Abstract
Traumatic brain injury (TBI) induces a cascade of primary and secondary events resulting in impairment of neuronal networks that eventually determines clinical outcome. The dynorphins, endogenous opioid peptides, have been implicated in secondary injury and neurodegeneration in rodent and human brain. To gain insight into the role of dynorphins in the brain's response to trauma, we analyzed short-term (1-day) and long-term (7-day) changes in dynorphin A (Dyn A) levels in the frontal cortex, hippocampus, and striatum, induced by unilateral left-side or right-side cortical TBI in mice. The effects of TBI were significantly different from those of sham surgery (Sham), while the sham surgery also produced noticeable effects. Both sham and TBI induced short-term changes and long-term changes in all three regions. Two types of responses were generally observed. In the hippocampus, Dyn A levels were predominantly altered ipsilateral to the injury. In the striatum and frontal cortex, injury to the right (R) hemisphere affected Dyn A levels to a greater extent than that seen in the left (L) hemisphere. The R-TBI but not L-TBI produced Dyn A changes in the striatum and frontal cortex at 7 days after injury. Effects of the R-side injury were similar in the two hemispheres. In naive animals, Dyn A was symmetrically distributed between the two hemispheres. Thus, trauma may reveal a lateralization in the mechanism mediating the response of Dyn A-expressing neuronal networks in the brain. These networks may differentially mediate effects of left and right brain injury on lateralized brain functions.
Key words: dynorphins, lateralization, neurodegeneration, neuronal networks, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of disability and death in children and young adults in Western countries, and mortality and morbidity in those with war-related injuries (Elder et al., 2010; Maas et al., 2008). TBI induces a broad range of short-term and long-term physical, behavioral, and cognitive impairments, depending on the severity of injury (Albensi, 2001; Berger et al., 1999). Both primary and secondary responses result in neurological deficits (Graham et al., 1995). The delayed secondary response is a complex cascade of cellular and molecular processes that includes impairment of energy metabolism and ionic homeostasis, the release of toxic molecules and excitatory neurotransmitters, and disruption of synaptic connectivity (Lyeth et al., 1990; McIntosh et al., 1998; Povlishock et al., 1999; Raghupathi et al., 2000; Scheff et al., 2005).
Along with effects on brain cortical areas, TBI causes atrophy and dysfunction of sub-cortical structures. Massive neuronal damage and cell loss occur in the cerebral cortex, hippocampus, and substantia nigra following experimental and clinical TBI (Adams et al., 1985; Anderson et al., 2005; Baldwin et al., 1997; Dietrich et al., 1994; Soares et al., 1995). Neuronal damage and degeneration in the hippocampus and thalamus correlate with the severity of post-traumatic motor dysfunction and cognitive deficits. Damage to the hippocampus may account for injury-related cognitive impairment (Bramlett et al., 1997; Colicos et al., 1996; Pierce et al., 1998). Striatal structures that participate in movement, emotional responses, and memory, undergo atrophic changes after TBI (Anderson et al., 1996; Shin et al., 2011). TBI also induces hypo-function of the striatal dopaminergic system (Shin et al., 2011).
Several lines of evidence suggest a role of endogenous dynorphins in central nervous system (CNS) injury. Dynorphin A (Dyn A), dynorphin B, and α-neoendorphin, collectively known as dynorphins, are endogenous κ-opioid receptor ligands. General and κ-receptor-selective opioid antagonists were reported to be beneficial in experimental models of spinal cord injury and TBI, suggesting that endogenous dynorphins contribute to secondary CNS injury (Behrmann et al., 1993; Faden et al., 1987; McIntosh et al., 1987; Vink et al., 1990). Furthermore, Dyn A, the most pathogenic dynorphin, may cause tissue injury and cell death, and may exacerbate the clinical severity of traumatic injury to the head or spinal cord (Faden, 1990,1996; Goody et al., 2003; Hauser et al., 2005; Headrick et al., 1995; Hu et al., 1996; McIntosh et al., 1994; Woods et al., 2006). Dyn A-induced tissue injury may involve both opioid and non-opioid components (Adjan et al., 2007; Bakshi et al., 1992; Faden, 1990; Hauser et al., 2005; Long et al., 1994; Woods et al., 2006).
We have recently identified missense mutations in the human prodynorphin gene coding for a precursor to dynorphins, and demonstrated that these mutations cause profound neurodegeneration in the cerebrum and cerebellum underlying the spinocerebellar ataxia type 23, a dominantly inherited neurodegenerative disorder (Bakalkin et al., 2010). Three out of four mutations are located in Dyn A. Generalized pathological changes including cerebral cortical and subcortical atrophy, and agenesis of the corpus callosum, were characteristics of patients carrying Dyn A mutations. The mutations apparently enhance the pathogenic potential of Dyn A, as is evident from analysis of the peptide's ability to induce neuron death (Bakalkin et al., 2010). This fact, along with the gross structural effects on brain morphology, indicate a fundamental role of wild-type Dyn A in modulating neuronal function and survival, and indirectly support the hypothesis that Dyn A has a pathogenic function in TBI and spinal cord injury. In this study, to gain better insight into the TBI mechanisms, we analyzed short-term (1-day) and long-term (7-day) changes in Dyn A levels in the frontal cortex, hippocampus, and striatum after unilateral cortical TBI. Several TBI effects in rodents and humans have been reported to be lateralized (Heilman et al., 1978; Kerkhoff, 2001; Levin et al., 1995; Morrow et al., 1981; Pavlovskaya et al., 2007; Pearlson and Robinson, 1981; Schaefer et al., 2009). Therefore, to avoid a biased assessment of unilateral TBI effects, we analyzed Dyn A levels in groups of animals subjected to left- (L-TBI) or right- (R-TBI), and to L- or R-sham surgery (Sham).
