Abstract
Background
Hepatic ischemia–reperfusion injury is a common clinical problem in hepatic surgery and transplantation. Several cellular and tissue structural and functional alterations are observed in such injury. The aim of this study was to evaluate the effect of dexmedetomidine on lipid peroxidation and erythrocyte deformability during ischemia–reperfusion injury in rats.
Methods
Twenty-four Wistar Albino rats were randomly separated into three groups as control (C), ischemia–reperfusion injury (I/R) and dexmedetomidine group (I/R-D). Ischemia was induced with portal clampage for 45 min and reperfusion period was 45 min after declampage. Group I/R-D received dexmedetomidine 100 µg/kg i.p. 30 min before portal clampage. Serum malondialdehyde and superoxide dismutase activities to document lipid peroxidation and erythrocyte deformability index were investigated.
Results
Serum superoxide dismutase and malondialdehyde activity levels were significantly higher and erythrocyte deformability index was decreased in hepatic ischemia–reperfusion group. However, these changes were observed to be prevented with dexmedetomidine treatment when given before portal clampage.
Conclusion
These findings clearly indicate that erythrocyte deformability index is decreased in hepatic ischemia reperfusion injury and has a potential role to prevent these alterations. The protective effect of dexmedetomidine on hepatic I/R injury is also decreased lipid peroxidation. Further experimental and clinical investigations may clarify the molecular mechanisms and clinical significance of these findings.
Keywords: hepatic ischemia–reperfusion injury, lipid peroxidation, malondialdehyde, superoxide dismutase, erythrocyte deformability, dexmedetomidine
Hepatic ischemia–reperfusion (I/R) injury is a common problem in trauma, hepatic surgery, and transplantation. Previous clinical and experimental studies have investigated several aspects of this clinical entity (1–4). Hepatic I/R injury is known to be a result of a complex mechanism which involves different cellular and molecular elements such as leukocytes, red blood cells (RBC), TNFα, NO, NF kappaB, and the others (2). Oxidative stress, free radical formation and lipid peroxidation, which play important roles in development of I/R injury, also cause membrane changes in RBCs (5). Erythrocyte deformability is crucial for the maintenance of normal circulation. Optimal deformability facilitates the passage of RBC through narrow capillaries in the microcirculation and reduces blood viscosity at high shear rates in large blood vessels (6).
Dexmedetomidine, a selective and potent α2-adrenoceptor agonist, was approved by the US Food and Drug Administration in 1999 for sedation of patients hospitalized in intensive care settings. Since then, a growing number of research articles have emerged reporting other possible indications, such as regional and general anesthesia (7, 8). Dexmedetomidine was reported to be effective in protecting against focal ischemia in rabbits, in cardiac I/R injury in rats in kidney I/R injury in rats, and in incomplete forebrain ischemia in rats (9–12). Despite its increased clinical use, frequently in critically ill patients, the effect of dexmedetomidine on liver I/R injury has not been yet investigated (13).
The primary aim of this study was to investigate hepatic I/R injury induced changes in erythrocyte deformability and the preventive role of dexmedetomidine on increased lipid peroxidation and erythrocyte deformability alterations in experimental hepatic I/R injury.
Materials and methods
Animals and experimental protocol
The experiments were performed in adherence to National Institutes of Health guidelines on the use of experimental animals. Twenty-four male Wistar rats weighing from 275 to 350 g were housed at constant temperature with 14/10 h periods of light and dark exposure. Animals were allowed access to standard rat chow and water ad libitum during an acclimatation period of at least 5 days prior to use in these experiments. Approval of the Institutional Ethics Committee was obtained. The experiments were performed in the Animal Experimental Laboratory of the Physiology Department in Kirikkale University.
