Abstract
Consequences of age on the effects of hyperbaric oxygen (HBO) on bone marrow (BM) derived stem cells and progenitors (SCPs) are largely unknown. We treated 2- and 18-month old C57BL/6 female mice by HBO. Hematopoietic stem cells and progenitors, enumerated as colony-forming units in culture, were doubled only in peripheral leukocytes and BM cells of young mice receiving HBO. In old mice colony-forming unit fibroblast numbers, a measure of mesenchymal stromal cells (MSCs) from BM, were high but unaffected by HBO. To further explore this finding, in BM-MSCs we quantified the transcripts of adipocyte early-differentiation genes peroxisome proliferator-activated receptor-γ, CCAAT/enhancer binding protein-β and fatty-acid binding protein 4; these transcripts were not affected by age or HBO. However, osteoblast gene transcripts runt-related transcription factor 2, osterix (OSX) and alkaline phosphatase (AP) were twofold to 20-fold more abundant in MSCs from old control mice relative to those of young control mice. HBO affected expression of osteoblast markers only in old MSCs (OSX gene expression was reduced by twofold and AP expression was increased threefold). Our data demonstrate the impact of aging on the response of BM SCPs to HBO and indicate the potentially different age-related benefit of HBO in wound healing and tissue remodeling.
Keywords: Aging, Hyperbaric oxygen, Hematopoietic progenitor cells, Mesenchymal stromal cells
Background
Allogeneic bone marrow (BM) transplantation (BMT) results in more deaths, more tissue injury and higher pro-inflammatory response in old mice and humans than in the young (Ordemann et al. 2002). Advanced age is accompanied by a marked decrease in the number of CD34+ hematopoietic stem cells and progenitors (HSCPs) and attenuated lymphoid differentiation (Lansdorp et al. 1993; de Haan and Van Zant 1999; Lee et al. 2005). These phenomena suggest that self-renewal and proliferative potential of hematopoietic cells are diminished with age.
Among modalities studied for the potential to mitigate the problems associated with BMT is treatment by hyperbaric oxygen (HBO), a method effective in therapy that requires tissue regeneration (Neuman and Thom 2008). HBO influences tissues by different mechanisms, including modulation of the inflammatory response after BMT (Xiao-Yu et al. 2008) and mobilization of vasculogenic and HSCPs into circulation (Thom et al. 2006; Milovanova et al. 2009).
Although older subjects are more likely to require the benefit of HBO, the role of age on the effectiveness of HBO has not been explored. The need for understanding the role of aging is buttressed by the adverse events caused by the standard methods of HSCPs mobilization by chemotherapeutics and/or growth factors that increase the risk of acute arterial thrombosis, angina, sepsis, and death (Takahashi et al. 1999) in the elderly much more than in the young (Nomellini et al. 2009). The effects of age on the putative HBO-effects on mesenchymal stromal cells (MSCs) and their differentiation potential are unknown. Consequently, we studied the effects of HBO on HSCPs and MSCs in a murine model.
Methods
Animals
Pathogen-free young (2 months) and old (18 months) female C57BL/6 mice from the National Institute of Aging colony at Harlan Laboratories (Indianapolis, IN) were maintained in an environmentally controlled facility at Mayo Clinic for at least one week prior to experiments. Immediately after death, mice were dissected and organs screened for visible tumors and/or gross abnormalities, but none was found. All experimental protocols followed the guidelines in “Principles of Laboratory Animal Care” (NIH publication No. 86-23, revised 1996) and were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Normobaric and HBO treatment
Mice (three to four animals per cage) were placed in an animal hyperbaric chamber (Mechidyne Systems, Inc. Houston, TX). Pressure of pure medical grade oxygen was increased to 2.8 atm absolute (ATA; 283.7 kPa) in the course of 6.5 min. After 90 min of HBO, pressure was reduced to 1.0 ATA (101.3 kPa) in 5.0 min (Quirinia and Viidik 1996; Thom et al. 2006). Control mice were exposed to air or normobaric oxygen to test the evidence that neither pure normobaric oxygen nor hyperbaric air had any effect on HSCPs numbers in circulation (Thom et al. 2006). The animals were exposed to five consecutive daily HBO treatments. Eighteen hours after the last treatment the mice were killed by CO2 inhalation. The results shown are representative of three independent experiments.
Blood cell count
EDTA-anticoagulated blood was drawn from the right ventricle. Complete blood cell counts were quantified using a Hemavet 850FS cell counter (Drew Scientific, Oxford, CT).
Colony-forming cell assays
Individual hematopoietic colony-forming units in culture (CFU-C) were enumerated in peripheral blood and BM cells. A leukocyte suspension was prepared from EDTA-anticoagulated peripheral blood by lysing erythrocytes with ammonium chloride. BM was obtained by flushing femora with α-minimum essential medium containing 2% fetal bovine serum (FBS). One million leukocytes or 2 × 105 BM cells were plated in 35-mm dishes containing methylcellulose and growth factors (MethoCult GF M3434 assay; StemCell Technologies, Vancouver, Canada) according to manufacturer’s protocol and placed at 37°C in humidified air containing 5% CO2. At day 12, colonies were counted and evaluated using an inverted microscope.