Methods
Experimental animals and surgical procedures
CF-1 young adult male mice weighing 29–31 g (Charles River, Portage, MI) were utilized in this study. This strain has previously been well characterized in terms of time course of TBI-induced neurodegeneration, and motor and cognitive impairment (Hall et al., 2005; Hunt et al., 2009; Kelso et al., 2006; Scheff et al., 1997; Thompson et al., 2010). The mice were group-housed (4 per cage) with a 12-h light/dark cycle with food and water available ad libitum. All animal procedures were reviewed and approved by the institutional animal care and use committee at the University of Kentucky, and carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize pain and discomfort.
Nine groups of animals were analyzed, including (1) naïve animals (n=9); (2 and 3) left- and right-sham-operated animals (L-Sham, n=9; R-Sham, n=10) sacrificed at 24 h; (4 and 5) L-TBI (n=17) and R-TBI (n=8) animals sacrificed at 24 h; (6 and 7) L-Sham (n=9) and R-Sham (n=13) animals sacrificed at 7 days; and (8 and 9) L-TBI (n=18) and R-TBI (n=10) animals sacrificed at 7 days. The 24-h and 7-day L-TBI groups were replicated, producing similar results (p>0.05), and then data for each time group were pooled. The mice were anesthetized with intraperitoneal (IP) tribromoethanol (Avertin 0.175 mg/kg; Sigma-Aldrich, St. Louis, MO) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The head was positioned in the horizontal plane with the nose bar set at −5. Using sterile procedures, the skin was retracted, and a 4-mm craniotomy was made lateral to the sagittal suture centered between the bregma and lambda. The skull cap was carefully removed without disruption of the underlying dura. The exposed somatosensory cortex was injured using a pneumatically-controlled impactor device (TBI 031; Precision Systems & Instrumentation, Fairfax Station, VA), as described previously (Hall et al., 2005; Hunt et al., 2009; Kelso et al., 2006; Scheff et al., 1997). The impactor rod tip (3-mm diameter) compressed the cortex at 3.5 m/sec to a depth of 0.3 mm with 400 msec dwell time. After injury, Surgiseal (Johnson and Johnson, Arlington, TX) was laid on the dura, and the skull cap was replaced. The skin was then sutured together, and the animals were placed on a heating pad to recover. Sham animals underwent the same procedures except for the cortical impact. The core body temperature of the animals was 36–37°C throughout the procedure. At the indicated time points, the mice were deeply anesthetized by isoflurane inhalation and decapitated, the brain was quickly removed and dissected, and tissues were snap-frozen and stored at −80°C. The frontal cortical tissue taken for the analysis included the lesioned region, and the contralateral undamaged area.
Radioimmunoassay
The Dyn A (1-17) radioimmunoassay (RIA) and anti-dynorphin antibodies were described elsewhere (Christensson-Nylander et al., 1985; Merg et al., 2006). Briefly, tissues were extracted in 1 M acetic acid (1:10 w/v), boiled at 95°C for 5 min, centrifuged at 14,000 rpm for 20 min, and the supernatant was run through an SP-Sephadex ion exchange C-25 column. The elution was performed in a stepwise manner with a mixture of pyridine and formic acid. Anti-Dyn A antibodies demonstrated 100% molar cross-reactivity with dynorphin A (9-17), and less than 0.1% molar cross-reactivity with dynorphin B, dynorphin A (1-8), α-neoendorphin, leu-enkephalin, and big dynorphin. Peptide levels are presented in fmol/mg tissue.
Statistical analyses
Data on Dyn A levels were first subjected to an overall two-way analysis of variance (ANOVA), with treatment as a between-subjects factor (9 levels: naive mice, L-Sham at 24 h, L-TBI at 24 h, R-Sham at 24 h, R-TBI at 24 h, L-Sham at 7 days, L-TBI at days, R-Sham at 7 days, and R-TBI at 7 days), and lateralization as a within-subjects factor (2 levels: ipsilateral hemisphere and contralateral hemisphere). Second, the significant main effects of treatment were followed by Bonferroni's post-hoc analyses of the mean content of Dyn A levels in two hemispheres (left+right/2). One-way ANOVA was used to better characterize any significant interactions observed in the two-way ANOVA. In these instances, the dependent variable was Dyn A levels, in which Dyn A content in the hemisphere (1) ipsilateral or (2) contralateral to the lesion (sham or TBI) were compared separately by one-way ANOVA. One-way ANOVAs were followed by Bonferroni's post-hoc analyses. Data are presented as mean±standard deviation (SD).
Results
The sham-operated (Sham) and naive groups were compared to determine whether the surgery itself might affect the results, while significant differences between the TBI and respective sham groups were the criteria for demonstrating an effect of TBI. TBI causes pronounced atrophy and dysfunction of cortical and sub-cortical structures, including the hippocampus, thalamus, and striatum (Adams et al., 1985; Anderson et al., 1996,2005; Baldwin et al., 1997; Bramlett et al., 1997; Colicos et al., 1996; Dietrich et al., 1994; Pierce et al., 1998; Shin et al., 2011; Soares et al., 1995; Thompson et al., 2010). Because the frontal cortex, hippocampus, and striatum additionally express moderate to high levels of dynorphins they were chosen for analysis. The 24-h and 7-day time points were selected because they correspond to the periods when (1) profound neurodegeneration and motor impairment are most evident; and when (2) the neurodegenerative changes subside and motor functions begin to recover, respectively (Fox et al., 1999; Hall et al., 2005; Hanell et al., 2010; Hunt et al., 2009; Kelso et al., 2006; Scheff et al., 1997; Thompson et al., 2010).
