Abstract
Neisseria meningitidis is a major cause of endemic cases and epidemics of meningitis and devastating septicemia. Although effective vaccines exist for several serogroups of pathogenic N. meningitidis, conventional vaccinology approaches have failed to provide a universal solution for serogroup B (MenB) which consequently remains an important burden of disease worldwide. The advent of whole-genome sequencing changed the approach to vaccine development, enabling the identification of potential vaccine candidates starting directly with the genomic information, with a process named reverse vaccinology. The application of reverse vaccinology to MenB allowed the identification of new protein antigens able to induce bactericidal antibodies. Three highly immunogenic antigens (fHbp, NadA and NHBA) were combined with outer membrane vesicles and formulated for human use in a multicomponent vaccine, named 4CMenB. This is the first MenB vaccine based on recombinant proteins able to elicit a robust bactericidal immune response in adults, adolescents and infants against a broad range of serogroup B isolates. This review describes the successful story of the development of the 4CMenB vaccine, with particular emphasis on the functional, immunological and structural characterization of the protein antigens included in the vaccine.
Keywords: Neisseria meningitidis B, reverse vaccinology, multicomponent vaccine, fHbp, NadA, NHBA
Introduction
Neisseria meningitidis is a major cause of meningitis and sepsis, two devastating diseases that can kill children and young adults within hours, despite the availability of effective antibiotics. It is a human-specific Gram-negative bacterium that is adapted to colonize the upper nasopharynx and spread directly from host to host, without requiring a reservoir outside humans. Carriage rates are very variable among human populations and depend on different factors such as age, coincident respiratory tract infection and social contacts; in Europe and the United States point-prevalence carriage rates have been estimated to range from 10 to 35% in young adults [1, 2]. In a small but significant number of infections the bacterium traverses the epithelium and reaches the bloodstream, causing septicemia. From the blood, meningococcus is able to cross the blood-brain barrier and infect the meninges, causing meningitis [3–5]. The ability to colonize and cause disease are dependent on the ability of the meningococcus to evade the human immune system [6].
With the exception of isolated case reports [7–9], a capsule made up of complex polysaccharides surrounds all currently known disease-causing meningococci and is one of the essential meningococcal attributes for pathogenesis. The capsular polysaccharide (CPS) inhibits bacterial adhesion by masking the action of meningococcal adhesins but in contrast is known to be important for bacterial survival in the blood [10]. The precise structure of the CPS defines the serogroup, the highest serological typing order in meningococcus. Indeed, N. meningitidis can be classified in 13 serogroups on the basis of the chemical composition of the CPS. However, more than 95% of total cases of invasive disease are caused by five major serogroups: A, B, C, Y and W135. Recently, a sixth serogroup, serogroup X, has also revealed an epidemic potential [11]. The distribution of the serogroups varies globally; large epidemics in Africa have been generally associated with serogroup A meningococci (see review in this issue by T. Aguado). Serogroup B meningococci, which are generally absent in sub-Saharan Africa, are the primary concern in industrialized countries. Outbreaks of serogroup C meningococcal disease occurs worldwide, especially in adolescents and young adults [12] and serogroup Y meningococci have emerged as an important cause of disease in North America in the past 10 years and more recently in Europe [1].
Although meningococcal disease in certain industrialized nations, including the United States, are at historic lows [13], the emergence of strains with epidemic potential can rapidly alter this scenario. Moreover, changes in serogroup circulation are unpredictable and can occur very quickly [14]. In light of these observations, vaccines conferring broad protection against N. meningitidis are of global importance. Vaccines against serogroups A, C, Y and W135 were developed in the 1960s by using the purified CPS as the antigens. More effective, second generation, conjugated vaccines have now been introduced, in which CPS components are conjugated to carrier proteins such as CRM197 - a non-toxic mutant of the diphtheria toxin [15]. The first conjugate vaccines targeting N. meningitidis were introduced in the United Kingdom in 1999 to control the ongoing hyperendemic level of disease in infants and children caused by group C meningococci. Monovalent MenC conjugate vaccines have shown immunogenicity and safety in all age groups. Routine vaccination programs have substantially reduced serogroup C disease in United Kingdom and other countries including Spain, Italy, Greece, France, Canada, Australia, Brazil, and Argentina [14]. Following the success of MenC vaccines, quadrivalent meningococcal conjugate vaccines, containing the polysaccharide from serogroups A, C, Y and W-135 conjugated to a protein carrier, have been developed. These vaccines offer the potential to broaden protection against meningococcal disease beyond that offered by monovalent MenC conjugate vaccines. Unlike the earlier polysaccharide vaccines, the quadrivalent meningococcal conjugate vaccine conjugated with CRM197, MenACWY-CRM, has been shown to be immunogenic in all age groups, including infants [16]. The chemical composition of the serogroup B CPS is a polysialic acid that resembles a molecule present on human tissue surfaces, thus making a serogroup B CPS-based vaccine poorly immunogenic and also presenting a possible cause of auto-immunity [17, 18], although this concern has recently been challenged [19]. Consequently, an alternative approach was pursued in order to develop a N. meningitidis vaccine protective against serogroup B (MenB).
Over the last forty years, great efforts have been directed towards the identification of meningococcus B antigens as the basis of a new vaccine. However, the high sequence variability of these proteins among different MenB strains represents a serious obstacle to the production of a globally effective anti-MenB vaccine [20–22]. Currently, the only licensed vaccines against serogroup B disease employ outer membrane vesicles (OMVs). Vaccines based on OMVs have been developed by taking advantage of the fact that meningococcus naturally produces and releases vesicles composed by outer membrane proteins, lipids and periplasmic components. A variety of ‘tailor-made’ MenB OMV vaccines have been developed and licensed to control regional epidemics that in contrast to endemic disease, tend to be caused by a single clone of N. meningitidis. OMV vaccines have been used in Norway [23], Cuba [24], Chile [25] and New Zealand [26]. They are able to induce protective antibodies against the homologous strain (i.e. the strain causing the epidemic and used to prepare the OMVs) in all age groups, and have proved successful in controlling epidemic disease in these countries [27]. However, the main limitation of OMV vaccines is that they are strain-specific and do not provide protection against heterologous strains. This is because the major protective antigen of the OMV-based vaccines is PorA which is the most abundant integral outer membrane protein and which is known to be highly variable across different isolates of N. meningitidis. For example, in the United States of America (USA), as many as 20 different PorA variants would be required to cover over 80% of invasive strains [28]. As a consequence there currently are no effective licensed vaccines currently available for the prevention of MenB disease, which is responsible for one third of meningococcal disease in the USA, and up to 80% of cases in Europe.
Second generation OMV vaccines have been developed in order to broaden the strain coverage. Meningococcal strains expressing six different PorA variants have been genetically engineered in order to produce the hexavalent PorA OMV vaccines [29]. More recently, several attempts have been made to produce multivalent OMV vaccines derived from meningococcal strains genetically manipulated to reduce LOS toxicity and increase expression of protein antigens such as Opc, fHbp and NadA [30–38]. However, since the use of multivalent OMV vaccines does not promise a simple universal solution, alternative approaches based on surface-exposed proteins were sought. Here we describe in detail the identification and characterization of the antigens ultimately included in the 4CMenB (4 Components against MenB) vaccine.
Discovery of MenB vaccine antigens by Reverse Vaccinology
The availability of whole genome sequences in the genomic era has radically changed the approach to vaccine development. The genome represents a list of virtually all the protein antigens that the pathogen can express at any time. It becomes possible to identify potentially surface-exposed proteins in a reverse manner, starting from the genome rather than from cultures of the microorganism [39]. The concept of reverse vaccinology was first applied to MenB. N. meningitidis is essentially an extracellular pathogen and the main protective response relies on circulating antibody. Complement-mediated bactericidal activity (measured by the serum bactericidal assay (SBA) using human complement) is the accepted correlate for in vivo protection and has been adopted in clinical trials of meningococcal vaccines as the surrogate for protection [40]. The sequence of the N. meningitidis virulent strain MC58 was determined by the shotgun strategy revealing over 2000 predicted proteins [41]. In order to identify novel vaccine antigens, the proteins were analyzed using bioinfomatics algorithms for their potential surface localization. Those proteins predicted to be surface-exposed or secreted were recombinantly expressed in Escherichia coli, purified and tested for their potential to induce bactericidal antibodies [42].