Rats were anesthetized with intramuscular ketamine 50 mg/kg. The chest and abdomen were shaved and each animal was fixed in a supine position on the operating table. The abdomen was cleaned with 1% polyvinyl iodine and when dry, the operating field was covered with a sterile drape and median laparotomy was performed. There were three experimental groups (Group C (sham-control; n=8), Group I/R (ischemia-reperfusion, n=8), and Group I/R-D (I/R-Dexmedetomidine; n=8)).
Sham operation was performed on the rats in Group A. The sham operation consisted of mobilization of the hepatic pedicle only. The rats in this group were sacrificed 90 min after the procedure. Hepatic I/R injury was induced in Groups I/R and I/R-D, respectively with hepatic pedicle clampage using a vascular clamp as in the previous study of Yaylak et al. (3). After an ischemia period of 45 min, the vascular clamp was removed. A reperfusion period was maintained for 45 min. In Group I/R-D, dexmedetomidine hydrochloride 100 mg/kg, (Precedex 100 µg/2 ml, Abbott®, Abbott Laboratory, North Chicago, IL, USA) was administrated via intraperitoneal route 30 min before clampage (14).
All the rats were given ketamine 100 mg/kg intraperitoneally and intracardiac blood samples were obtained. Heparinized total blood samples were used to prepare erythrocyte packs. Deformability measurements were done using erythrocyte suspensions with 5% hematocrit in phosphate buffered saline (PBS) buffer.
Biochemical measurements
Blood samples with etylenediamine tetra acetic acid were centrifuged and plasma was stored at −80°C until the day of study. Experiments were studied at the upper phase obtained after centrifugation. Superoxide dismutase (SOD) and malondialdehyde (MDA) levels in plasma were measured biochemically.
Deformability measurements
Erythrocyte deformability was measured using a constant flow filtrometer system (MP 30, Biopac Systems Inc., Commat, USA). Erythrocyte suspension that was delivered at 1 ml/min flow rate was passed through a nucleopore-polycarbonate filter of 5 µm in diameter, and alterations in the filtration pressure corresponding to different flow rates were measured. The alterations in the pressure were transferred to computer medium with an MP 30 data equation system. The ratio of the values of filtration pressure for the cellular suspension and buffer were calculated, and the relative resistance was calculated.
Statistical analysis
Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) 12.0 program was used for statistical analysis. Variations in plasma oxidative state parameters and erythrocyte deformability between study groups were assessed by using Kruskal–Wallis test. Bonferroni adjusted Mann–Whitney U test was used after significant Kruskal–Wallis to determine which group differs from the other. Results were expressed as mean±standard deviation (Mean±SD). Statistical significance was set at a p-value <0.05 for all analyses and p<0.033 (0.1/3) for Bonferroni adjusted Mann–Whitney U test.
Results
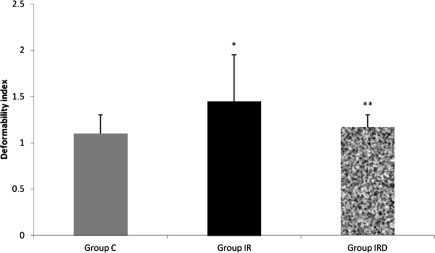
Relative resistance, which reflects erythrocyte deformability, was increased in Group I/R. Deformability index was higher in I/R group than the other groups (Group C–Group I/R p=0.005; Group I/R–Group I/R-D p=0.028), (Fig. 1). However, with dexmedetomidine treatment deformability index was found to be similar with the (Groups C and I/R-D, p=0.574).
Fig. 1.
Comparison of the Mean±SD erythrocyte deformability values between the groups (Mann–Whitney U test, Group C–Group I/R p=0.005; Group I/R–Group I/R-D p=0.028).
Note: *p<0.05 compared to the Group C; **p<0.05 compared to the Group I/R.