MSC frequency was evaluated by colony-forming unit fibroblast (CFU-F) assay using the complete MesenCult mouse medium 05501 (StemCell Technologies) according to manufacturer’s protocol. One million leukocytes or BM cells were plated in duplicate for each mouse and incubated in six-well plates (2.0 mL/well). Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C. After 10 to 13 days in culture, the medium was decanted, adherent colonies washed with PBS twice, air-dried for 5 min, covered with methanol and incubated at room temperature for 5 more minutes. Then methanol was decanted, colonies were air dried for 5 min and the well covered with Giemsa Staining Solution. After 5 min, the wells were washed with water and air-dried. Colonies were counted using an inverted microscope.
Isolation and culture of splenic macrophages
Splenic macrophages were isolated by plastic adherence (Boehmer et al. 2005; Gomez et al. 2010). Spleens were aseptically removed and the cells disassociated by passing through a nylon mesh in RPMI 1640 medium, supplemented with 5% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM glutamine (culture medium; GIBCO-BRL, Grand Island, NY). Following red blood cell lysis with ACK Lysis Buffer (Invitrogen, Carlsbad, CA), white blood cells were counted in a hemocytometer; their viability was determined by trypan blue exclusion. Two million cells/well were seeded in 96-well plates in 200 μL of culture medium. After incubation for two hours at standard tissue culture conditions, non-adherent cells were aspirated and discarded; adherent cells were washed twice with warm phosphate buffer saline. This method resulted in adherent cells that were 98% positive for Mac-3 and Di-I-acetylated low-density lipoprotein uptake (Faunce et al. 1998). Adherent cells were treated in 200 μL of culture medium containing 100 ng/mL LPS from Escherichia coli 0111:B4 (Sigma, St. Louis, MO). Supernatants were collected after 18 h and stored at −80°C.
Measurement of pro-inflammatory cytokines
Concentrations of tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) in macrophage supernatants were measured by commercial ELISA kits (OptEIA; BD Pharmingen, San Diego, CA) according to manufacturer’s instructions. The lower detection limit of the kits was 15.6 pg/mL.
Real-time PCR for early differentiation markers
BM cells were cultured in complete MesenCult mouse medium 05501 for 10 days; the medium was replaced twice a week. Total RNA was isolated using TRIzol Reagent (Invitrogen) and quantified with a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). One microgram of RNA was reverse transcribed with Superscript III Reverse Transcriptase (Invitrogen). Following reverse transcription, real-time PCR was performed using LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics, Indianapolis, IN). The forward and reverse primers were designed using the PrimerTime qPCR Assay (Integrated DNA Technologies, Skokie, IL). Primer sequences are shown in Table 1. Each primer master mix consisted of one forward and one reverse primer (10 μM each), SYBR Green I Master Mix (2× concentration), and sterile water. Five μL of the cDNA was aliquoted to each well of the LightCycler 480 Multiwell Plate (Roche Diagnostics); 15 μL of the primer master mix was added to cDNA. Each reaction was run in duplicate in a final volume of 20 μL. In the LightCycler 480 real-time PCR apparatus, the pre-incubation at 95°C took five min and was followed by 45 amplification cycles at 95°C for 30 s and then 60°C for 30 s. The results for individual genes were normalized based on the expression of the housekeeping gene TATA-binding protein (Syed et al. 2010) run in the same plate. Data are expressed as the factor (“fold”) of change relative to the expression in young control animals.
Table 1.
Primer sequences employed in analysis of transcript levels for early markers of adipocytic and osteoblastic differentiation of mesenchymal stromal cells
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| PPAR-γ | GAGGAGTCCCTTCCCTCATC | TCCTCGAAGGTTAAGGCTGA |
| C/EBP-β | GTTTCGGGACTTGATGCAAT | GCCCGGCTAGACAGTTACAC |
| aP2 | GAATGTGTTATGAAAGGCGTGAC | AAATTTCCATCCATCCAGGCCTCT |
| RUNX2 | CCAGGAAGACTGCAAGAAGG | TCCTGCATGGACTGTGGTTA |
| OSX | GGAGGTTTCACTCCATTCCA | TAGAAGGAGCAGGGGACAGA |
| AP | CACAGATTCCCAAAGCACCT | GGGATGGAGGAGAGAAGGTC |
| TBP | CTCAGTTACAGGTGGCAGCA | CAGCACAGAGCAAGCAACTC |
Markers of adipocytic differentiation: PPAR-γ Peroxisome proliferator-activated receptor-γ, C/EBP-β CCAAT/enhancer binding protein-β, aP2 fatty-acid binding protein 4. Markers of osteoblastic differentiation: RUNX2 runt-related transcription factor 2, OSX osterix, AP alkaline phosphatase. Housekeeping gene: TBP TATA-binding protein
Statistical analysis
Groups consisted of no more than four mice. The total number of animals studied was 13, 3 and 7 young mice in normobaric air, normobaric oxygen, and HBO, respectively and 9, 3 and 10 old mice in normobaric air, normobaric oxygen, and HBO, respectively. As the number of animals in study groups differed, we analyzed the data using a two-way ANOVA of logarithmically transformed data. p values were obtained as pair-wise comparisons between group means using the Fisher’s Least Significant Difference method. Because the overall global p value was 0.009, the Fisher’s Protected Least Significant Difference method provided correct control over type I error (false positives); hence, the pair-wise p values less than 0.05 could be considered statistically significant.