Each purified recombinant protein was used to immunize mice and the antibody response was analyzed by Western blot analysis using both total cell extracts and purified outer membrane proteins to verify protein expression. Surface localization of the target protein was confirmed by enzyme-linked immunosorbent assay (ELISA) and flow cytometry using intact, whole bacteria. Finally, the SBA was used to evaluate the complement-mediated killing activity of the antibodies. Of the 91 proteins found to be positive in at least one of these assays, 28 were able to induce antibodies with bactericidal activity.
The antigens selected by reverse vaccinology were prioritized based on their ability to induce broad protection (i.e., against a diverse collection of strains) as inferred by SBA or the ability of specific antibodies against the antigens to confer passive protection in the infant rat or mouse models [43]. The proteins that met these prioritization criteria and were ultimately selected were called Genome-derived Neisseria Antigens (GNA) 2132 (Neisserial Heparin Binding Antigen, or NHBA), GNA1870 (factor H binding protein, or fHbp) and GNA1994 (Neisseria adhesin A, or NadA) (Fig. 1). Two additional antigens, GNA2091 and GNA1030, were also selected because they induced protective immunity but only in some of the assays. The antigens were combined in a multicomponent vaccine with the aim of inducing better and broader protection. Moreover, in order to facilitate large-scale manufacturing of the vaccine, four of the selected antigens were combined as two fusion proteins. Among the several protein–protein fusions generated, the best performing combinations in terms of production and immunogenicity were NHBA plus GNA1030 and GNA2091 with fHbp. NadA did not perform well when fused to other proteins, probably due to disruption of its trimeric organization.
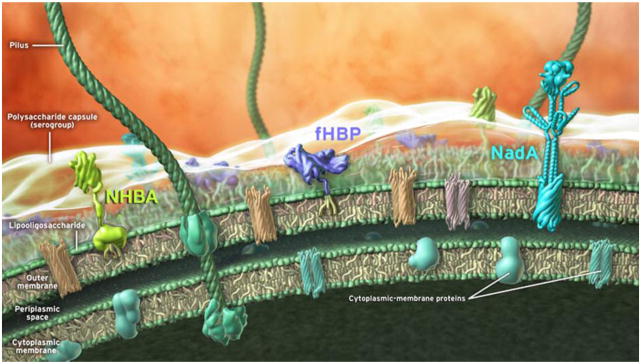
Figure 1. Schematic representation of the 4CMenB vaccine antigens on the surface of N. meningitidis.
The different bacterial compartments (outer membrane, periplasmic space, cytoplasmic membrane) and the main antigens identified through reverse vaccinology approach (NHBA, fHbp and NadA) are depicted. The representation of NHBA and fHbp in the picture is derived from the NMR structural data available and reported as cartoon. NadA is a model based on the structural homology with other members of the Oca family. Other components of the meningococcal membranes are also shown (pilus, polysaccharide capsule, lipooligosaccharide and integral inner and outer membrane proteins).
In order to assess fully whether the vaccine formulation developed was able to induce protection against a large proportion of the MenB strains, a large panel of clinical isolates representing as much as possible the diversity of the bacterial population was collected. Sera obtained by immunizing mice with the vaccine were tested in a bactericidal assay against a panel of 85 meningococcal strains. Preclinical characterization showed that the vaccine induced bactericidal antibodies against 78% of the strains [43].
The final vaccine formulation contains 50μg of each recombinant protein NHBA-GNA1030, NadA and GNA2091-fHbp (vaccine named ‘rMenB’). In addition, the 3 recombinant proteins were also formulated with 25μg of OMV-based vaccine MeNZB (from N. meningitidis strain NZ98/254, expressing PorA serosubtype P1.4) in order to provide broader serogroup B strain coverage, due to the PorA variant contained in the OMVs (vaccine named 4CMenB). Both vaccines, with or without OMVs, are formulated with aluminium hydroxide. The addition of the MeNZB component was driven by the positive results obtained with the OMV-based vaccine, which was shown to be safe and efficacious in the control of the clonal meningococcal serogroup B epidemic in New Zealand [26]. The rationale for combining the three main antigens was to increase the spectrum of vaccine coverage, minimizing the possibility of bacterial evasion and the emergence of selection mutants.
Protein antigens in the 4CMenB vaccine
The immunogenic protein antigens included in the 4CMenB vaccine (fHbp, NadA, and NHBA) have been functionally and immunologically characterized. Several studies have demonstrated their roles as virulence factors in meningococcal pathogenesis and, more importantly, they are the key immunogens in the vaccine (Table 1).
Table 1.
Relevant publications concerning the 4CMenB vaccine antigens
| Antigen | Content - main message | Reference |
|---|---|---|
| fHbp | ||
| Function | ||
| Discovery of GNA1870. Identification of the three genetic and immunogenic variants of GNA1870. | [44] | |
| GNA1870 is the fH binding protein of N. meningitidis | [50] | |
| Characterization of fHbp deletion mutants: they were sensitive to killing in ex vivo human whole blood and serum models of meningococcal bacteremia with respect to the isogenic wild-type strains. | [51] | |
| fHbp binds only human fH suggesting that N. meningitidis evolved to survive and grow only in human blood and explaining why this pathogen is strictly human specific. | [57] | |
| fHbp is regulated by oxygen limitation in a FNR-dependent manner. | [60] | |
| Immunogenicity | ||
| Characterization of 12 fHbp subvariants for their level of surface exposure and ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. | [69] | |
| Evaluation of the contribution of amino acid sequence variability of fHbp to the strain coverage of sub-variants 1. | [59] | |
| Identification of the fHbp protein region containing bactericidal epitopes. | [64] | |
| The ability of fHbp vaccines to elicit protective antibodies, can be enhanced if the antibody repertoire is of high avidity and includes fH-blocking activity. | [67] | |
| N. meningitidis strains can cause invasive disease even if they do not express fHbp. | [62] | |
| Structure | ||
| Structure determination by NMR spectroscopy and functional epitope mapping of the full-length fHbp. | [53, 112] | |
| Crystal structure of mature fHbp determined at 2 Å resolution. | [54] | |
| The crystal structure of the complex between human fH and fHbp of N. meningitidis. fHbp mimics host cell carbohydrates in binding fH. | [55] | |
| Rational designed of a chimeric fHbp able to induce cross-protective bactericidal antibodies against the three variants. | [56] | |
| NHBA | ||
| Function | ||
| The application of the reverse vaccinology approach identify the GNA2132 as a surface- exposed lipoprotein able to induce bactericidal antibodies in mice. | [42] | |
| NHBA antigen induces protective immunity in humans and it is recognized by sera of patients after meningococcal disease. The protein binds heparin in vitro through an Arg-rich region and this property correlates with increased survival of the unencapsulated bacterium in human serum. | [74] | |
| Immunogenicity | ||
| NHBA antiserum passively protected infant rats against meningococcal bacteremia after challenge with different meningococcus strains. | [65] | |
| Cooperative serum bactericidal activity exists between human Ab against antigens fHbp and NHBA. | [70] | |
| Structure | ||
| Structure of NHBA C-terminal domain solved by NMR spectroscopy; it consists of an eight strands β-barrel that closely resembles to the C-terminal domains of other surface-exposed lipoproteins (e.g. fHbp and TbpB). | [81] | |
| NadA | ||
| Function | ||
| Characterization of NadA as antigen able to induce bactericidal antibodies and protective immunity in the infant rat model. | [90] | |
| NadA is an adhesin and invasin of N. meningitidis. | [94] | |
| NadA expressed in a noninvasive Yersinia enterocolitica mutant strain binds β-1 integrin | [95] | |
| NadA expression is regulated by the NadR repressor, and may be induced during colonization of the oropharynx by the 4-hydroxyphenylacetic acid, a metabolite of aromatic amino acid catabolism that is secreted in saliva.. | [102] | |
| NadA interacts with human Heat Shock Protein 90 (Hsp90) interfering with the adhesion and invasion process mediated by NadA. | [96] | |
| Immunogenicity | ||
| Presence and conservation of NadA in carrier isolates of MenB. | [99] | |
| NadA is recognized by serum antibodies of young children convalescing after meningococcal disease. | [100] |
fHbp
Factor H binding protein (fHbp or GNA1870) was first identified as a surface-exposed lipoprotein during the screening of the MC58 genome [42, 44]. It was also identified independently as a potential vaccine antigen (designated as LP2086) using the entirely different approach of membrane fractionation [45] and became a component of another MenB vaccine in clinical development [46].