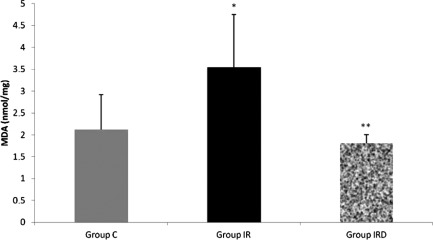
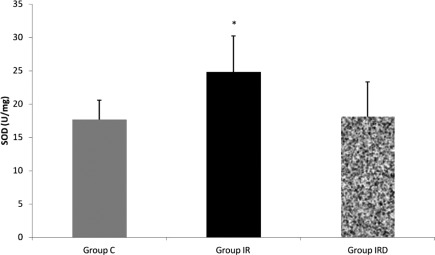
MDA activity was higher in I/R group than the other groups (Group C–Group I/R p=0.027; Group I/R–Group I/R-D p=0.011) (Fig. 2). SOD activity was also higher in I/R group than the control group (Group C–Group I/R p=0.029), (Fig. 3). However, dexmedetomidine treatment in I/R-D group resulted with similar SOD and MDA levels with the control group (Figs. 2 and 3).
Fig. 2.
Comparison of the Mean±SD malondialdehyde (MDA; nmol/mg) levels between the groups (Mann–Whitney U test, Group C–Group I/R p=0.027; Group I/R–Group I/R-D p=0.011).
Note: *p<0.05 compared to the Group C; **p<0.05 compared to the Group I/R.
Fig. 3.
Comparison of the Mean±SD levels of superoxide dismutase (SOD; U/mg) between the groups (Mann–Whitney U test, Group C–Group I/R p=0.029; Group I/R–Group I/R-D p=0.108).
Note: *p<0.05 compared to the Group C.
Discussion
Hepatic I/R injury is defined as the prolongation and aggravation of ischemic tissue damage with reperfusion (15). Several cellular and molecular changes in hepatic I/R injury have been investigated in clinical and experimental studies. However, in this study, we have reported (for the first time, to our knowledge) the protective effect of dexmedetomidine on erythrocyte deformability alterations in experimental hepatic I/R injury model in the rat. Dexmedetomidine, which is a highly potent and selective α2-adrenoreceptor agonist, was administered before induction of ischemia, and resulted in decreased SOD and MDA levels, which are known to play crucial role in development of hepatic I/R injury.
Hepatic I/R injury induced lipid peroxidation generates a complex variety of products. Many of these react with several protein products and DNA and causes local and systemic toxic and mutagenic effects (16). A major target site of lipid peroxidation is the cellular membrane, which contains polyunsaturated fatty acids. Toxicity of partially reduced oxygen species arises from the peroxidation of polyunsaturated fatty acid of membrane phospholipids. The final event leading to structural and functional tissue damage is the loss of disintegration of cellular membrane. In hepatic I/R injury this mechanism is exacerbated after reperfusion with microcirculatory derangements and altered mitochondrial functions (17).
Hematological factors are sensitive to metabolic changes and may be altered with the changes in tissue perfusions due to several factors: such as bleeding, sepsis, and cardiovascular problems. Disorders in the hematological state may result with changes plasma viscosity (18). Erythrocyte deformability and plasma viscosity are important factors that affect organ and tissue perfusion (19). Optimal delivery of oxygen and vital molecules to the final organ capillaries and clearance of metabolic wastes, erythrocytes must be able to extend and curve, and have the capability to move in these areas. This capacity is termed as ‘deformability’ (20). There is equilibrium in free radical production and antioxidant defense system that suppress production of oxygen free radical in the body. Oxidative damages occur when this equilibrium is disrupted. Increased lipid peroxidation is also one of the results of increased oxidative stress (21). It was reported that, some parameters of erythrocyte functions and membrane integrity were impaired in increased lipid peroxidation in vivo and in vitro studies (22). The products that arise due to lipid peroxidation associated with increased oxidative stress significantly affect membrane permeability and micro viscosity. Thus, diminished deformability capacity and survival of the erythrocytes are observed (23). SOD, CAT, and GSH-Px are responsible in cellular antioxidant defense mechanisms. These enzymes eliminate superoxide anions and hydrogen peroxides, and prevent free radical production (24). SOD is the primary defensive enzyme against oxygen derived free radical production and catalyses H2O2 conversion (25). Oxygen radicals generated in response to I/R have been implicated in the micro vascular dysfunction and parenchymal cell injury of the intestine and liver (26, 27). The TBA assay, which measures MDA, is a simple and easy-to-use method for assessment of the degree of injury attributable to the free radicals produced by ischemia/reperfusion, and is frequently used in laboratory (28). In vitro treatment of the erythrocytes with MDA has been reported to result with decreased erythrocyte deformability and survival (23, 25, 29, 30). In this current study, increased MDA levels were accompanied with decreased erythrocyte deformability index in hepatic I/R group. Dexmedetomidine treatment was also observed to protective against increased MDA production in hepatic I/R injury. Also this finding was considered significant, which may explain the protection of erythrocyte deformability with dexmedetomidine treatment prior to hepatic ischemia.