Frontal cortex
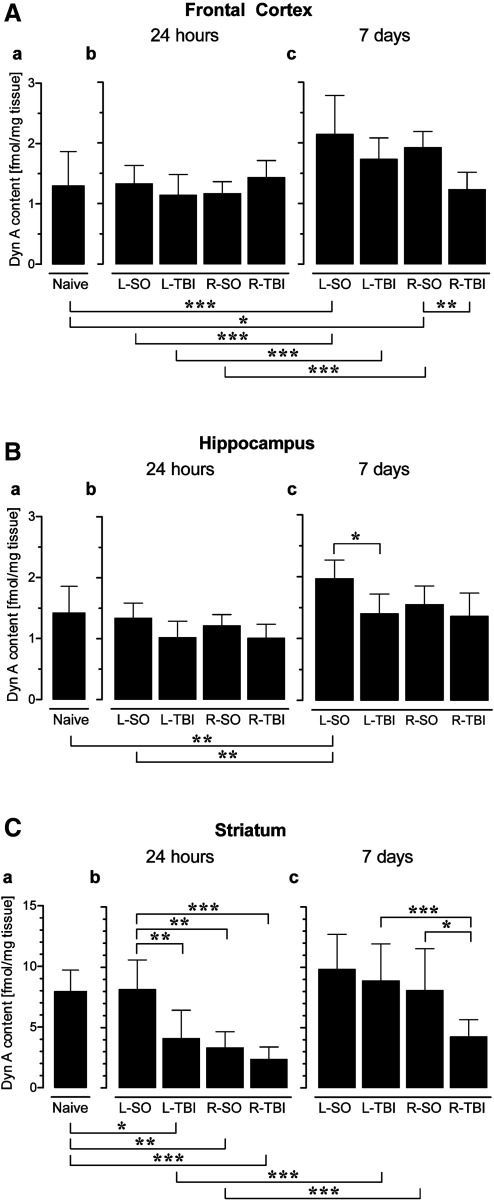
The overall two-way ANOVA revealed a significant main effect of treatment [F(8,86)=9.1, p<0.001]. Although a significant main effect of lateralization was not observed [F(1,86)=0.74, p=0.392], a significant treatment×lateralization interaction was noted [F(8,86)=16.01, p<0.001]. The significant main effect of treatment was further analyzed by Bonferroni's post-hoc analysis of the Dyn A mean hemispheric content (Fig. 1A). At the 7 day time point, the L- and R-Sham animals demonstrated higher Dyn A levels compared to (1) the naïve (untreated) mice (L-Sham 7 days versus untreated mice: p<0.001; R-Sham 7 days versus naïve mice: p<0.05), and (2) the 24-h groups (L-Sham 24 h versus L-Sham 7 days: p<0.001; R-Sham 24 h versus R-Sham 7 days: p<0.001). Temporal differences were also noted for the L-TBI group (L-TBI 24 h versus L-TBI 7 days: p<0.001). The R-TBI group had a significant effect compared to the R-Sham group (p<0.01) at 7 days after the operation. No effect of the L-TBI in the 7-day group, nor L- and R-TBI in the 24-h groups was evident.
FIG. 1.
Impact of left and right sham operation (L-SO and R-SO), or cortical traumatic brain injury (L-TBI and R-TBI) on Dyn A levels in the frontal cortex, hippocampus, and striatum at 24 h and 7 days after the operation. Dyn A levels are shown as mean values for the left and right hemispheres (L+R/2). ANOVA testing (9 levels) was followed by Bonferroni's post-hoc analyses (*p<0.05, **p<0.01, ***p<0.001). Data bars represent means±SD (SD, standard deviation; ANOVA, analysis of variance; Dyn A, dynorphin A).
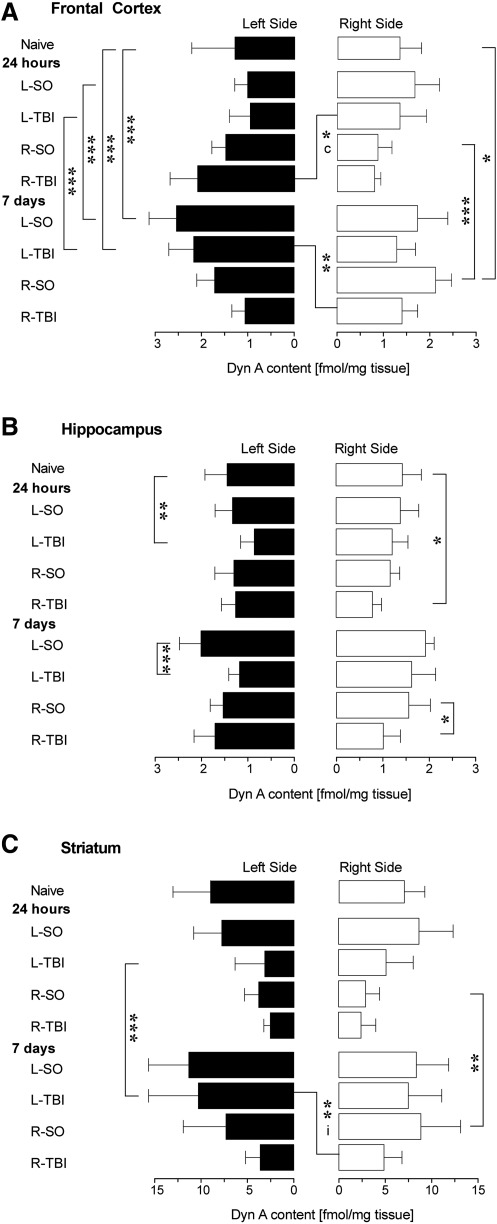
A one-way ANOVA (9 levels) performed separately for the ipsilateral [F(8,86)=20.3, p<0.001] and contralateral [F(8,86)=3.7, p<0.001] hemispheres, revealed significant effects of treatment (Fig. 2A). In the ipsilateral hemisphere of the 7-day groups, the Dyn A levels were significantly higher after L-Sham, R-Sham, and L-TBI compared to naïve mice (p<0.001; p<0.05; p<0.001), and to the respective 24-h groups (p<0.001; p<0.001; p<0.001). In the ipsilateral hemisphere at the 7-day time point, Dyn A levels differed significantly between the L-TBI and R-TBI groups (p<0.01). At 24 h, the R-TBI group compared to the L-TBI animals showed significantly higher Dyn A levels in the contralateral hemisphere (p<0.05). No other significant differences were evident between the naïve and injury (Sham or TBI) groups, and between the Sham and TBI groups at the two time points, when the contralateral hemisphere was analyzed.