fHbp binds to human factor H, an inhibitor of the alternative complement pathway. Evasion of the human complement system is critical for meningococci to cause invasive disease. The observation that persons deficient in various complement components are highly predisposed to invasive meningococcal disease provides epidemiologic evidence for the role of complement in host defense against this infection [47, 48]. The contribution of factor H to the ability of meningococci to resist killing by human serum was first provided by Schneider and colleagues, who showed that depleting factor H from human serum resulted in enhanced killing of meningococci [49]. In a subsequent report, Madico et al identified the meningococcal ligand for factor H as GNA1870, which was subsequently renamed factor H binding protein (fHbp) [50]. Several reports thereafter have confirmed that deleting fHbp results in increased susceptibility of most strains of N. meningitidis to killing in either serum or in whole blood [51].
The three-dimensional (3D) solution structure of fHbp has been determined by nuclear magnetic resonance (NMR) spectroscopy [52, 53], and by X-ray crystallography [54]. The NMR and X-ray structures are very similar (pairwise root mean square deviation of the Cα trace is 2.6Å) and reveal that the protein is composed of two domains: an N-terminal domain of 8 beta-strands forming a highly curved anti-parallel beta-sheet (approximating a beta-barrel) and a C-terminal domain that is a well-defined beta-barrel of 8 anti-parallel beta strands. The 2 domains are connected by a short linker, which together with several predominantly hydrophobic inter-domain contacts, results in minimal orientational flexibility between the two domains (see Figure 2). Although there are over 300 different sequence variants of fHbp known, multiple-sequence alignments show that residues contributing to the hydrophobic cores of each domain are well conserved, suggesting that the 3D fold will be the same in all variants, even with sequence identity as low as 63% in some cases.
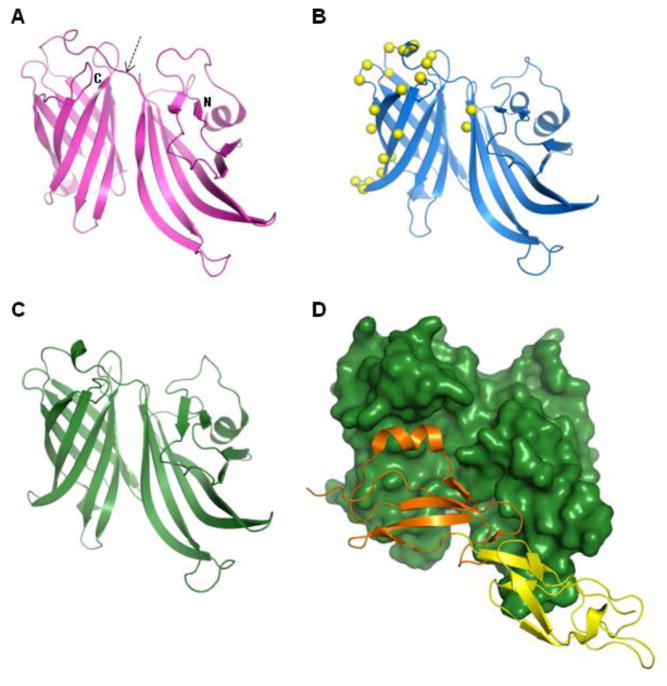
Figure 2. Structures of fHbp.
The currently known structures of the factor H binding protein (fHbp) from N. meningitidis serogroup B all share the same overall fold: two beta-barrel domains (one domain N-terminal and the other C-terminal, as indicated) connected by a short linker (arrowed), evident in panels A-C.(A) The NMR solution structure of a variant 1 fHbp from strain MC58 (PDB entry 2KC0) and (B) the crystal structure of an engineered form of fHbp variant 1 (strain MC58) containing 22 point mutations (PDB entry 2Y7S); the crystal structure of fHbp variant 1 has also been determined in complex with domains 6 and 7 of human Factor H (PDB entry 2W80): panel (C) shows the fHbp alone, panel (D) shows the same orientation of fHbp as a surface representation (dark green) with bound Factor H domains 6 (orange) and 7(yellow) shown as ribbons.
fHbp has a long N-terminal stretch containing a signal sequence and a lipo-box motif (LxxC). There are approximately 10 residues between the Cys and the folded N-terminal domain of fHbp and this linker region is highly flexible and has been removed from the protein in most structural studies. The structure of the lipidated form of the micelle-associated protein [53] shows no significant differences when compared with the other known structures, suggesting that this N-terminal region serves simply to anchor the protein on the exterior bacterial surface, thus exposing fHbp to the solution and making it accessible to the host immune system (Fig. 1).
The structure of fHbp in complex with domains 6 and 7 of factor H was determined by X-ray crystallography, providing detailed insights into the function of fHbp [55]. It is noteworthy that the sites in fH that interact with fHbp are similar to the glycosaminoglycan binding region in domains 6 and 7, thus revealing an example of host mimicry by the bacterial protein. The interaction buries a relatively large total surface area ~2860 Å2, involving both the N- and C-terminal domains of fHbp, creating a high-affinity binding (KD 5–30nM) [55, 56]. The binding to fHbp appears to be specific for human fH [57] and, interestingly the structure of the complex revealed that the amino acid differences between human and rhesus fH domain 6 lay in or around the contact interface between human fH and fHbp. The high affinity and the large surface area buried by the fH-fHbp interaction, and the human-specific nature of the fH-fHbp interaction might have implications for the immunogenicity of the fHbp antigen. The interaction between human fH and fHbp could potentially prevent recognition by the human immune system of surface-exposed fHbp epitopes that are targets for bactericidal antibodies. This hypothesis was tested in a transgenic BALB/c mouse that expressed human fH [58]. Transgenic human fH mice that were immunized with the wild-type fHbp variant 1 protein (capable of binding human fH) mounted a lower IgG response and showed significantly lower SBA titers than wild-type mice (murine fH does not bind to fHbp). Moreover, the human fH transgenic mice immunized with the R41S mutant fHbp protein (protein mutated at position Arg 41, which does not bind fH) displayed higher SBA titers than transgenic mice immunized with wild-type fHbp. Further, the level of human fH expressed by the transgenic mice correlated inversely with the SBA titers elicited by the wild-type fHbp protein, but not the R41S mutant. The clinical implications of these findings in humans immunized with fHbp remains unclear at this time.