Results
HBO affects circulating blood cells in young, but not old mice
To determine the effects of age and HBO on circulating cells, we exposed mice to pure oxygen at 2.8 ATA for 90 min on each of five consecutive days and compared the effects to control mice breathing normobaric air (Table 2). HBO-treated young mice exhibited an increase in lymphocyte and monocyte counts relative to control air-breathing mice (p < 0.05), but blood counts of total white blood cells, basophils, eosinophils, red blood cells and platelets did not differ. Peripheral blood counts in old air-breathing mice were similar to young air-breathing mice, except for elevated monocyte and platelet levels (p < 0.05). However, HBO did not affect the cell densities in old mice.
Table 2.
Effects of age on circulating blood cells in young and old mice
| Young | Old | |||
|---|---|---|---|---|
| Air | HBO | Air | HBO | |
| White blood cells | 5.5 ± 0.6 | 8.5 ± 0.6 | 6.2 ± 1.1 | 6.1 ± 0.8 |
| Lymphocytes | 4.2 ± 0.5 | 6.5 ± 0.4* | 3.8 ± 0.7 | 4.0 ± 0.4 |
| Monocytes | 0.2 ± 0.0 | 0.3 ± 0.0* | 0.3 ± 0.0** | 0.3 ± 0.0 |
| Neutrophils | 1.2 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.4 | 1.3 ± 0.1 |
| Basophils | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Eosinophils | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Red blood cells | 7.8 ± 0.1 | 8.0 ± 0.4 | 7.9 ± 0.1 | 7.3 ± 0.3 |
| Platelets | 648 ± 26.8 | 722 ± 40.9 | 954 ± 24.1** | 900 ± 74.5 |
Young and old mice were treated by 100% oxygen at 2.8 ATA for 90 min on five consecutive days. Controls breathed normobaric air. Complete blood counts were determined using a Hemavet 850FS cytometer (DREW Scientific, Oxford, CT). All cell numbers are expressed as thousands per μL and shown as mean ± SEM. * Significant compared to animals breathing normobaric air. ** Significant compared to young animals breathing normobaric air. For lymphocytes, p < 0.05 for the difference between young controls and young hyperbaric oxygen (HBO)-treated animals; for monocytes, probabilities for comparisons of control and HBO-treated young mice and young control mice versus old control were < 0.05 as was the probability for the difference of platelet numbers between young controls and old controls
Effects of age on hematopoietic progenitors
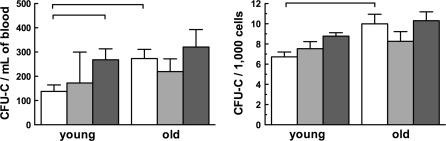
Because HBO, but not normobaric oxygen, mobilizes SCPs in young mice (Thom et al. 2006; Milovanova et al. 2009), we explored the effects of HBO on SCP mobilization in old mice by determining the number of hematopoietic colony forming units in culture (CFU-C). For additional control, we exposed the animals to normobaric oxygen under conditions otherwise identical to those exposed to HBO. We found no effect of normobaric oxygen compared to normobaric air in the young and old (p > 0.05), but the blood of old control mice yielded twice as many CFU-Cs as the blood of young mice (p < 0.05; Fig. 1). HBO treatment doubled this number in young mice (p < 0.05); the increase in old mice did not reach statistical significance. The number of CFU-Cs from BM cells was higher in old control mice than in young control mice (p < 0.05; Fig. 1). HBO effect on BM cells was similar to the effect on circulating cells: it affected young mice (p < 0.05), but not old mice. Again, normobaric oxygen had no effect in either group (p > 0.05). These results suggest that aging increases the number of CFU-C-generating cells on its own and that HBO has no effect on these cells in old mice. As normobaric oxygen did not affect proliferation as the ultimate measure of cell function, we focused further studies on the effects of HBO.
Fig. 1.