Erythrocyte deformability and erythrocyte membrane rigidity were reported to be affected with several agents that induce lipid peroxidation. In this study, dexmedetomidine treatment was shown to have protective effects against hepatic I/R injury induced erythrocyte deformability changes. Altered erythrocyte deformability not only changes the oxygen delivery capacity of the erythrocytes but also the survival of the circulating erythrocytes (22, 23).
As a conclusion the results of this study clearly demonstrate that erythrocyte deformability is significantly altered in experimental hepatic I/R injury in the rat. Increased SOD and MDA production, which causes lipid peroxidation and cellular membrane alterations may play an important role in erythrocyte deformability alterations. In addition, dexmedetomidine was observed to protect against these alterations in hepatic I/R injury, when given before induction of ischemia. Other aspects of these findings including clinical significance and practical applications, merit further experimental and clinical investigation.
Conflict of interest and funding
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Siriussawakul A, Zaky A, Lang JD. Role of nitric oxide in hepatic ischemia–reperfusion injury. World J Gastroenterol. 2010;16:6079–86. doi: 10.3748/wjg.v16.i48.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alchera E, Dal Ponte C, Imarisio C, Albano E, Carini R. Molecular mechanisms of liver preconditioning. World J Gastroenterol. 2010;16:6058–67. doi: 10.3748/wjg.v16.i48.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaylak F, Canbaz H, Caglikulekci M, Dirlik M, Tamer L, Ogetman Z, et al. Liver tissue inducible nitric oxide synthase (iNOS) expression and lipid peroxidation in experimental hepatic ischemia reperfusion injury stimulated with lipopolysaccharide: the role of aminoguanidine. J Surg Res. 2008;148:214–23. doi: 10.1016/j.jss.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Bedirli N, Ofluoglu E, Kerem M, Utebey G, Alper M, Yilmazer D, et al. Hepatic energy metabolism and the differential protective effects of sevoflurane and isoflurane anesthesia in a rat hepatic ischemia–reperfusion injury model. Anesth Analg. 2008;106:830–7. doi: 10.1213/ane.0b013e3181616fc9. [DOI] [PubMed] [Google Scholar]
- 5.Grisham MB, Granger DN. Free radicals: reactive metabolites of oxygen as mediators of postischemic reperfusion injury. In: Martson A, Bulkley GB, Fiddian-Green RG, Haglung U, editors. Splanchnic ischemia and multiple organ failure. St. Louis: Mosby; 1989. pp. 135–144. [Google Scholar]
- 6.Peto K, Nemeth N, Brath E, Takacs IE, Baskurt OK, Meiselman HJ, et al. The effects of renal ischemia–reperfusion on hemorheological factors: preventive role of allopurinol. Clin Hemorheol Microcirc. 2007;37:347–58. [PubMed] [Google Scholar]
- 7.McCutcheon CA, Orme RM, Scott DA, Davies MJ, McGlade DP. A comparison of dexmedetomidine versus conventional therapy for sedation and hemodynamic control during carotid endarterectomy performed under regional anesthesia. Anesth Analg. 2006;102:668–75. doi: 10.1213/01.ane.0000197777.62397.d5. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Maier CM, Sun GH, Kunis DM, Giffard RG, Steinberg GK. Neuroprotection by the N-methyl-D-aspartate receptor antagonist CGP 40116: in vivo and in vitro studies. J Neurochem. 1995;65:652–9. doi: 10.1046/j.1471-4159.1995.65020652.x. [DOI] [PubMed] [Google Scholar]