The fHbp protein can be classified into three genetic and immunogenic variants: fHbp-1, fHbp-2 and fHbp-3 [44], which are not cross-protective, and can be further divided into subvariants fHbp-1.x, fHbp-2.x and fHbp-3.x. Sequence conservation within each variant ranges from 92 to 100%, while between the variants the conservation can be as low as 63%. This diversity has an important impact on the immunological properties of fHbp, since members of each variant induce a strong protective immune response against meningococcal strains carrying homologous alleles but are ineffective against strains that express distantly related variants [44]. In a different nomenclature scheme based on genetic information, the variants have been grouped into family A (variants 2–3) and family B (variant 1) [45]. In order to evaluate the cross-reactivity within fHbp variant 1 of antibodies induced by immunization with the vaccine subvariant fHbp-1.1, post-immunization sera from different age groups were tested in SBA against a panel of recombinant isogenic Neisseria strains expressing the same amounts of different fHbp variant 1 proteins. This approach enabled the potential interfering effects from the other vaccine antigens to be avoided. These data demonstrated a broad cross-reactivity in adults but sera from infants were more susceptible to amino acidic variation. Nevertheless, analysis of natural isolates expressing diverse fHbp variants showed that these strains are killed by infants’ sera, demonstrating the importance of the other components of the 4CMenB vaccine [59].
The level of fHbp expressed by N. meningitidis varies widely among strains and is regulated by oxygen limitation in a FNR-dependent manner [44, 60]. Although fHbp is expressed in nearly all N. meningitidis strains examined thus far [44, 61], a few strains that do not express fHbp have been identified recently [62]. In the context of N. meningitidis pathogenesis, this finding suggests that these isolates may have alternative systems for recruiting human fH in order to escape the host complement system. Of note, Neisseria surface protein A (NspA) that is expressed by all meningococcal strains examined for presence of this protein has also been shown to bind factor H [63]. The redundancy in factor H binding mechanisms by meningococci highlights the critical role of this strategy to limit complement activation on the bacterial surface. While immune pressure (for example, the presence of anti-fHbp antibodies that may be elicited following vaccination) could select for strains that do not express fHbp, this selection would occur at the expense of a key virulence mechanism generating bacterial strains missing an important virulence factor.
fHbp is able to induce bactericidal antibodies and confer passive protection in an infant rat model of bacteremia [44]. Several monoclonal antibodies have been generated against fHbp [64–66]; using a panel of monoclonal antibodies, Granoff and colleagues have characterized the functional basis for the activity of antibodies directed against fHbp. Interplay between the following variables likely contributes to the efficacy of anti-fHbp antibodies in the killing of meningococcus: i) the ability of the antibody to block fH binding to the bacterial surface (antibodies that block fH binding show greater bactericidal activity) [67]; ii) subclass of the Ab (murine IgG3 > IgG2a/b, human IgG3 > IgG1 > IgG2 [68]; iii) presence of antibodies directed against distinct fHbp epitopes (synergy is seen with combinations of anti-fHbp mAbs directed against non-competing epitopes) [65] and iv) the amount of fHbp expressed on the bacterial surface (epitope density) [68]. Moreover, it has been demonstrated that the bactericidal response induced by fHbp is dependent on amino acidic sequence diversity; different fHbp sub-variants induce different levels of cross reactivity suggesting that the selected fHbp subvariant is important for the breath and magnitude of bactericidal response [69]. Germane to fHbp vaccine development is the observation that antibodies directed against distinct fHbp epitopes can synergize to effect bactericidal activity, including strains that express very low amounts of antigen [65], which portends the need for a robust polyclonal response against multiple surface exposed bactericidal fHbp epitopes for broad bactericidal activity. In the context of the 4CMenB vaccine, it was demonstrated that a cooperative serum bactericidal activity exists between human antibodies against fHbp and another antigen of the vaccine, NHBA [70].
To identify the immunogenic regions of fHbp, a number of epitope mapping studies have been performed, using NMR spectroscopy [53, 71], scanning of the full-length protein via synthetic linear peptides [64] and other ELISA-based techniques [72, 73]. Interestingly, these studies revealed that amino acids contributing to the immunogenicity of variant 1 or variants 2 and 3 are located in non-overlapping areas, which is perhaps not surprising given the relatively high degree of sequence variability. This structural and functional variation between different variant families has important implications for vaccine design, since the choice of fHbp variant to include will determine the number of MenB strains that are effectively covered by a fHbp-based vaccine. The extensive structural and immunological characterizations of fHbp inspired a recent proof-of-concept study that demonstrated that the immunogenic regions from all three variants can be incorporated into a single molecule [56]. The overall structure of this engineered fHbp is not significantly changed from the variant 1 scaffold, but displays on its surface multiple immunogenic regions capable of inducing broad cross-protection against multiple strains of MenB harboring variants 2 and 3 (Fig 2B). It is conceivable that this successful approach could be applied in other cases where antigenic sequence variation is a hurdle in vaccine development.
NHBA
NHBA (Neisserial Heparin Binding Antigen, or GNA2132) is a surface-exposed lipoprotein also discovered by the application of reverse vaccinology [42]. The protein is specific for Neisseria species and recent studies have demonstrated that the protein binds heparin in vitro through an arginine-rich region [74]. Upon binding heparin, which is often used as a surrogate for host polyanions in in vitro assays, unencapsulated bacteria showed increased survival in human serum [74]. These data may point to a role for NHBA in protection of unencapsulated meningococci (as found in the nasopharynx) against complement. Heparin binding is a common feature of several bacterial virulence factors and vaccine components [75]. The binding of heparin to bacteria has been reported to increase resistance to the bactericidal activity of normal human serum [76]. The interactions between heparin and the complement system are complex and involve several of its component proteins [77], including complement inhibitors such as factor H, C4b-binding protein and vitronectin. It is conceivable that the establishment of an NHBA–heparin complex on the meningococcus cell surface could recruit complement inhibitors, which in turn act to prevent complement activation.
In vivo, NHBA is likely to bind glysoaminoglycans (such as heparan sulfate) that are present in mucosal secretions. Several bacterial adhesins are reported to bind heparin and heparan sulfate including the Nm Opc adhesin [78], N. gonorrheae Opa proteins [76], heparin-binding hemagglutinin adhesin of Mycobacterium tuberculosis [79] and the filamentous hemagglutinin of Bordetella pertussis, a component of licensed acellular pertussis vaccines [80]. In this context NHBA might contribute to the interaction of meningococcus with the host cells.
The primary amino acid sequence of NHBA comprises approximately 450 residues. The N-terminal region (residues 1 to ~230) is annotated as an intrinsically unfolded region by commonly used structure prediction algorithms. Between the N- and C-terminal regions is a central arginine-rich motif (residues 235–245 in strain 2996) that is implicated in binding to heparin [74]. Adjacent to the Arg-rich motif are two predicted cleavage sites for NalP and lactoferrin. The lack of order in the N-terminal region of NHBA has resulted in a shift of focus of structural studies to the C-terminal region. The structure of the C-terminal region of NHBA has been determined recently by NMR spectroscopy [81]. The NHBA structure spanning residues 246–428 exhibits a single 8-stranded anti-parallel beta-barrel (Figure 3) with high thermal stability. This fold resembles members of the lipocalin superfamily which are typically transporters of small hydrophobic molecules [82]. In particular, the NHBA fold clearly shows structural similarity to transferrin binding protein B (TbpB) [83] (Fig 3B) and to the C-terminal domain of fHbp (Fig 3C) which, together with a very low sequence similarity, suggests that the proteins may be evolutionarily related but are unlikely to share the same function.
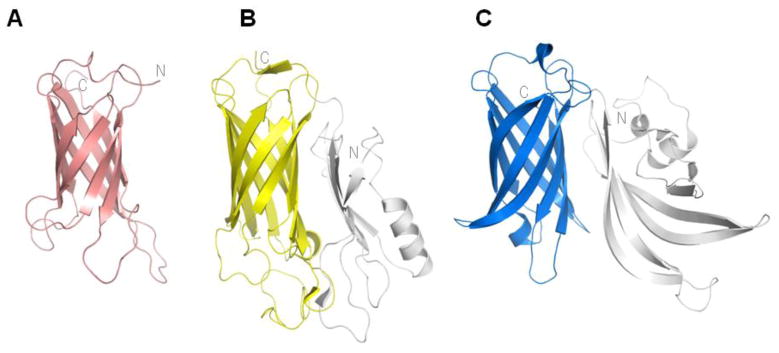
Figure 3. NHBA and structurally similar proteins.