Hyperbaric oxygen and age affect hematopoietic propensity of circulating and bone marrow cells. Mice breathed normobaric air (controls; white bars) or normobaric oxygen (lightly shaded bars) or hyperbaric oxygen (HBO; heavily shaded bars) for five consecutive days. Eighteen hours after final HBO treatment, animals were killed and hematopoietic stem cells and progenitors (HSCPs) were quantified as colony-forming units in culture (CFU-C) by peripheral blood leukocytes (left panel) and bone marrow (BM) cells (right panel). Mice were treated in groups of no more than four. The total number of animals studied was 13, 3 and 7 young mice in normobaric air, normobaric oxygen, and HBO, respectively and 9, 3 and 10 old mice in normobaric air, normobaric oxygen, and HBO, respectively. Pooled data for all mice that underwent the same treatment are shown as mean values ± SEM. Horizontal lines indicate the differences among groups that are statistically significant (p < 0.05)
HBO suppresses expression of proinflammatory cytokines by activated macrophages
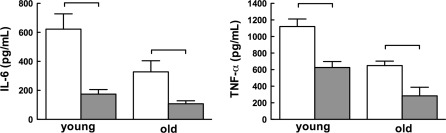
Most CFU-Cs developed from blood and BM cells were CFU-granulocyte/macrophage (data not shown); this indicated that most cells were committed to differentiation into myeloid cells. This observation compelled us to determine the effects of HBO on myeloid cell function. We isolated splenic macrophages, activated them by lipopolysaccharide (LPS) and measured expression of IL-6 and TNF-α, cytokines previously tested in studies of HBO effects (Lahat et al. 1995; van den Blink et al. 2002; Benson et al. 2003; Fildissis et al. 2004; Buras et al. 2006; Thom 2009). We found no detectable cytokines in the media conditioned by LPS-free macrophages (data not shown), but in the presence of LPS macrophages expressed high levels of IL-6 and TNF-α (Fig. 2 ). In agreement with others, macrophages from old mice secreted less of these cytokines (Renshaw et al. 2002; Boehmer et al. 2005; Chelvarajan et al. 2005; Gomez et al. 2010). HBO reduced the mean concentration of IL-6 by 72% in the media conditioned by macrophages from young mice (p < 0.05) and by 67% by macrophages from old mice (p < 0.05). TNF-α secretion was similarly reduced by 44% (p < 0.05) and 56% (p < 0.05), respectively. These results show that systemic HBO exposure reduces in vitro pro-inflammatory cytokine expression in macrophages from young and old mice alike.
Fig. 2.
Hyperbaric oxygen suppresses secretion of inflammatory cytokines. Splenic macrophages obtained from young and old mice shown in Fig. 1 were cultured for 18 h with lipopolysaccharide (LPS; 100 ng/mL). Supernatants were assayed for interleukin-6 (IL-6) (left panel) and TNF-α (right panel). Data are shown as mean values ± SEM for all mice that underwent the same treatment. Horizontal lines indicate the differences among groups that are statistically significant (p < 0.05)
Effect of age on HBO on MSCs
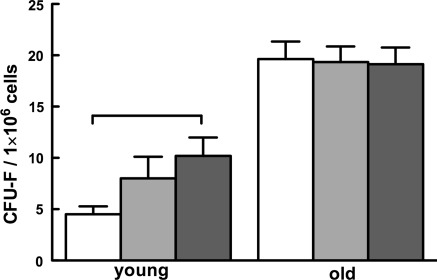
Based on the recent interest in the potential role of MSCs in regenerative medicine, we set to determine if HBO affects this major group of cells as well. Consequently, we quantified the MSC frequency in peripheral leukocytes and BM by the CFU-F assay. We found no CFU-F-generating cells in the blood of young air-breathing and HBO-treated mice. However, leukocytes of old air-breathing mice yielded low, but detectable numbers of CFU-Fs (1.0 ± 0.8 CFU-F/1 × 106 leukocytes); the number of CFU-Fs did not change by HBO (p > 0.05).
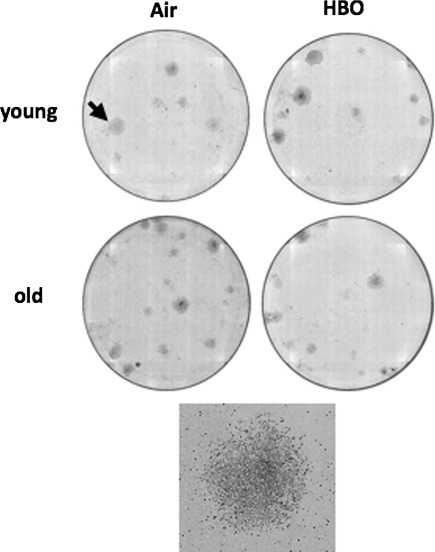
In distinction to leukocytes, BM cells gave rise to typical CFU-Fs (Fig. 3). One million BM cells of young mice yielded 4.5 ± 0.8 CFU-Fs (Fig. 4). Surprisingly, HBO doubled the number of CFU-Fs in young mice (p < 0.05). Less unexpected was the finding that the number of CFU-F-generating cells in the BM of old mice was fourfold higher relative to young animals (p < 0.051), while HBO did not affect that number (p > 0.05). These findings suggest that HBO affects BM MSC and that the number of CFU-F-generating cells increases with age.
Fig. 3.