FIG. 2.
Impact of L-Sham (L-SO), R-Sham (R-SO), L-TBI, or R-TBI on Dyn A levels in the frontal cortex, hippocampus, and striatum, analyzed separately in the ipsilateral and contralesional regions at 24 h and 7 days after surgery. ANOVA (9 levels) was followed by Bonferroni's post-hoc analyses (*p<0.05, **p<0.01, ***p<0.001). Data bars represent means±SD (SD, standard deviation, ANOVA, analysis of variance; Dyn A, dynorphin A).
Altogether, these results (Figs. 1A and 2A) demonstrate that the sham surgery induces upregulation of Dyn A predominantly in the ipsilateral frontal cortex, and that this effect evolves over time. The L-TBI did not produce any effect compared to L-Sham animals. In contrast to the Sham and L-TBI groups, the R-TBI group saw a decrease of Dyn A levels at 7 days after trauma (Fig. 1A). In the 24-h groups, the L-TBI and R-TBI did not produce any effects compared to their respective sham groups.
Hippocampus
The overall two-way ANOVA revealed a significant main effect of treatment [F(8,82)=9.3, p<0.001], a significant main effect of lateralization [F(1,82)=2.18, p<0.001], and a significant treatment×lateralization interaction [F(8,82)=4.45, p<0.001]. The significant main effect of treatment was further analyzed by Bonferroni's post-hoc analysis of the Dyn A mean hemispheric content (Fig. 1B). The Dyn A levels were elevated after 7 days in the L-Sham group compared to naïve animals (p<0.01), and to the 24-h L-Sham group (p<0.01). At 7 days, the effects of the L-TBI resulted in significantly decreased Dyn A levels compared to the L-Sham animals (p<0.05). No other significant differences were noted.
A one-way ANOVA (9 levels) performed for the ipsilateral [F(8,83)=11.7, p<0.001] and contralateral [F(8,82)=4.3, p<0.001] hemispheres separately, revealed significant sham and TBI effects, preferentially in the ipsilateral hemisphere (Fig. 2B). At 24 h, the L-TBI and R-TBI groups had significantly decreased Dyn A levels compared to those in the naïve animals (p<0.01 and p<0.05, respectively). Similarly, at the 7-day time point, the L-TBI and R-TBI compared to the L-Sham and R-Sham animals had significantly decreased Dyn A levels in the ipsilateral hemisphere (L-Sham 7 days versus L-TBI 7 days: p<0.001; R-Sham 7 days versus R-TBI 7 days: p<0.05). No significant effects were noted for the contralateral hemisphere. Thus, the sham surgery did not produce effects in the hippocampus at the 24-h time point, while at the 7-day time point the L-Sham animals had elevated mean levels in the hippocampus compared to the naive animals (Fig. 1B). The L-TBI and R-TBI predominantly affected the hippocampus of the ipsilateral hemisphere.
Striatum
The overall two-way ANOVA revealed a significant main effect of treatment [F(8,91)=12.69, p<0.001]. While there was no significant main effect of lateralization [F(1, 91)=1.41, p=0.238], there was a trend toward a marked treatment×lateralization interaction [F(8, 91)=1.84, p=0.080]. The significant main effect of treatment was further analyzed by Bonferroni's post-hoc analysis of the mean Dyn A hemispheric content (Fig. 1C). Compared to the naïve mice, the L-Sham animals at both time points, and the R-Sham animals at the 7-day point, had no significant effects. In contrast, 24 h after the R-Sham surgery, Dyn A levels were decreased compared to the levels in the naïve and L-Sham groups (p<0.01 and p<0.01, respectively). At 24 h, both L-TBI and R-TBI had decreased peptide levels compared to the naive animals (p<0.05 and p<0.001, respectively), and the L-Sham animals (p<0.01 and p<0.001, respectively). Effects of the L-TBI and R-Sham were time-dependent; the initial Dyn A downregulation at 24 h was followed by recovery observed in the 7-day animals. At the 7-day time point, the R-TBI animals had significantly decreased peptide levels compared to the R-Sham and L-TBI groups (p<0.05 and p<0.001, respectively), while no other significant effects were evident.
For consistency and because there was a trend toward a significant interaction when treatment×lateralization were compared, a one-way ANOVA (9 levels) was performed for the ipsilateral [F(8,92)=10.1, p<0.001] and contralateral [F(8,91)=5.3, p<0.001] hemispheres separately, which revealed significant sham and TBI effects (Fig. 2C). No significant effects were seen in the L-Sham and R-Sham groups versus naïve mice, and the L-TBI and R-TBI groups versus their respective sham groups. Similarly to the mean Dyn A hemispheric content analyses, a significant time effect for the ipsilateral hemisphere was noted for the R-Sham animals (R-Sham 24 h versus R-Sham 7 days: p<0.01), and the L-TBI animals (L-TBI 24 h versus L-TBI 7 days: p<0.001). At 7 days, R-TBI compared to L-TBI resulted in significantly lower Dyn A levels in the ipsilateral hemisphere (p<0.01).
Thus, the left side injury (sham and TBI) excluding the L-TBI animals at 24 h, did not produce significant effects on Dyn A content in the striatum. In contrast, the R-Sham at 24 h, and the R-TBI at 7 days, produced a significant decrease in the Dyn A content compared to their respective controls, including the naïve and R-Sham groups. We may thus conclude that the right-side sham and TBI produced more robust effects compared to the left-side injuries. The right-side effects depended on the type of injury (sham or TBI), and were time-dependent.