(A) The structure of the conserved C-terminal region of NHBA (GNA2132) (PDB code 2LFU) from N. meningitidis serogroup B strain 2996, determined by NMR spectroscopy. The NHBA C-terminal domain structure (A) is architecturally related to the beta-barrel domains (shown as coloured regions) in the transferrin-binding protein B (TbpB) from Actinobacillus pleuropneumoniae (B)(PDB code 3PQS) and in fHbp (blue) C).
The gene coding for NHBA is present in all the N. meningitidis strains tested thus far [61, 84–86] encoding for a substantial number of peptides that have some association with clonal complexes and sequence types [61]. The 4CMenB vaccine contains the NHBA peptide 2, which is the most frequently expressed peptide based on molecular epidemiology studies recently conducted in different countries. Gene sequence analysis from genetically diverse group B strains reveals variable segments of NHBA although the N- and C-terminal regions are highly conserved [42, 61]. Serum antibodies from mice immunized with recombinant NHBA are able to bind to the surface of diverse N. meningitidis strains and elicit complement-mediated bactericidal activity [42, 43]. Moreover, anti-NHBA antibody elicited deposition of human C3b on the bacterial surface and passively protected infant rats against meningococcal bacteremia after challenge with different N. meningitidis strains [87]. NHBA is recognized by sera of patients after meningococcal disease [74], is able to induce bactericidal antibodies in human [74, 88] and has also been implicated in opsonophagocytic protection [89]. Preclinical studies suggest that antibodies elicited against the vaccine peptide are cross-protective against meningococcal strains expressing different NHBA peptides [43].
Since 4CMenB is a multicomponent vaccine, there might be the possibility of cooperativity of antibodies directed against the different antigens. The ability of antibodies against NHBA and fHbp to interact and augment protective immunity has been investigated recently and it was demonstrated that a cooperative serum bactericidal activity exists between human antibodies against fHbp and NHBA suggesting that a vaccine composed by multiple components might increase the breath of strain coverage [70].
NadA
NadA (Neisseria adhesin A or GNA1994) was identified as a member of the ‘Oca’ (oligomeric coiled-coil adhesin) family of bacterial trimeric autotransporter adhesins [90], which are characterized by a common secretory mechanism of the extracellular ‘passenger’ domains and their subsequent trimerization on the bacterial surface [91–93]. NadA trimers mediate adhesion to and entry into epithelial cells [94]. A putative protein receptor molecule which is differentially expressed by different human epithelial cell lines seems to mediate the binding of the trimeric NadA [94]. Recent analysis using a noninvasive Yersinia enterocolitica mutant strain expressing NadA revealed that β-1 integrin can act as a receptor for NadA [95]. NadA can also interact with human Heat Shock Protein 90 (Hsp90), which may interfere with the adhesion and invasion process mediated by NadA [96].
The form of NadA and a properly folded N-terminal ‘head’ domain are necessary for in vitro cell-binding activity. In order to preserve its functional organization and conformational structure, which could potentially also be implicated in an efficacious immune response, NadA was included in the MenB vaccine as single trimeric soluble protein, devoid of the membrane anchor domain.
Although the 3D structure of NadA is currently unknown, sequence analyses of the Oca family members provide some insight into NadA organization and function. An N-terminal leader peptide of 23 residues, not present in the mature vaccine antigen, is followed by a ‘head’ domain of around 70 residues. The head domain is followed by a long region of 200–250 residues that is strongly predicted to form a homotrimeric coiled-coil region. The extracellular elongated stalk and head domains confer the characteristic ‘drumstick’ appearance on the bacterial cell surface with specific adhesive capabilities localized within the head [97, 98]. In the native protein, the coil regions are followed by the C-terminal domain of 55 residues that share high sequence similarity (~60% identity) with a region of the Yersinia YadA and Moraxella UspA2 proteins [97]. In YadA, this C-terminal domain is made of four amphipathic beta-strands and acts as a membrane anchor, a structure and function likely to be conserved in NadA. Although it can be produced in a soluble and stable form, the large size of the trimeric, mature ectodomain of NadA (3 x ~300 residues) makes it a challenging target for NMR spectroscopy. Meanwhile, the long coiled-coil region comprising the majority of the ectodomain is likely to be rather flexible, which may explain why a crystallographic structure of NadA has not yet been determined.
The nadA gene is found in three out of the four known hypervirulent lineages of serogroup B and C strains, whereas it is mostly absent from carrier strains and is not found in N. gonorrhoeae nor in the commensal species N. lactamica and N. cinerea [90]. NadA is well conserved, and five variants have been identified [86, 90, 99]. The three main variants (NadA-1, NadA-2, and NadA-3) show highly conserved sequences and accordingly produce cross-bactericidal activity [90]. The other two variants (NadA-4 and NadA-5) are less common, and are associated with carrier strains [99] and with ST-269 strains (NadA-5) respectively [86]. Based on molecular epidemiology studies, nadA results more frequently associated with disease isolates than with carriage isolates [61, 86, 90, 99]. Antibodies raised against NadA are able to induce passive protection in infant rats challenged with different MenB strains [90]. Moreover, sera from convalescent patients after meningococcal disease are able to recognize NadA, suggesting that the protein is expressed and immunogenic during infection in humans [100]. The level of NadA expression is highly variable because nadA is subject to complex regulatory controls and is highly dependent on environmental signals [90, 101, 102]. NadA is expressed at different levels during growth, reaching a maximum in stationary phase [90]. Expression of NadA is phase variable because of a tetranucleotide tract (TAAA) located upstream of the nadA promoter [101]. Moreover, it has recently been shown that expression of NadA is controlled by a transcriptional regulator, NadR [102]. Under the in vitro conditions of growth of the bacteria used to perform serum bactericidal assay, NadA antigen is repressed by the NadR repressor protein. NadA expression can be de-repressed in the presence of 4-hydroxyphenyl acetate (4-HPA), an aromatic amino acid catabolite that is secreted in saliva [102]. Once repression mediated by NadR is relieved and NadA is expressed at high levels, strains normally resistant to killing by 4CMenB immune sera are rendered highly susceptible to killing in SBA [103].
Evaluating the immunogenicity of the vaccine components and the impact of the 4CMenB vaccine in strain coverage
The 4CMenB vaccine is a multicomponent vaccine able to elicit bactericidal antibodies against the four key antigens combined therein. The bactericidal activity of antibodies using human complement as the complement source is widely accepted as a surrogate marker of protection against meningococcal disease [40, 104]. In an SBA assay using immune sera derived from humans vaccinated with the 4CMenB, bacteria are killed by combinations of antibodies elicited by the different antigens. This feature of the SBA makes it difficult to tease out the contribution of each individual vaccine antigen to the overall function of the vaccine.
In order to assess the immunogenicity of each of the 4CMenB components, it was necessary to measure antigen-specific bactericidal antibodies through the use of N. meningitidis strains selectively recognized by antibodies against each of the antigen. The genetic diversity of the 4CMenB vaccine antigens was recently studied in large collections of N. meningitidis strains isolated in different countries around the world [61, 85, 105] and this information was taken into consideration in order to select MenB strains matched for the individual components. Then a solution phase interaction assay between the antigens of interest and the antibodies present in the vaccinees’ sera was developed. In this assay, antibodies directed against a specific antigen are sequestered by the addition of the purified recombinant antigen and thus are be available to bind to its cognate antigen on bacteria in the SBA assay. Using this competitive inhibition bactericidal assay we selected MenB strains that are predominantly killed by human antibodies that recognize specific protein components of the 4CMenB vaccine [88]. The strains identified to specifically assess functional antibody responses induced in humans by fHbp, NadA, NHBA and the immunodominant P1.4 PorA component of OMV NZ are virulent strains of serogroup B meningococci that were isolated from cases of invasive disease [88]. For example, strain H44/76 express homologous fHbp (variant 1.1) but heterologous NHBA and PorA and lacked NadA, thus any bactericidal activity observed can be ascribed to fHbp.