Age and HBO affect bone marrow-derived MSCs. MSC frequency in leukocytes and BM was evaluated by colony formation. One million leukocytes or BM cells from animals breathing normobaric air or treated by HBO were cultured for up to 13 days and stained by Giemsa when CFU-fibroblast (CFU-F) colonies were counted under the microscope. Shown are representative Giemsa-stained plates of BM-derived CFU-Fs; the arrow in the upper left plate points to the randomly selected colony that is magnified in the square panel to demonstrate the morphology typical for CFU-Fs
Fig. 4.
Hyperbaric oxygen and age affect the frequency of mesenchymal stromal cells in bone marrow. Mesenchymal stromal cells (MSCs) from mice in Fig. 1 were quantified as CFU-fibroblast (CFU-F) colonies. Horizontal lines indicate the difference between groups that is statistically significant (p < 0.05)
HBO modifies expression of early differentiation genes in MSCs
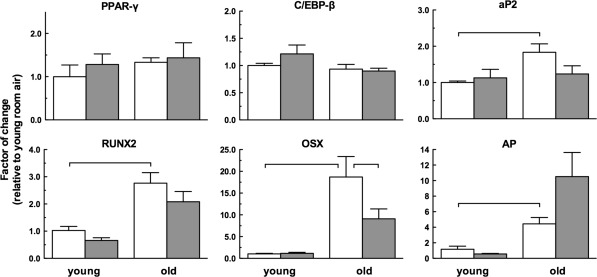
To gain insight whether age-related and HBO-related changes in CFU-F-generating cells in Figs. 3 and 4 are reflected in the differentiation potential of these cells, we analyzed transcript levels of genes associated with early differentiation into adipocytes and osteoblasts. Genes of adipocytic differentiation (Gregoire et al. 1998) included the peroxisome proliferator-activated receptor-γ (PPAR-γ); CCAAT/enhancer binding protein-β (C/EBP-β); and fatty-acid binding protein 4 (aP2); while those of osteogenic differentiation (van Straalen et al. 1991; Karsenty 2008) were the runt-related transcription factor 2 (RUNX2); osterix (OSX); and alkaline phosphatase (AP). We measured transcript levels by quantitative PCR and for each transcript normalized the data of all groups to its expression level in young air-breathing mice. Interestingly, expression of PPAR-γ and C/EBP-β was affected neither by age nor by HBO, while the level of aP2 transcripts in old mice was elevated relative to young mice (p < 0.05); the reduction by HBO did not reach statistical significance (Fig. 5). In contrast, osteoblastic differentiation markers were affected both by age and HBO. The levels of gene transcripts in old mice were threefold, 19-fold and fourfold higher for RUNX2, OSX and AP, respectively (p < 0.05 for all three transcripts).
Fig. 5.
Hyperbaric oxygen modulates transcription of osteogenic, but not adipogenic differentiation genes in bone marrow mesenchymal stromal cells of old mice. Transcripts associated with early adipogenic differentiation [peroxisome proliferator-activated receptor-γ (PPAR-γ); CCAAT/enhancer binding protein (C/EBP)-β; and, fatty acid binding protein 4 (aP2)]; and early osteogenic differentiation [runt-related transcription factor 2 (RUNX2); osterix (OSX); and, alkaline phosphatase (AP)] were quantified by real-time PCR in bone marrow mesenchymal stromal cells obtained from mice in Fig. 1. Horizontal lines indicate the differences among groups that are statistically significant (p < 0.05)
While the effects of HBO on transcript levels were marginal in young mice, they were prominent in the old. There was no change in RUNX2 levels, levels of transcripts for OSX were reduced by one half (p < 0.05) in contrast to doubling of AP transcripts (not statistically significant). Overall, these results indicate age and HBO affect the expression of osteoblastic early differentiation genes in MSCs.
Discussion
The purpose of this work is to probe the effects of senescence on the ability of HSCPs and MSCs to respond to HBO. The impetus for the study is the need to understand if efficacy of the standard HBO therapy protocols depends on age and, hence, the protocols might have to be tailored to patient age. Based on the general understanding of differences in HSCPs and MSCs between the young and the old (Lansdorp et al. 1993; de Haan and Van Zant 1999; Lee et al. 2005; Sethe et al. 2006; Gazit et al. 2008; Roobrouck et al. 2008), the underlying hypothesis has been that HBO may affect HSCPs and MSCs in an age-dependent manner.
For this study we selected 2-month old and 18-month old mice. Based on the allometric relationships between body size and expected longevity, the younger group would be roughly equivalent to human adolescents, while the older to an octogenarian (Lindstedt and Calder 1981). In this study we focused on a single gender of mice (females); additional experiments in males should define the effect of age and gender on HBO-mediated mobilization of SCPs. Some gender-specific differences might be anticipated based on gender-related response of old mice to stressors (Gomez et al. 2009).