Discussion
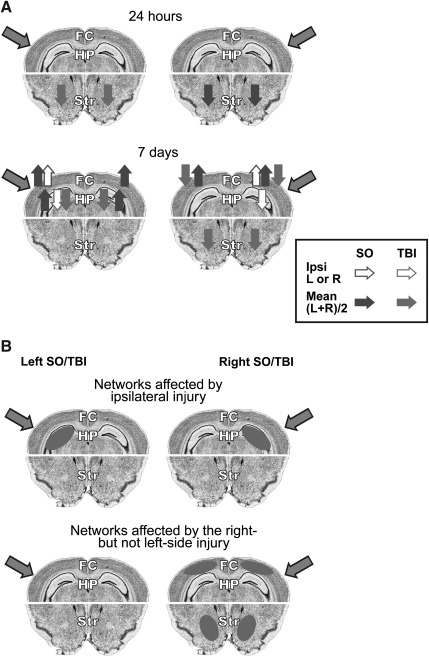
The main findings of this study, including transient changes in Dyn A levels in the mouse brain induced by sham surgery or TBI at 24 h, and more long-lasting responses evident at 7 days, are summarized in Figure 3. While the differences between TBI and sham animals were significant, the sham surgery also produced noticeable effects compared to the naïve animals.
FIG. 3.
(A) Summary of the effects of sham (SO) and TBI on Dyn A levels in the frontal cortex, hippocampus, and striatum. Gray arrows show damaged hemispheres. Solid arrows denote the direction of statistically significant effects of the sham or TBI; the open arrows point to effects in the hemisphere ipsilateral to the injury, while the filled arrows indicate changes in the content of the peptide averaged for the left and right hemispheres [(L+R)/2]. (B) Model for the hemispheric localization of the Dyn A-expressing neuronal networks affected by sham (SO) or TBI on the ipsilateral side (upper panel), or the networks affected by the right-side but not left-side injury (lower panel). Diagrams of coronal sections of a mouse brain were adopted from the Allen Mouse Brain Atlas (http://mouse.brain-map.org; Allen Institute for Brain Science, Seattle, WA), using section levels of −2.48 mm (for frontal cortex and HP), and+1.545 mm (for Str) from the bregma (TBI, traumatic brain injury; HP, hippocampus; Str, striatum; FC, frontal cortex; Ipsi, ipsilateral).
Both left-sided and right-sided trauma was studied because of the evidence for lateralized neurochemical and behavioral responses to unilateral brain injury in both rodents and humans (Heilman et al., 1978; Kerkhoff, 2001; Levin et al., 1995; Morrow et al., 1981; Pavlovskaya et al., 2007; Pearlson and Robinson, 1981; Schaefer et al., 2009). Previous studies have shown that region-specific differences in norepinephrine turnover after somatosensory TBI were dependent on the side of injury in rats (Levin et al., 1995). These lateralized effects had both behavioral and biochemical correlates. Right but not left somatosensory lesions produced behavioral hyperactivity and bilaterally decreased cerebral and locus ceruleus norepinephrine concentrations (Pearlson and Robinson, 1981). Identical lesions of the left frontal cerebral cortex produce neither the hyperactivity nor a decrease in norepinephrine levels. In humans, left hemisphere damage produced deficits in controlling the trajectory of arm movement, but not in achieving final position. In contrast, the right-hemisphere-damaged group showed deficits in accuracy of final position, but not in the ability to coordinate complex movement of a limb with multiple joints (Schaefer et al., 2009). Furthermore, lateralization of the lesion was identified as the critical factor in emotional hyporeactivity in brain-injured patients; right-hemisphere-damaged patients were psychophysiologically hypoaroused compared with left-hemisphere-damaged subjects (Heilman et al., 1978; Morrow et al., 1981).
By analogy to c-Fos activation in various experimental paradigms (Murphy et al., 2004), Dyn A may be considered a marker of neuronal circuits affected by pain (Mika et al., 2011; Schwarzer, 2009), stress (Knoll and Carlezon, 2010), substance abuse (Wee and Koob, 2010), brain and spinal cord injury (Faden, 1990; Goody et al., 2003; Hauser et al., 2005; Headrick et al., 1995; Hu et al., 1996; McIntosh et al., 1994). In this context, the overall effects of the unilateral injury may be explained as involving two types of Dyn A neuronal networks. The first type may respond to a unilateral brain injury preferentially on the side ipsilateral to the injury (Fig. 3B, upper panel). This response, seen in the hippocampus, does not depend on the side of the impact, and does not involve the contralateral hemisphere. The second type of circuits may have differential sensitivity to right- and left-sided injury, with a prevailing response induced by right-sided damage (Fig. 3B, lower panel). In such circuits, R-TBI but not L-TBI decreased Dyn A levels in the striatum and frontal cortex at the 7-day time point, and the R-Sham but not L-Sham in the striatum at the 24-h time point. The effects produced by the right-sided injuries are approximately similar in the left and right hemispheres. These two types of networks apparently differ in their topological organization in the brain. The first may operate in the hippocampus, while the second network is represented to a greater degree in the striatum and frontal cortex. The Dyn A-mediated effects seen in these networks may contribute to impairments of specific behaviors that may (the second type), and may not (the first type), depend on the side of injury. These two putative Dyn A circuits remain speculative, and have not yet been characterized by immunohistochemistry or other methods, which is a limitation of the present study.
Paw preference in rats and mice, which may result from the dominant hemisphere of the rodent brain (Gao and Zhang, 2008; Neveu and Merlot, 2003; Sullivan et al., 2012), is thought to be similar to handedness in humans. In rats, ischemia in the dominant hemisphere causes more significant neurobehavioral consequences than in the non-dominant hemisphere (Gao and Zhang, 2008). Hemispheric dominance may influence the lateralized response in Dyn A circuitry following TBI. The effects of TBI on the Dyn A networks and how the Dyn system affects behavior may be analyzed using genetic and pharmacological approaches. For example, mice deficient in Pdyn, or mouse strains that express this gene at different levels (Gieryk et al., 2010), may provide excellent models for such studies in the future.