This minimal panel of 4 strains can be used to assess whether a sero-response has occurred to the vaccine and has two main advantages: it eliminates the need for region-specific panels which becomes prohibitive for a global vaccine and it also reduces the logistic challenge posed by the limited amount of serum available from infant clinical trials. Clinical data using this panel of strains showed that most human subjects made bactericidal antibodies against each of the major components demonstrating that each of the antigens are able to evoke a protective bactericidal response [106–109]. In a phase I trial, healthy adults received 3 doses of 4CMenB [109]. Pre- and post-vaccination sera were assayed for bactericidal activity using human complement and a hSBA titer ≥1:4 was used as the correlate of protection. 4CMenB demonstrated a strong immunogenicity against the three reference strains, H44/76, 5/99 and NZ98/254 (the M10713 strains was not available at the time of the study). In a study of laboratory workers that received 4CMenB followed by Men ACWY-CRM, bactericidal immune responses were evident after each dose of 4CMenB as assessed by hSBA against the reference strains demonstrating that the vaccine antigens were immunogenic in this population [107]. These analyses were subsequently confirmed in a phase IIb/III study conducted in adolescents aged 11–17 years where, after two doses given either 1, 2 or 6 months apart, 99–100% of subjects achieved an hSBA ≥ 1:4 against the reference strains.
Importantly, immunogenicity has also been demonstrated in infants, the age group at highest risk of disease. In Phase II studies, UK infants were enrolled and randomly assigned to receive the 4CMenB or rMenB (4CMenB without OMV-NZ) vaccine in two clinical trials. One study followed an early infant schedule at 2, 4, 6 and 12 months with a further cohort receiving a single dose at 12 months of age [106] and one followed a late infant schedule at 6, 8 and 12 months [108]. Following three doses, both vaccines were immunogenic against the indicator strains selected to assess the immunogenicity of the antigens. However, in both studies, broader responses were observed following immunization with 4CMenB than rMenB. These data have also been subsequently confirmed in larger phase III studies conducted in infants (see review in this issue by Dull and McIntosh).
Assessing the potential strain coverage of a multicomponent protein-based MenB vaccine is complicated due to the number of naturally occurring protein variants among different serogroup B strains that may vary not only in sequence and but also in expression. Conventional typing methods for meningococci, such as serotyping and serosubtyping of PorA or multilocus sequence typing, are not necessarily relevant to the novel antigens in the 4CMenB vaccine and none of the existing serological or genetic typing systems correlate with SBA titers. Therefore, assessing the potential strain coverage of such a multicomponent vaccine requires a reliable means of phenotyping different isolates of MenB bacteria that correlates with their susceptibility to killing by vaccine elicited antibodies. The Meningococcal Antigen Typing System (MATS) is a method that, for any given meningococcal isolate, can be used to quantify the level of expression and antigenic reactivity for each protein antigen and relates it to the probability that the isolate will be killed by the sera of an immunized person [110]. MATS uses ELISA assays to measure specifically the expression levels of each of the three 4CMenB vaccine recombinant antigens NadA, fHbp, and NHBA, and also determines the PorA subtype by serological analysis or by PCR sequencing of the hypervariable region VR2. To evaluate whether the relative antigen content determined by MATS correlates with bactericidal activity, SBA with exogenous human complement was performed against 57 group B meningococcal strains (with known antigen genotypes and relative antigen content) using pooled serum from infant human subjects immunized four times with the 4CMenB. By correlating the killing of meningococcal isolates to their antigen content, it was possible to evaluate the minimal level of expression of at least one target antigen (named Positive Bactericidal Threshold, PBT) to allow bactericidal antibody killing. Strains that carry the P1.4 PorA subtype are considered killed by vaccine-induced immune sera based on the data obtained with the OMV-NZ vaccine. Using MATS, N. meningitidis strains can be assessed for their expression of vaccine antigens and thus their predicted susceptibility to vaccine-elicited immune sera. MATS can be easily performed in large panel of strains, making it possible to survey large collections of MenB isolates in order to determine the potential for strain coverage by the 4CMenB vaccine of a target geographic region (see review in this issue by U. Vogel). Currently MATS is being used by several meningococcal reference laboratories to establish the potential coverage of the 4CMenB vaccine in different countries in Europe, North and South America and Australia.
Conclusions
The search for a universal vaccine against MenB has been a long and arduous process, in part because conventional approaches to vaccine development failed to provide an effective solution for this deadly pathogen. The availability of the genomic sequence of a pathogen provides the possibility to identify vaccine candidates and virulence factors in silico, irrespective of protein abundance or cultivation of the bacteria. The validity of reverse vaccinology was demonstrated for group B meningococci, and has been successfully applied in the development of vaccines against several bacterial pathogens including group B streptococcus, group A streptococcus and pneumococcus [111].
The MenB example shows the successful application of reverse vaccinology in the identification of a large number of previously unidentified antigens and has dramatically changed the landscape of antigen discovery. Immunological and molecular epidemiology studies were instrumental in prioritizing the antigens and in the definition of the antigen combination that was further used in development of the human vaccine. The successful combination, named 4CMenB, contains three protein antigens that are all involved in important steps of the meningococcal pathogenesis, as well as the OMVs. While the vaccine was in clinical development, research efforts were focused on the functional, immunological and structural characterization of the main protein antigens fHbp, NadA, and NHBA. The data obtained suggest that antibodies to the antigens of 4CMenB vaccine might have two modes of action: (i) by directly activating classical complement pathway and (ii) indirectly by interfering with colonization of invasive strains and/or by preventing binding of fH on the bacterial surface. Here we have reviewed the relevant structural, functional and immunological data obtained during the development of the 4CMenB vaccine. The clinical data obtained recently strongly support the efficacy of this vaccine, and suggest that its implementation will be of great benefit in the prevention of meningococcal disease.