Using the selected model of senescence, we confirmed that the levels of CFU-C-forming cells in the blood and marrow of old mice were higher than in the young mice. A pertinent finding is that HBO affected the proliferative capacity of HSCPs and MSCs in the young, but not in old mice, in line with the observation that systemic hyperoxia enhances proliferation of human (Thom et al. 2006) and murine BM-derived HSCPs (Thom et al. 2006; Milovanova et al. 2009). After a single HBO treatment Thom et al. detected a twofold increase in CFU-Cs in murine peripheral blood leukocytes and BM cells. They found HSCP mobilization by measuring expression of CD34 and Sca-1 in peripheral blood cells (Thom et al. 2006; Milovanova et al. 2009); our results confirm the latter finding by colony forming and demonstrate that the cells are functional and add considerable weight to evidence that HBO mobilizes HSCPs.
Mobilizing agents induce rapid emigration of stem cells from the BM. However, under most circumstances mobilization requires cell proliferation in the BM (Nakamura et al. 2004). The higher basal level of HSCPs in old mice could be associated with the age-related expansion of the SCP pool that originates in the increased autonomous cell renewal capacity (Gazit et al. 2008). In contrast, old age is linked to a decline in SCP function (Sudo et al. 2000; Geiger and Van Zant 2002), including less efficient hematopoiesis (Chen et al. 2000), reduced differentiation potential (Rossi et al. 2005) and impaired homing (Xing et al. 2006). Altogether, these studies suggest that age-related reduction of HSCP activity could be offset by the increase in HSCP numbers (Gazit et al. 2008). Our results are in line with other phenomena in aging, e.g., the pro-inflammatory phenotype, even in the absence of insult (“inflamm-aging”) that results in an inherent activation rendering the system less able to respond to perturbation (de Haan and Van Zant 1999; Franceschi et al. 2000; Gomez et al. 2005).
Our finding that HBO does not mobilize HSCPs in the old is at variance with mobilization by granulocyte-colony stimulating factor (Xing et al. 2006) that reduces HSCP adhesion to BM stroma even in the old (Geiger and Van Zant 2009). This difference suggests qualitatively and/or quantitatively distinct mechanisms in HBO-mediated and G-CSF-mediated HSCP mobilization in old mice. In response to HBO, nitric oxide levels increase and trigger a cascade mechanism that mobilizes SPCs from BM by the release into circulation of cytokines such as cKit ligand (stem cell factor, SCF; Thom et al. 2006). The effects of aging on this mechanism are unknown, but HSPC mobilization by chemotherapeutics and/or growth factors increases the risk of acute arterial thrombosis, angina, sepsis, and death (Takahashi et al. 1999) in the old much more than in the young (Nomellini et al. 2009). Consequently, it will be important to clarify the effects of aging on the components of the proposed HBO-mediated cascade involved in SCP mobilization. Additional insight might be gained from studies of the effects of aging and HBO on the expression of SDF-1 and CXCR4, the critical regulators of SPC function and homing, and of nitric oxide-mediated mechanism of HSCP mobilization by HBO.
Aging increases the differentiation potential of myeloid cells [reviewed in (Linton and Dorshkind 2004)], but results also in their functional defects [reviewed in (Gomez et al. 2008)]. Our studies confirmed that LPS-activated macrophages from old mice express less proinflammatory cytokines IL-6 and TNF-α relative to young cells (Renshaw et al. 2002; Boehmer et al. 2005; Chelvarajan et al. 2005; Gomez et al. 2010). Interestingly, HBO reduced IL-6 and TNF-α expression to a similar extent in the young and the old, in line with some (Benson et al. 2003; Buras et al. 2006; Thom 2009), but not all observations (Lahat et al. 1995; van den Blink et al. 2002; Fildissis et al. 2004). Apparently, HBO can control acute inflammation following injury and sepsis (Huang et al. 2005; Oter et al. 2005; Neuman and Thom 2008), but its effects on increased morbidity and mortality associated with aberrant inflammatory responses in the old are largely unknown and, therefore, very much worthy of additional research. While HBO-mediated mobilization of HSCPs into circulation has been known for some time (Thom et al. 2006), HBO effects on MSCs have not been studied. We found no CFU-F-forming cells among peripheral blood leukocytes under any condition, except in old mice where we did detect low levels of these cells. The significance of this observation will require further study. However, interestingly in BM, HBO increased the CFU-F number in young mice.
MSCs and the effects of aging on them (Sethe et al. 2006; Roobrouck et al. 2008) are gaining interest because of the putative applicability of MSCs in regenerative therapy and tissue engineering (Parekkadan and Milwid 2010). We found detectable levels of circulating MSCs (measured by the ability to generate CFU-F colonies) only in old air-breathing mice. In the BM, these cells were detectable in young mice and at a fourfold higher level in old mice. This result differs from the reports of age-related decrease in the total number of CFUs or the absence of age effects [reviewed in (Sethe et al. 2006)]. Further MSC evaluation by techniques like flow cytometry may be needed to detect fractions of MSCs especially sensitive to the culture conditions used in CFU-F assays and to settle the discord among the results.
