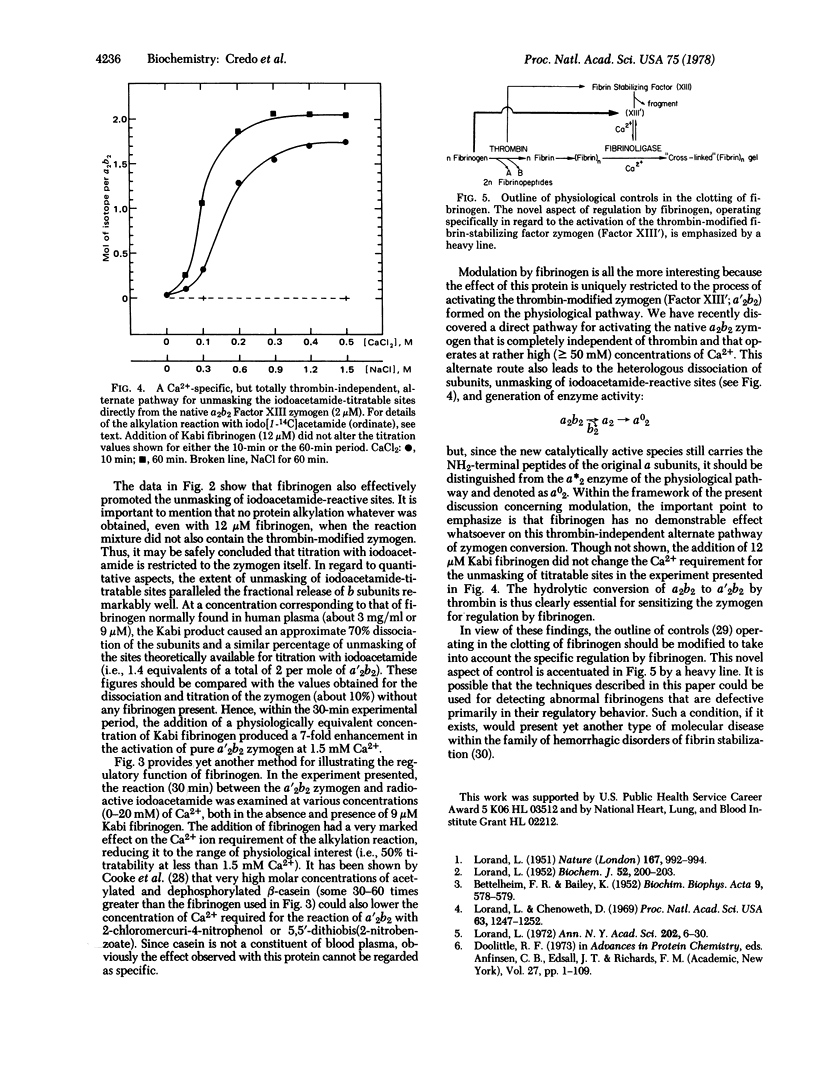
Abstract
Fibrinogen displays a regulation of considerable physiological significance by lowering the Ca2+ requirement for the conversion of the fibrin-stabilizing factor (Factor XIII) zymogen to the range of concentrations of this ion found in plasma. Fibrinogen modulates both Ca2+-dependent steps in the complex process of zymogen activation, involving the heterologous dissociation of subunits of the thrombin-modified zymogen (Factor XIII') species : formula: (see text) and the unmasking of iodoacetamide titratable sites during generation of transamidating activity : formula: (see text). It is interesting that a thrombin-independent pathway of zymogen activation : formula: (see text), which we found to operate at Ca2+ concentrations above 50 mM, is not affected by the presence of fibrinogen. Regulation by fibrinogen thus appears to be specific for controlling only the physiological pathway of zymogen conversion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETTELHEIM F. R., BAILEY K. The products of the action of thrombin on fibrinogen. Biochim Biophys Acta. 1952 Nov;9(5):578–579. doi: 10.1016/0006-3002(52)90213-8. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Lewis M. S., Folk J. E. Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem. 1974 Feb 10;249(3):940–950. [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. Calcium and the assays of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):71–78. doi: 10.1042/bj1410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Pestell T. C., Holbrook J. J. Calcium and thiol reactivity of human plasma clotting factor XIII. Biochem J. 1974 Sep;141(3):675–682. doi: 10.1042/bj1410675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974 Aug 27;13(18):3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Lorand L. Fibrin-stabilizing factor (factor XIII). Methods Enzymol. 1976;45:177–191. doi: 10.1016/s0076-6879(76)45018-8. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Stenberg P., Chou C. H., Gray A., Brown K. L., Lorand L. Titration and subunit localization of active center cysteine in fibrinoligase (thrombin-activated fibrin stabilizing fector). Biochem Biophys Res Commun. 1973 May 1;52(1):51–56. doi: 10.1016/0006-291x(73)90952-2. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Cooke R. D., Kingston I. B. The amino acid sequence around the reactive cysteine residue in human plasma Factor XII. Biochem J. 1973 Dec;135(4):901–903. doi: 10.1042/bj1350901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- LORAND L. 'Fibrino-peptide'; new aspects of the fibrinogen-fibrin transformation. Nature. 1951 Jun 16;167(4259):992–993. doi: 10.1038/167992a0. [DOI] [PubMed] [Google Scholar]
- LORAND L. Fibrino-peptide. Biochem J. 1952 Oct;52(2):200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAND L., KONISHI K. ACTIVATION OF THE FIBRIN STABILIZING FACTOR OF PLASMA BY THROMBIN. Arch Biochem Biophys. 1964 Apr;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- Lorand L., Chenoweth D. Intramolecular localization of the acceptor cross-linking sites in fibrin. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1247–1252. doi: 10.1073/pnas.63.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L. Fibrinoligase: the fibrin-stabilizing factor system of blood plasma. Ann N Y Acad Sci. 1972 Dec 8;202:6–30. doi: 10.1111/j.1749-6632.1972.tb16319.x. [DOI] [PubMed] [Google Scholar]
- Lorand L., Gray A. J., Brown K., Credo R. B., Curtis C. G., Domanik R. A., Stenberg P. Dissociation of the subunit structure of fibrin stabilizing factor during activation of the zymogen. Biochem Biophys Res Commun. 1974 Feb 27;56(4):914–922. doi: 10.1016/s0006-291x(74)80275-5. [DOI] [PubMed] [Google Scholar]
- Lorand L. Introduction to clotting and lysis in blood plasma. Methods Enzymol. 1976;45:31–37. doi: 10.1016/s0076-6879(76)45006-1. [DOI] [PubMed] [Google Scholar]
- Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968 Jan;7(1):208–223. doi: 10.1021/bi00841a026. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iwanaga S., Suzuki T., Mikuni Y., Konishi K. Amino acid sequence of the peptide released from bovine factor XIII following activation by thrombin. Biochem Biophys Res Commun. 1974 May 7;58(1):250–256. doi: 10.1016/0006-291x(74)90919-x. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The subunit structures of human plasma and platelet factor XIII (fibrin-stabilizing factor). J Biol Chem. 1971 Sep 25;246(18):5851–5854. [PubMed] [Google Scholar]
- Smoake J. A., Song S. Y., Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Distribution and developmental changes of the enzyme and its protein activator in mammalian tissues and cells. Biochim Biophys Acta. 1974 Apr 25;341(2):402–411. doi: 10.1016/0005-2744(74)90233-2. [DOI] [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry. 1974 Feb 12;13(4):750–756. doi: 10.1021/bi00701a018. [DOI] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]