Acknowledgments
We wish to thank Peter Dull for useful discussions and for comments on the manuscript. SR is supported by National Institutes of Health grants AI054544, AI084048 and AI32725.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007 Jan;31(1):3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, et al. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005 Apr 15;191(8):1263–71. doi: 10.1086/428590. [DOI] [PubMed] [Google Scholar]
- 3.Caugant DA, Maiden MC. Meningococcal carriage and disease-Population biology and evolution. Vaccine. 2009 May 20; doi: 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009 Jun 24;27( Suppl 2):B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009 Apr;7(4):274–86. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 6.Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis. 2009 Jul;9(7):418–27. doi: 10.1016/S1473-3099(09)70132-X. [DOI] [PubMed] [Google Scholar]
- 7.Findlow H, Vogel U, Mueller JE, Curry A, Njanpop-Lafourcade BM, Claus H, et al. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J Infect Dis. 2007 Apr 1;195(7):1071–7. doi: 10.1086/512084. [DOI] [PubMed] [Google Scholar]
- 8.Hoang LM, Thomas E, Tyler S, Pollard AJ, Stephens G, Gustafson L, et al. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005 Mar 1;40(5):e38–42. doi: 10.1086/427875. [DOI] [PubMed] [Google Scholar]
- 9.Vogel U, Claus H, von Muller L, Bunjes D, Elias J, Frosch M. Bacteremia in an immunocompromised patient caused by a commensal Neisseria meningitidis strain harboring the capsule null locus (cnl) J Clin Microbiol. 2004 Jul;42(7):2898–901. doi: 10.1128/JCM.42.7.2898-2901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007 May;15(5):233–40. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Mainassara HB, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007 Mar 1;44(5):657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 12.Caugant DA. Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect Genet Evol. 2008 Sep;8(5):558–65. doi: 10.1016/j.meegid.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011 Aug 5;60(30):1018–9. [PubMed] [Google Scholar]
- 14.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010 Mar;9(3):285–98. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 15.Pace D. Quadrivalent meningococcal ACYW-135 glycoconjugate vaccine for broader protection from infancy. Expert Rev Vaccines. 2009 May;8(5):529–42. doi: 10.1586/erv.09.18. [DOI] [PubMed] [Google Scholar]
- 16.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. Jama. 2008 Jan 9;299(2):173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 17.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987 Jun 15;138(12):4402–7. [PubMed] [Google Scholar]
- 18.Nedelec J, Boucraut J, Garnier JM, Bernard D, Rougon G. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J Neuroimmunol. 1990 Sep-Oct;29(1–3):49–56. doi: 10.1016/0165-5728(90)90146-e. [DOI] [PubMed] [Google Scholar]
- 19.Robbins JB, Schneerson R, Xie G, Ake-Hanson L, Miller MA. Capsular polysaccharide vaccine for Group B Neisseria meningitidis, Escherichia coli K1, and Pasteurella haemolytica A2. Proceedings of the National Academy of Sciences of the United States of America. 2011 Nov 1;108(44):17871–5. doi: 10.1073/pnas.1114489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai X, Borrow R. Genetic shifts of Neisseria meningitidis serogroup B antigens and the quest for a broadly cross-protective vaccine. Expert Rev Vaccines. 2010 Oct;9(10):1203–17. doi: 10.1586/erv.10.116. [DOI] [PubMed] [Google Scholar]
- 21.Sadarangani M, Pollard AJ. Serogroup B meningococcal vaccines-an unfinished story. Lancet Infect Dis. 2010 Feb;10(2):112–24. doi: 10.1016/S1473-3099(09)70324-X. [DOI] [PubMed] [Google Scholar]
- 22.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010 Apr 22;362(16):1511–20. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 23.Fredriksen JH, Rosenqvist E, Wedege E, Bryn K, Bjune G, Froholm LO, et al. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991 Dec;14(2):67–79. discussion -80. [PubMed] [Google Scholar]
- 24.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991 Dec;14(2):195–207. discussion 8–10. [PubMed] [Google Scholar]
- 25.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, et al. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine. 1995 Jun;13(9):821–9. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 26.Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005 Mar 18;23(17–18):2191–6. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 27.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009 Jun 24;27( Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 28.Tondella ML, Popovic T, Rosenstein NE, Lake DB, Carlone GM, Mayer LW, et al. Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. The Active Bacterial Core Surveillance Team. J Clin Microbiol. 2000 Sep;38(9):3323–8. doi: 10.1128/jcm.38.9.3323-3328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996 Jul;14(10):1001–8. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 30.Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011 Feb 4;29(7):1413–20. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE, 3rd, et al. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine. 2010 Oct 8;28(43):6970–6. doi: 10.1016/j.vaccine.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Koeberling O, Delany I, Granoff DM. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol. 2011 May;18(5):736–42. doi: 10.1128/CVI.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koeberling O, Giuntini S, Seubert A, Granoff DM. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol. 2009 Feb;16(2):156–62. doi: 10.1128/CVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor h-binding protein and genetically attenuated endotoxin. J Infect Dis. 2008 Jul 15;198(2):262–70. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, et al. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine. 2011 Jun 24;29(29–30):4728–34. doi: 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koeberling O, Welsch JA, Granoff DM. Improved immunogenicity of a H44/76 group B outer membrane vesicle vaccine with over-expressed genome-derived Neisserial antigen 1870. Vaccine. 2007 Feb 26;25(10):1912–20. doi: 10.1016/j.vaccine.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 37.Pinto VB, Moran EE, Cruz F, Wang XM, Fridman A, Zollinger WD, et al. An experimental outer membrane vesicle vaccine from N. meningitidis serogroup B strains that induces serum bactericidal activity to multiple serogroups. Vaccine. 2011 Oct 13;29(44):7752–8. doi: 10.1016/j.vaccine.2011.07.124. [DOI] [PubMed] [Google Scholar]
- 38.Zollinger WD, Donets MA, Schmiel DH, Pinto VB, Labrie JE, 3rd, Moran EE, et al. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine. 2010 Jul 12;28(31):5057–67. doi: 10.1016/j.vaccine.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001 Mar 21;19(17–19):2688–91. doi: 10.1016/s0264-410x(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 40.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006 Jun 12;24(24):5093–107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 41.Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000 Mar 10;87(5459):1809–15. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 42.Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000 Mar 10;287(5459):1816–20. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 43.Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jul 18;103(29):10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003 Mar 17;197(6):789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infection and immunity. 2004 Apr;72(4):2088–100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson AS, Jansen KU, Eiden J. New frontiers in meningococcal vaccines. Expert Rev Vaccines. 2011 May;10(5):617–34. doi: 10.1586/erv.11.50. [DOI] [PubMed] [Google Scholar]
- 47.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991 Jul;4(3):359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010 Oct;23(4):740–80. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006 Jun 15;176(12):7566–75. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 50.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006 Jul 1;177(1):501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infection and immunity. 2009 Jan;77(1):292–9. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantini F, Veggi D, Dragonetti S, Savino S, Scarselli M, Romagnoli G, et al. Solution structure of the factor H-binding protein, a survival factor and protective antigen of Neisseria meningitidis. J Biol Chem. 2009 Apr 3;284(14):9022–6. doi: 10.1074/jbc.C800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mascioni A, Bentley BE, Camarda R, Dilts DA, Fink P, Gusarova V, et al. Structural Basis for the Immunogenic Properties of the Meningococcal Vaccine Candidate LP2086. J Biol Chem. 2009 Mar 27;284(13):8738–46. doi: 10.1074/jbc.M808831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cendron L, Veggi D, Girardi E, Zanotti G. Structure of the uncomplexed Neisseria meningitidis factor H-binding protein fHbp (rLP2086) Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 May 1;67(Pt 5):531–5. doi: 10.1107/S1744309111006154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009 Apr 16;458(7240):890–3. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarselli M, Arico B, Brunelli B, Savino S, Di Marcello F, Palumbo E, et al. Rational design of a meningococcal antigen inducing broad protective immunity. Sci Transl Med. 2011 Jul 13;3(91):91ra62. doi: 10.1126/scitranslmed.3002234. [DOI] [PubMed] [Google Scholar]