The effects of oxygen tension on MSCs have not been clearly established either. On one side, hypoxia decreased proliferation and differentiation of BM-derived MSCs (Mohyeldin et al. 2010). On the other side, HBO-stimulated bone formation and healing (processes involving MSCs) did not enhance the osteogenic ability of MSCs in spinal fusion in a rabbit model (Fu et al. 2010). We found that HBO increased the number of CFU-Fs in the young BM. We demonstrated that young MSCs retained their differentiation potential (measuring adipogenic differentiation; data not shown), but that HBO had no effect on this process. This observation adds to those who suggest that in young mice HBO can change the numbers of BM-derived MSCs without affecting their differentiation potential (Fu et al. 2010).
Lineage commitment of MSCs is controlled by an array of intracellular and extracellular signals in the BM milieu, including the activation of phenotype-specific transcription factors (Karsenty 2008). Our results show that transcript levels of genes involved in adipogenic differentiation were unaffected by age, but that transcript levels of early osteoblastic differentiation genes were higher in old MSCs. These results differ from description of higher levels of aP2 transcripts (an adipocyte differentiation gene) in MSCs from old mice (no gender specified) and lower levels of transcripts of osteoclast-specific transcription factors including RUNX2 (Moerman et al. 2004). However, our findings are in line with the expression of significantly higher transcript levels for all osteoblast differentiation marker genes in hematopoietic lineage-negative (lin−) cells from old female C57/BL6 mice (Syed et al. 2010). Since lin− cells are highly enriched in osteoblastic progenitors that can mineralize in vitro, form bone in vivo, and express bone-related genes, our results are comparable with the demonstration of aging effects on osteoblast progenitors (Syed et al. 2010). Quantifying the baseline expression of osteogenic and adipogenic differentiation markers provided a snapshot of the effects of aging and HBO on the MSC differentiation potential. Expression of these genes during MSC differentiation coupled with other differentiation assays (e.g., in vitro mineralization and in vivo bone formation for osteoblasts and in vitro lipid formation for adipocytes) will provide more definite evidence for the effects of age and HBO on lineage commitment by MSCs.
Observation of MSC-restricted expression of early differentiation genes affected by HBO in old mice only warrants in-depth studies of the involved intracellular signaling networks. Study of signaling involving extracellular mediators, in particular the transforming growth factor-β, bone morphogenetic proteins 2 and 4 with a known role in controlling adipocyte versus osteoblast formation (Gregoire et al. 1998; Karsenty 2008) will help define the mechanisms involved in the effects of HBO in MSCs, particularly in the old.
In a murine model of aging we studied the effects of HBO on HSCPs and MSCs. We demonstrated that aging affected the ability of mice to mobilize HSCPs from the BM and suggest an age-related defect in mobilization by HBO; mechanistic aspects of this phenomenon require further elucidation. Our results suggest that HBO therapy protocols may have to be adjusted by age or eventually individualized. In addition, these results indicate the potentially different benefit of HBO in wound healing and tissue remodeling in the old and the young.
Acknowledgments
The authors are indebted to Mr. Richard Clarke, Baromedical Research Foundation, Columbia, South Carolina, for the generous loan of the hyperbaric chamber; to Dr. Nancy Nadon, Biological Resources Branch, National Institute on Aging for allowing access to mice from the NIH rodent colonies; Dr. Paul Claus, Division of Preventive, Occupational and Aerospace Medicine, Mayo Clinic, for leadership and discussions; Messrs. Robert Fuqua and Jaime Campos, Mayo Clinic Hyperbaric and Altitude Medicine, for help with the hyperbaric chamber; Ms. Peggy A. Bulur for help with experiments; Dr. Vernon S. Pankratz, Biomedical Statistics, Mayo Clinic, for help with statistical analysis, and Dr. Virginia Shapiro, Department of Immunology, Mayo Clinic, for the access to Hemavet 850FS cytometer. Division of Preventive, Occupational and Aerospace Medicine, Department of Internal Medicine, Mayo Clinic provided financial support for this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AP
Alkaline phosphatase
- aP2
Fatty-acid binding protein 4
- BM
Bone marrow
- BMT
Bone marrow transplant
- C/EBP-β
CCAAT/enhancer binding protein-β
- CFU-C
Colony-forming units in culture
- CFU-F
Colony-forming unit fibroblast
- HSCP
Hematopoietic stem cells and progenitors
- HBO
Hyperbaric oxygen
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- MSC
Mesenchymal stromal cell
- OSX
Osterix
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- RUNX2
Runt-related transcription factor 2
- SCP
Stem cells and progenitor
- TNF-α
Tumor necrosis factor α
References
- Benson RM, Minter LM, Osborne BA, Granowitz EV. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol. 2003;134:57–62. doi: 10.1046/j.1365-2249.2003.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/S0301-472X(99)00157-5. [DOI] [PubMed] [Google Scholar]