The expression of Pdyn and dynorphins is upregulated in animals under several neuropathological paradigms, including experimental models of chronic pain (Hauser et al., 2005), suggesting a pathogenic role for these peptides (Bakalkin et al., 2010; Hauser et al., 2005). In the present study, all sham surgeries except R-Sham at the 24-h time point resulted in elevation of Dyn A levels, which is consistent with a nociceptive response. Considering that in models of spinal cord and brain injury, endogenous dynorphins may contribute to secondary CNS injury (Behrmann et al., 1993; Faden et al., 1987; Faden, 1990; Goody et al., 2003; Hauser et al., 2005; Headrick et al., 1995; Hu et al., 1996; McIntosh et al., 1994; McIntosh et al., 1987; Vink et al., 1990), it was anticipated that Dyn A would be upregulated in the TBI animals and contribute to neural injury via positive feedback. However, the present findings do not support this hypothesis. Instead of activation, TBI resulted in downregulation of Dyn A at both time points and in all three brain areas analyzed (Fig. 3A). The downregulation of Dyn A, as well as the lateralized nature of the TBI effects, support a “network” hypothesis for the role of Dyn A in TBI. In this scenario, Dyn A neurons are organized into two functionally discrete, but spatially overlapping, circuits in which one of the circuits functionally mediates the effects of R-TBI, but not L-TBI, on animal behavior.
The “Dyn network” hypothesis may also be applied to TBI-impaired locomotion. Unilateral, brain injury-induced motor deficits are generally characterized by a loss in performance of the contralesional forelimb and/or hindlimb. Our previous studies demonstrated that spinal neurons, which maintain muscular tone of the left and right hindlimbs, differ in their response to κ-agonist application (Bakalkin and Kobylyansky, 1989; Bakalkin et al., 1986,1989; Chazov et al., 1981). Thus dynorphin, or bremazocine, a synthetic κ-opioid agonist induced a preferential flexion of the right hindlimb. Neuropeptides are capable of volume transmission in the brain and spinal cord (Banghart and Sabatini, 2012; Duggan, 2000; Khachaturian et al., 1985), and diffusion through the cerebrospinal fluid. Conceivably, TBI-induced changes in dynorphins may propagate to the spinal cord via the cerebrospinal fluid. Therefore, we may speculate that the TBI-induced side-specific alterations in dynorphin levels in the spinal cord may shift the balance in activity of symmetric motor neurons in favor of persistent alterations in reflexes of the left or right leg, hence contributing to the unilateral motor deficit.
In summary, unilateral TBI and sham surgery induced changes in the levels of Dyn A. Decreased levels on the side ipsilateral to injury were evident in the hippocampus. Changes in Dyn A in the striatum and frontal cortex were specific to the side of trauma, and may contribute to the lateralized behavioral effects of the right versus left cortical injury (Kerkhoff, 2001; Levin et al., 1995; Pavlovskaya et al., 2007; Pearlson and Robinson, 1981; Schaefer et al., 2009). Future research should be directed towards an examination of Dyn A distribution within the damaged area, identification of neuronal networks expressing this peptide in the damaged brain, and establishing the functional role of lateralized neuronal Dyn A circuits in the normal and pathological brain.
Acknowledgments
This work was supported by grants from the Swedish Science Research Council to G.B., DA015097 to P.E.K., and from the NIH NS39828 to S.W.S.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams J.H. Doyle D. Graham D.I. Lawrence A.E. McLellan D.R. Gennarelli T.A. Pastuszko M. Sakamoto T. The contusion index: a reappraisal in human and experimental non-missile head injury. Neuropathol. Appl. Neurobiol. 1985;11:299–308. doi: 10.1111/j.1365-2990.1985.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Adjan V.V. Hauser K.F. Bakalkin G. Yakovleva T. Gharibyan A. Scheff S.W. Knapp P.E. Caspase-3 activity is reduced after spinal cord injury in mice lacking dynorphin: differential effects on glia and neurons. Neuroscience. 2007;148:724–736. doi: 10.1016/j.neuroscience.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Albensi B.C. Models of brain injury and alterations in synaptic plasticity. J. Neurosci. Res. 2001;65:279–283. doi: 10.1002/jnr.1151. [DOI] [PubMed] [Google Scholar]
- Anderson C.V. Wood D.M. Bigler E.D. Blatter D.D. Lesion volume, injury severity, and thalamic integrity following head injury. J. Neurotrauma. 1996;13:35–40. doi: 10.1089/neu.1996.13.35. [DOI] [PubMed] [Google Scholar]
- Anderson K.J. Miller K.M. Fugaccia I. Scheff S.W. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Bakalkin G. Kobylyansky A.G. Nagornaya L.V. Yarygin K.N. Titov M.I. Met-enkephalin-induced release into the blood of a factor causing postural asymmetry. Peptides. 1986;7:551–556. doi: 10.1016/0196-9781(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Bakalkin G. Kobylyansky A.G. Opioids induce postural asymmetry in spinal rat: the side of the flexed limb depends upon the type of opioid agonist. Brain Res. 1989;480:277–289. doi: 10.1016/0006-8993(89)90193-5. [DOI] [PubMed] [Google Scholar]
- Bakalkin G. Pivovarov A.S. Kobylyansky A.G. Yarygin K.N. Akparov V. Ipsilateral responses induced by factors present in left and right hemispheres. Int. J. Neurosci. 1989;47:217–230. doi: 10.3109/00207458908987436. [DOI] [PubMed] [Google Scholar]