- 57.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infection and immunity. 2009 Feb;77(2):764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011 Mar 15;186(6):3606–14. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunelli B, Del Tordello E, Palumbo E, Biolchi A, Bambini S, Comanducci M, et al. Influence of sequence variability on bactericidal activity sera induced by Factor H binding protein variant 1.1. Vaccine. 2011 Jan 29;29(5):1072–81. doi: 10.1016/j.vaccine.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 60.Oriente F, Scarlato V, Delany I. Expression of factor H binding protein of meningococcus responds to oxygen limitation through a dedicated FNR-regulated promoter. Journal of bacteriology. 2010 Feb;192(3):691–701. doi: 10.1128/JB.01308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009 May 11;27(21):2794–803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 62.Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin Vaccine Immunol. 2011 Jun;18(6):1002–14. doi: 10.1128/CVI.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6(7):e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giuliani MM, Santini L, Brunelli B, Biolchi A, Arico B, Di Marcello F, et al. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infection and immunity. 2005 Feb;73(2):1151–60. doi: 10.1128/IAI.73.2.1151-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008 Apr 1;197(7):1053–61. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 66.Welsch JA, Rossi R, Comanducci M, Granoff DM. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol. 2004 May 1;172(9):5606–15. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 67.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infection and immunity. 2011 Sep;79(9):3751–9. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giuntini S, Reason DC, Granoff DM. The Combined Roles of Human IgG Subclass, Alternative Complement Pathway Activation, and Epitope Density on Bactericidal Activity of Antibodies to Meningococcal Factor H Binding Protein. Infection and immunity. 2011 Nov 7; doi: 10.1128/IAI.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seib KL, Brunelli B, Brogioni B, Palumbo E, Bambini S, Muzzi A, et al. Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infection and immunity. 2011 Feb;79(2):970–81. doi: 10.1128/IAI.00891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vu DM, Wong TT, Granoff DM. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and Neisserial heparin binding antigen. Vaccine. 2011 Feb 24;29(10):1968–73. doi: 10.1016/j.vaccine.2010.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scarselli M, Cantini F, Santini L, Veggi D, Dragonetti S, Donati C, et al. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J Mol Biol. 2009 Feb 13;386(1):97–108. doi: 10.1016/j.jmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Beernink PT, Granoff DM. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infection and immunity. 2008 Jun;76(6):2568–75. doi: 10.1128/IAI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor h-binding protein. Infection and immunity. 2008 Sep;76(9):4232–40. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, et al. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010 Feb 23;107(8):3770–5. doi: 10.1073/pnas.0915162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infection and immunity. 1997 Jan;65(1):1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen T, Swanson J, Wilson J, Belland RJ. Heparin protects Opa+ Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infection and immunity. 1995 May;63(5):1790–5. doi: 10.1128/iai.63.5.1790-1795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahu A, Pangburn MK. Identification of multiple sites of interaction between heparin and the complement system. Mol Immunol. 1993 May;30(7):679–84. doi: 10.1016/0161-5890(93)90079-q. [DOI] [PubMed] [Google Scholar]
- 78.de Vries FP, Cole R, Dankert J, Frosch M, van Putten JP. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Molecular microbiology. 1998 Mar;27(6):1203–12. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 79.Menozzi FD, Rouse JH, Alavi M, Laude-Sharp M, Muller J, Bischoff R, et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996 Sep 1;184(3):993–1001. doi: 10.1084/jem.184.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hannah JH, Menozzi FD, Renauld G, Locht C, Brennan MJ. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infection and immunity. 1994 Nov;62(11):5010–9. doi: 10.1128/iai.62.11.5010-5019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esposito V, Musi V, de Chiara C, Veggi D, Serruto D, Scarselli M, et al. Structure of the C-terminal domain of NHBA, one of the main antigens of a novel vaccine against N. meningitidis. J Biol Chem. 2011 Sep 29; doi: 10.1074/jbc.M111.289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000 Oct 18;1482(1–2):9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 83.Calmettes C, Yu RH, Silva LP, Curran D, Schriemer DC, Schryvers AB, et al. Structural variations within the transferrin binding site on transferrin-binding protein B, TbpB. J Biol Chem. 2011 Apr 8;286(14):12683–92. doi: 10.1074/jbc.M110.206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacobsson S, Hedberg ST, Molling P, Unemo M, Comanducci M, Rappuoli R, et al. Prevalence and sequence variations of the genes encoding the five antigens included in the novel 5CVMB vaccine covering group B meningococcal disease. Vaccine. 2009 Mar 4;27(10):1579–84. doi: 10.1016/j.vaccine.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 85.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, et al. Characterisation of fHbp, nhba (gna2132), nadA, porA, Sequence Type and the genomic presence of IS1301 in group B meningococcal ST269 clonal complex case-isolates from England and Wales. J Clin Microbiol. 2009 Sep 16; doi: 10.1128/JCM.00936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, et al. Characterization of fHbp, nhba (gna2132), nadA, porA, and sequence type in group B meningococcal case isolates collected in England and Wales during January 2008 and potential coverage of an investigational group B meningococcal vaccine. Clin Vaccine Immunol. 2010 Jun;17(6):919–29. doi: 10.1128/CVI.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003 Dec 1;188(11):1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 88.Giuliani MM, Biolchi A, Serruto D, Ferlicca F, Vienken K, Oster P, et al. Measuring antigen-specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine. 2010 May 18; doi: 10.1016/j.vaccine.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 89.Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol. 2008 May;15(5):799–804. doi: 10.1128/CVI.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002 Jun 3;195(11):1445–54. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cotter SE, Surana NK, St Geme JW., 3rd Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 2005 May;13(5):199–205. doi: 10.1016/j.tim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 92.Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 2006 Jun;14(6):264–70. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Surana NK, Cutter D, Barenkamp SJ, St Geme JW., 3rd The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J Biol Chem. 2004 Apr 9;279(15):14679–85. doi: 10.1074/jbc.M311496200. [DOI] [PubMed] [Google Scholar]
- 94.Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, Taddei A, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Molecular microbiology. 2005 Feb;55(3):687–98. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 95.Nagele V, Heesemann J, Schielke S, Jimenez-Soto LF, Kurzai O, Ackermann N. Neisseria meningitidis adhesin NadA targets beta1 integrins: functional similarity to Yersinia invasin. J Biol Chem. 2011 Jun 10;286(23):20536–46. doi: 10.1074/jbc.M110.188326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montanari P, Bozza G, Capecchi B, Caproni E, Barrile R, Norais N, et al. Human heat shock protein (Hsp) 90 interferes with Neisseria meningitidis adhesin A (NadA)-mediated adhesion and invasion. Cell Microbiol. 2011 Nov 8; doi: 10.1111/j.1462-5822.2011.01722.x. [DOI] [PubMed] [Google Scholar]
- 97.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. Embo J. 2000 Nov 15;19(22):5989–99. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roggenkamp A, Ackermann N, Jacobi CA, Truelzsch K, Hoffmann H, Heesemann J. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. Journal of bacteriology. 2003 Jul;185(13):3735–44. doi: 10.1128/JB.185.13.3735-3744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Comanducci M, Bambini S, Caugant DA, Mora M, Brunelli B, Capecchi B, et al. NadA diversity and carriage in Neisseria meningitidis. Infection and immunity. 2004 Jul;72(7):4217–23. doi: 10.1128/IAI.72.7.4217-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Litt DJ, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, et al. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J Infect Dis. 2004 Oct 15;190(8):1488–97. doi: 10.1086/424464. [DOI] [PubMed] [Google Scholar]
- 101.Martin P, Makepeace K, Hill SA, Hood DW, Moxon ER. Microsatellite instability regulates transcription factor binding and gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2005 Mar 8;102(10):3800–4. doi: 10.1073/pnas.0406805102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Metruccio MM, Pigozzi E, Roncarati D, Berlanda Scorza F, Norais N, Hill SA, et al. A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements. PLoS Pathog. 2009 Dec;5(12):e1000710. doi: 10.1371/journal.ppat.1000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biolchi A, Fagnocchi L, Pigozzi E, Brunelli B, Boccadifuoco G, donnelly J, et al. In vitro levels of NadA expression may underestimate the potential effectiveness of immune responses against NadA in vivo EMGM 2011. Ljubljana, Slovenia: EMGM; 2011. p. 153. [Google Scholar]
- 104.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–48. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine. 2011 Jun 24;29(29–30):4739–44. doi: 10.1016/j.vaccine.2011.04.092. [DOI] [PubMed] [Google Scholar]
- 106.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010 Nov 15;51(10):1127–37. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 107.Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol. 2011 Mar;18(3):483–6. doi: 10.1128/CVI.00304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010 Nov;29(11):e71–9. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 109.Toneatto D, Oster P, Deboer AC, Emerson A, Santos GF, Ypma E, et al. Early clinical experience with a candidate meningococcal B recombinant vaccine (rMenB) in healthy adults. Hum Vaccin. 2011 Jul 1;7(7):781–91. doi: 10.4161/hv.7.7.15997. [DOI] [PubMed] [Google Scholar]
- 110.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2010 Nov 9;107(45):19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serruto D, Serino L, Masignani V, Pizza M. Genome-based approaches to develop vaccines against bacterial pathogens. Vaccine. 2009 May 26;27(25–26):3245–50. doi: 10.1016/j.vaccine.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 112.Cantini F, Veggi D, Dragonetti S, Savino S, Scarselli M, Romagnoli G, et al. Solution structure of the factor H binding protein, a survival factor and protective antigen of Neisseria meningitidis. J Biol Chem. 2009 Feb 4; doi: 10.1074/jbc.C800214200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]