- de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Glucocorticoids protect against suppression of T cell responses in a murine model of acute ethanol exposure and thermal injury by regulating IL-6. J Leukoc Biol. 1998;64:724–732. doi: 10.1002/jlb.64.6.724. [DOI] [PubMed] [Google Scholar]
- Fildissis G, Venetsanou K, Myrianthefs P, Karatzas S, Zidianakis V, Baltopoulos G. Whole blood pro-inflammatory cytokines and adhesion molecules post-lipopolysaccharides exposure in hyperbaric conditions. Eur Cytokine Netw. 2004;15:217–221. [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fu TS, Ueng SW, Tsai TT, Chen LH, Lin SS, Chen WJ. Effect of hyperbaric oxygen on mesenchymal stem cells for lumbar fusion in vivo. BMC Musculoskelet Disord. 2010;11:52. doi: 10.1186/1471-2474-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45:218–224. doi: 10.1053/j.seminhematol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- Geiger H, Van Zant G. Lympho-hematopoietic stem cells and their aging. In: Fulop T Jr, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on immunosenescence. Basic understanding and clinical applications. New York: Springer Press; 2009. pp. 573–588. [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CR, Nomellini V, Kovacs EJ. Immunosenescence and sex hormones. In: Fulop T Jr, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on immunosenescence. Basic understanding and clinical applications. New York: Springer Press; 2009. pp. 799–831. [Google Scholar]
- Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V, Kovacs EJ. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediat Inflamm. 2010;2010:475139. doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Huang TY, Tsai PS, Wang TY, Huang CL, Huang CJ. Hyperbaric oxygen attenuation of lipopolysaccharide-induced acute lung injury involves heme oxygenase-1. Acta Anaesthesiol Scand. 2005;49:1293–1301. doi: 10.1111/j.1399-6576.2005.00812.x. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- Lahat N, Bitterman H, Yaniv N, Kinarty A, Bitterman N. Exposure to hyperbaric oxygen induces tumour necrosis factor-alpha (TNF-alpha) secretion from rat macrophages. Clin Exp Immunol. 1995;102:655–659. doi: 10.1111/j.1365-2249.1995.tb03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Fletcher MD, Tarantal AF. Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatr Res. 2005;58:315–322. doi: 10.1203/01.PDR.0000169975.30339.32. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL, Calder WA. Body size, physiologycal time, and longevity in homeothermic animals. Q Rev Biol. 1981;56:1–16. doi: 10.1086/412080. [DOI] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Milovanova TN, Bhopale VM, Sorokina EM, Moore JS, Hunt TK, Hauer-Jensen M, Velazquez OC, Thom SR. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol. 2009;106:711–728. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tajima F, Ishiga K, Yamazaki H, Oshimura M, Shiota G, Murawaki Y. Soluble c-kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol. 2004;32:390–396. doi: 10.1016/j.exphem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Neuman TS, Thom SR. Physiology and medicine of Hyperbaric Oxygen Therapy. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordemann R, Hutchinson R, Friedman J, Burakoff SJ, Reddy P, Duffner U, Braun TM, Liu C, Teshima T, Ferrara JL. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oter S, Edremitlioglu M, Korkmaz A, Coskun O, Kilic D, Kisa U, Yaren H, Bilgic H. Effects of hyperbaric oxygen treatment on liver functions, oxidative status and histology in septic rats. Intensive Care Med. 2005;31:1262–1268. doi: 10.1007/s00134-005-2701-6. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirinia A, Viidik A. The impact of ischemia on wound healing is increased in old age but can be countered by hyperbaric oxygen therapy. Mech Ageing Dev. 1996;91:131–144. doi: 10.1016/0047-6374(96)01782-4. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, Oursler MJ, Khosla S. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res. 2010;25:2438–2446. doi: 10.1002/jbmr.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/8462. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106(3):988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–H1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- van den Blink B, van der Kleij AJ, Versteeg HH, Peppelenbosch MP. Immunomodulatory effect of oxygen and pressure. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:193–197. doi: 10.1016/S1095-6433(01)00547-5. [DOI] [PubMed] [Google Scholar]
- van Straalen JP, Sanders E, Prummel MF, Sanders GT. Bone-alkaline phosphatase as indicator of bone formation. Clin Chim Acta. 1991;201:27–33. doi: 10.1016/0009-8981(91)90021-4. [DOI] [PubMed] [Google Scholar]
- Xiao-Yu S, Lu-Ning S, Ning–Ning Z, Hai-Peng Z. Effect of Hyperbaric Oxygen on Acute Graft-Versus-Host disease after Allogeneic Bone marrow Transplantation. J Exp Hematol. 2008;16:623–626. [PubMed] [Google Scholar]
- Xing Z, Ryan MA, Daria D, Nattamai KJ, Van Zant G, Wang L, Zheng Y, Geiger H. Increased hematopoietic stem cell mobilization in aged mice. Blood. 2006;108:2190–2197. doi: 10.1182/blood-2005-12-010272. [DOI] [PMC free article] [PubMed] [Google Scholar]