- 10.Kocoglu H, Karaaslan K, Gonca E, Bozdogan O, Gulcu N. Preconditioning effects of dexmedetomidine on myocardial ischemia/reperfusion injury in rats. Curr Ther Res Clin Exp. 2008;69:150–8. doi: 10.1016/j.curtheres.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia–reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70–4. doi: 10.1080/08860220802546487. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–32. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Snapir A, Posti J, Kentala E, Koskenvuo J, Sundell J, Tuunanen H, et al. Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology. 2006;105:902–10. doi: 10.1097/00000542-200611000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Engelhard K, Werner C, Eberspächer E, Bachl M, Blobner M, Hildt E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–31. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke H, Leamsters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 16.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 17.Giakoustidis D, Papageorgiou G, Iliadis S, Giakoustidis A, Kostopoulou E, Kontos N, et al. The protective effect of alpha-tocopherol and GdCl3 against hepatic ischemia/reperfusion injury. Surg Today. 2006;36:450–6. doi: 10.1007/s00595-005-3162-9. [DOI] [PubMed] [Google Scholar]
- 18.Muller R, Musikic P. Hemorheology in surgery: a review. Angiology. 1987;38:581–92. doi: 10.1177/000331978703800802. [DOI] [PubMed] [Google Scholar]
- 19.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. 1987;253:H898–903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]
- 20.Zinchuk VV. Erythrocyte deformability: physiological aspects. Usp Fiziol Nauk. 2001;32:66–78. [PubMed] [Google Scholar]
- 21.Therond P, Bonnefont-Rousselot D, David-Spraul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care. 2000;3:373–84. doi: 10.1097/00075197-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers FA. Red cell membrane damage. J Heart Valve Dis. 1998;7:387–95. [PubMed] [Google Scholar]
- 23.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169–88. doi: 10.2165/00139709-200423030-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mates JM, Perez-Gomez C, Castro IN. Antioxidant enzymes and human disease. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 25.Yerer MB, Yapislar H, Aydogan S, Yalcin O, Baskurt O. Lipid peroxidation and deformability of red blood cells in experimental sepsis in rats: The protective effects of melatonin. Clin Hemorheol Microcirc. 2004;30:77–82. [PubMed] [Google Scholar]
- 26.Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–52. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Yagmurdur MC, Ozdemir A, Topaloglu S, Kilinç K, Ozenç A. Effects of alpha tocopherol and verapamil on liver and small bowel following mesenteric ischemia–reperfusion. Turk J Gastroenterol. 2002;13:40–6. [PubMed] [Google Scholar]
- 28.Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995;57:311–32. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 29.Hanci V, Erol B, Bektaş S, Mungan G, Yurtlu S, Tokgöz H. Effect of dexmedetomidine on testicular torsion/detorsion damage in rats. Urol Int. 2010;84:105–11. doi: 10.1159/000273476. [DOI] [PubMed] [Google Scholar]
- 30.Yagmurdur H, Ozcan N, Dokumaci F, Kilinc K, Yilmaz F, Basar H. Dexmedetomidine reduces the ischemia–reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33:941–7. doi: 10.1016/j.jhsa.2008.01.014. [DOI] [PubMed] [Google Scholar]