- Bakalkin G. Watanabe H. Jezierska J. Depoorter C. Verschuuren-Bemelmans C. Bazov I. Artemenko K.A. Yakovleva T. Dooijes D. Van de Warrenburg B.P. Zubarev R.A. Kremer B. Knapp P.E. Hauser K.F. Wijmenga C. Nyberg F. Sinke R.J. Verbeek D.S. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am. J. Hum. Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R. Ni R.X. Faden A.I. N-methyl-D-aspartate (NMDA) and opioid receptors mediate dynorphin-induced spinal cord injury: behavioral and histological studies. Brain Res. 1992;580:255–264. doi: 10.1016/0006-8993(92)90952-6. [DOI] [PubMed] [Google Scholar]
- Baldwin S.A. Gibson T. Callihan C.T. Sullivan P.G. Palmer E. Scheff S.W. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical dissector method for cell counting. J. Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- Banghart M.R. Sabatini B.L. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron. 2012;73:249–259. doi: 10.1016/j.neuron.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann D.L. Bresnahan J.C. Beattie M.S. A comparison of YM-14673, U-50488H, and nalmefene after spinal cord injury in the rat. Exp. Neurol. 1993;119:258–267. doi: 10.1006/exnr.1993.1028. [DOI] [PubMed] [Google Scholar]
- Berger E. Leven F. Pirente N. Bouillon B. Neugebauer E. Quality of life after traumatic brain injury: A systematic review of the literature. Restor. Neurol. Neurosci. 1999;14:93–102. [PubMed] [Google Scholar]
- Bramlett H.M. Green E.J. Dietrich W.D. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Chazov E.I. Bakalkin G. Yarigin K.N. Trushina E.D. Titov M.I. Smirnov V.N. Enkephalins induce asymmetrical effects on posture in the rat. Experientia. 1981;37:887–889. doi: 10.1007/BF01985696. [DOI] [PubMed] [Google Scholar]
- Christensson-Nylander I. Nyberg F. Ragnarsson U. Terenius L. A general procedure for analysis of proenkephalin B derived opioid peptides. Regul. Pept. 1985;11:65–76. doi: 10.1016/0167-0115(85)90032-1. [DOI] [PubMed] [Google Scholar]
- Colicos M.A. Dixon C.E. Dash P.K. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Duggan A.W. Neuropeptide spread in the brain and spinal cord. Prog. Brain Res. 2000;125:369–380. doi: 10.1016/S0079-6123(00)25026-7. [DOI] [PubMed] [Google Scholar]
- Elder G.A. Mitsis E.M. Ahlers S.T. Cristian A. Blast-induced mild traumatic brain injury. Psychiatr. Clin. North Am. 2010;33:757–781. doi: 10.1016/j.psc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Neurotoxic versus neuroprotective actions of endogenous opioid peptides: implications for treatment of CNS injury. NIDA Res. Monogr. 1996;163:318–330. [PubMed] [Google Scholar]
- Faden A.I. Opioid and nonopioid mechanisms may contribute to dynorphin's pathophysiological actions in spinal cord injury. Ann. Neurol. 1990;27:67–74. doi: 10.1002/ana.410270111. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Takemori A.E. Portoghese P.S. Kappa-selective opiate antagonist nor-binaltorphimine improves outcome after traumatic spinal cord injury in rats. Cent. Nerv. Syst. Trauma. 1987;4:227–237. doi: 10.1089/cns.1987.4.227. [DOI] [PubMed] [Google Scholar]
- Fox G.B. LeVasseur R.A. Faden A.I. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- Gao H. Zhang M. Asymmetry in the brain influenced the neurological deficits and infarction volume following the middle cerebral artery occlusion in rats. Behav. Brain Funct. 2008;4:57. doi: 10.1186/1744-9081-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieryk A. Ziolkowska B. Solecki W. Kubik J. Przewlocki R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl.) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- Goody R.J. Martin K.M. Goebel S.M. Hauser K.F. Dynorphin A toxicity in striatal neurons via an alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor mechanism. Neuroscience. 2003;116:807–816. doi: 10.1016/s0306-4522(02)00563-8. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Adams J.H. Nicoll J.A. Maxwell W.L. Gennarelli T.A. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5:397–406. doi: 10.1111/j.1750-3639.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Sullivan P.G. Gibson T.R. Pavel K.M. Thompson B.M. Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hanell A. Clausen F. Bjork M. Jansson K. Philipson O. Nilsson L.N. Hillered L. Weinreb P.H. Lee D. McIntosh T.K. Gimbel D.A. Strittmatter S.M. Marklund N. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J. Neurotrauma. 2010;27:1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K.F. Aldrich J.V. Anderson K.J. Bakalkin G. Christie M.J. Hall E.D. Knapp P.E. Scheff S.W. Singh I.N. Vissel B. Woods A.S. Yakovleva T. Shippenberg T.S. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headrick J.P. Faden A.I. Vink R. Intrathecal dynorphin-A infusion in rat spinal cord causes energy depletion, edema and neurologic dysfunction. Neurochem. Int. 1995;26:489–495. doi: 10.1016/0197-0186(94)00153-l. [DOI] [PubMed] [Google Scholar]
- Heilman K.M. Schwartz H.D. Watson R.T. Hypoarousal in patients with the neglect syndrome and emotional indifference. Neurology. 1978;28:229–232. doi: 10.1212/wnl.28.3.229. [DOI] [PubMed] [Google Scholar]
- Hunt R.F. Scheff S.W. Smith B.N. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp. Neurol. 2009;215:243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.H. Lee F.C. Wan X.S. Chen Y.T. Jen M.F. Dynorphin neurotoxicity induced nitric oxide synthase expression in ventral horn cells of rat spinal cord. Neurosci. Lett. 1996;203:13–16. doi: 10.1016/0304-3940(95)12246-x. [DOI] [PubMed] [Google Scholar]
- Kelso M.L. Wehner J.M. Collins A.C. Scheff S.W. Pauly J.R. The pathophysiology of traumatic brain injury in alpha7 nicotinic cholinergic receptor knockout mice. Brain Res. 2006;1083:204–210. doi: 10.1016/j.brainres.2006.01.127. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Prog. Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Khachaturian H. Lewis M.E. Schafer M.K. Watson S.J. Anatomy of the CNS opioid systems. Trends Neurosci. 1985;8:111–119. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Knoll A.T. Carlezon W.A., Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B.E. Brown K.L. Pawar G. Dunn-Meynell A. Widespread and lateralization effects of acute traumatic brain injury on norepinephrine turnover in the rat brain. Brain Res. 1995;674:307–313. doi: 10.1016/0006-8993(95)00032-l. [DOI] [PubMed] [Google Scholar]
- Long J.B. Rigamonti D.D. Oleshansky M.A. Wingfield C.P. Martinez-Arizala A. Dynorphin A-induced rat spinal cord injury: evidence for excitatory amino acid involvement in a pharmacological model of ischemic spinal cord injury. J. Pharmacol. Exp. Ther. 1994;269:358–366. [PubMed] [Google Scholar]
- Lyeth B.G. Jenkins L.W. Hamm R.J. Dixon C.E. Phillips L.L. Clifton G.L. Young H.F. Hayes R.L. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Stocchetti N. Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Fernyak S. Yamakami I. Faden A.I. Central and systemic kappa-opioid agonists exacerbate neurobehavioral response to brain injury in rats. Am. J. Physiol. 1994;267:R665–R672. doi: 10.1152/ajpregu.1994.267.3.R665. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Hayes R.L. DeWitt D.S. Agura V. Faden A.I. Endogenous opioids may mediate secondary damage after experimental brain injury. Am. J. Physiol. 1987;253:E565–E574. doi: 10.1152/ajpendo.1987.253.5.E565. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Saatman K.E. Raghupathi R. Graham D.I. Smith D.H. Lee V.M. Trojanowski J.Q. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: pathogenetic mechanisms. Neuropathol. Appl. Neurobiol. 1998;24:251–267. doi: 10.1046/j.1365-2990.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- Merg F. Filliol D. Usynin I. Bazov I. Bark N. Hurd Y.L. Yakovleva T. Kieffer B.L. Bakalkin G. Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J. Neurochem. 2006;97:292–301. doi: 10.1111/j.1471-4159.2006.03732.x. [DOI] [PubMed] [Google Scholar]
- Mika J. Obara I. Przewlocka B. The role of nociceptin and dynorphin in chronic pain: implications of neuro-glial interaction. Neuropeptides. 2011;45:247–261. doi: 10.1016/j.npep.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Morrow L. Vrtunski P.B. Kim Y. Boller F. Arousal responses to emotional stimuli and laterality of lesion. Neuropsychologia. 1981;19:65–71. doi: 10.1016/0028-3932(81)90045-2. [DOI] [PubMed] [Google Scholar]
- Murphy M. Greferath U. Nag N. Nithianantharajah J. Wilson Y.M. Tracing functional circuits using c-Fos regulated expression of marker genes targeted to neuronal projections. Front. Biosci. 2004;9:40–47. doi: 10.2741/1203. [DOI] [PubMed] [Google Scholar]
- Neveu P.J. Merlot E. Cytokine stress responses depend on lateralization in mice. Stress. 2003;6:5–9. doi: 10.1080/1025389031000087472. [DOI] [PubMed] [Google Scholar]
- Pavlovskaya M. Groswasser Z. Keren O. Mordvinov E. Hochstein S. Hemispheric visual attentional imbalance in patients with traumatic brain injury. Brain Cogn. 2007;64:21–29. doi: 10.1016/j.bandc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Pearlson G.D. Robinson R.G. Suction lesions of the frontal cerebral cortex in the rat induce asymmetrical behavioral and catecholaminergic responses. Brain Res. 1981;218:233–242. doi: 10.1016/0006-8993(81)91303-2. [DOI] [PubMed] [Google Scholar]
- Pierce J.E. Smith D.H. Trojanowski J.Q. McIntosh T.K. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Buki A. Koiziumi H. Stone J. Okonkwo D.O. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir. Suppl. 1999;73:15–20. doi: 10.1007/978-3-7091-6391-7_3. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Graham D.I. McIntosh T.K. Apoptosis after traumatic brain injury. J. Neurotrauma. 2000;17:927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- Schaefer S.Y. Haaland K.Y. Sainburg R.L. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Price D.A. Hicks R.R. Baldwin S.A. Robinson S. Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- Schwarzer C. 30 Years of dynorphins—new insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.S. Bray E.R. Zhang C.Q. Dixon C.E. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011;1369:208–215. doi: 10.1016/j.brainres.2010.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H.D. Hicks R.R. Smith D. McIntosh T.K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M. Chehab S.L. Dufresne M.M. Laplante F. Role of sex in the neurochemical and neuroendocrine correlates of paw preference in the rat. Neuroscience. 2012;202:192–201. doi: 10.1016/j.neuroscience.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Thompson S.N. Carrico K.M. Mustafa A.G. Bains M. Hall E.D. A pharmacological analysis of the neuroprotective efficacy of the brain- and cell-permeable calpain inhibitor MDL-28170 in the mouse controlled cortical impact traumatic brain injury model. J. Neurotrauma. 2010;27:2233–2243. doi: 10.1089/neu.2010.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R. McIntosh T.K. Rhomhanyi R. Faden A.I. Opiate antagonist nalmefene improves intracellular free Mg2+, bioenergetic state, and neurologic outcome following traumatic brain injury in rats. J. Neurosci. 1990;10:3524–3530. doi: 10.1523/JNEUROSCI.10-11-03524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S. Koob G.F. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl.) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A.S. Kaminski R. Oz M. Wang Y. Hauser K. Goody R. Wang H.Y. Jackson S.N. Zeitz P. Zeitz K.P. Zolkowska D. Schepers R. Nold M. Danielson J. Graslund A. Vukojevic V. Bakalkin G. Basbaum A. Shippenberg T. Decoy peptides that bind dynorphin noncovalently prevent NMDA receptor-mediated neurotoxicity. J. Proteome Res. 2006;5:1017–1023. doi: 10.1021/pr060016+. [DOI] [PMC free article] [PubMed] [Google Scholar]