The role of 18F-fluorodeoxyglucose positron emission tomography–computed tomography in the staging of early invasive primary breast cancer was evaluated.
Keywords: Breast cancer, Computed tomography, Spiral, Positron emission tomography, Staging
Abstract
Introduction.
Currently, there is a lack of data on the role of combined positron emission tomography–computed tomography (PET–CT) in the staging of early invasive primary breast cancer. We therefore evaluated the role of 18F-fluorodeoxyglucose (18F-FDG)-PET–CT in this patient population.
Methods.
We prospectively recruited 70 consecutive patients (69 women, one man; mean age, 61.9 ± 8.1 years) with early primary breast cancer for staging with 18F-FDG-PET–CT. All PET–CT images were interpreted by two readers (independently of each other). A third reader adjudicated any discrepancies. All readers had ≥5 years of specific experience. Ethics board approval and informed consent were obtained.
Results.
The mean clinical follow-up was 22.7 ± 12.6 months. The primary tumor was identified with PET–CT in 64 of 70 patients. Of the unidentified lesions, surgical pathology revealed two intraductal carcinomas, one invasive tubular carcinoma, and three invasive lobular carcinomas. Undiagnosed multifocal breast disease was shown in seven of 70 patients. PET–CT identified avid axillary lymph nodes in 19 of 70 patients, compared with 24 of 70 confirmed during surgery. There were four patients who were axillary node positive on PET but had no axillary disease at surgery.
Five patients were reported with avid metastases. Two of those patients were treated for metastatic disease (nodal, lung, and liver in one and bone metastases in the other) following further imaging and clinical assessment. In the other three patients, lesions (lung, n = 1; pleural, n = 1; paratrachael node, n = 1) were subsequently diagnosed as benign lesions.
Conclusion.
Integrated 18F-FDG-PET–CT may have a role in staging patients presenting with early breast cancer.
Introduction
Breast cancer is a common disease with a highly variable outcome [1], and therefore accurate tumor staging is important. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) has been shown to be helpful in the staging of many malignancies [2]. This reflects PET's inherent sensitivity over other imaging modalities [3]. However, the role of PET in the staging of primary breast cancer is less clear and there is only limited evidence, as recently highlighted [4]. Initial studies showed the potential for PET in this respect, but most studies were from stand-alone scanners [5–8], and there is recognition that data from PET–computed tomography (CT) is needed [9]. PET–CT over stand-alone PET has shown added value for many malignancies [10, 11], and such findings have also been suggested for breast cancer [12]. These findings are intuitive; the CT component aids localization of PET findings, whereas it may identify soft tissue and bone changes beyond the spatial resolution of radionuclide imaging [13]. The role of 18F-FDG-PET–CT in recurrent breast cancer and in treatment response is recognized [14–16], and the use of PET–CT to phenotype breast cancer by imaging metabolic flow relationships has been shown [17, 18].
Current recommendations do not incorporate the routine use of 18F-FDG-PET–CT for staging primary breast cancer [2]. However, the direct scientific evidence to support this recommendation is limited. There have been prospective investigations with 18F-FDG-PET–CT in patients with advanced primary breast cancer [19, 20] and in high-risk or recurrent patients [21–23]. Other studies combined whole-body 18F-FDG-PET–CT with PET–CT mammography [24] or compared diffusion-weighting magnetic resonance imaging (MRI) and PET–CT [25], but the data were obtained in patients with known metastatic disease.
The lack of evidence for the role of PET–CT in primary breast cancer has also led to a recent call for more research [4]; this is particularly true for early disease. Subsequent retrospective data suggesting that PET–CT may indeed have a role in breast cancer staging have recently become available, which further highlighted the need for prospective investigations [26]. Here, we present the findings from a prospective study investigating the role of 18F-FDG-PET–CT in the staging of patients with early primary breast carcinoma.
Materials and Methods
Patients
We prospectively recruited 70 consecutive eligible consenting patients (69 women and one man; mean age, 61.9 ± 8.1 years) with early primary unilateral invasive breast cancer for 18F-FDG-PET–CT staging. Eligibility included patients with primary tumors sized 1.0–3.5 cm (mean size, 2.1 ± 0.7 cm) as measured on imaging. Patients aged <45 years, pregnant patients, and patients with inflammatory breast carcinoma were excluded. The only other exclusion was patients declining to take part. All patients had undergone triple assessment including mammography, ultrasound, and core biopsy prior to recruitment. These tests were performed before the PET–CT study. The mean time from PET–CT to surgery was 2.4 ± 2.1 weeks. Although some patients at our institutions receive MRI clinically, breast MRI was not part of this study. In this patient cohort, bone scintigraphy, liver ultrasound, and CT were not performed because these patients had early disease.
Axillary node staging was performed using sentinel lymph node scintigraphy (performed after the PET–CT exam) in 59 patients. In nine patients, ultrasound-guided nodal biopsy confirmed metastasis (prior to the PET–CT examination). The remaining two patients had large multicentric lesions with enlarged axillary nodes on ultrasound. However, subsequent axillary clearance showed no metastasis. A clinical and histological profile of the study patients is presented in Table 1 and in supplemental online Table 1.
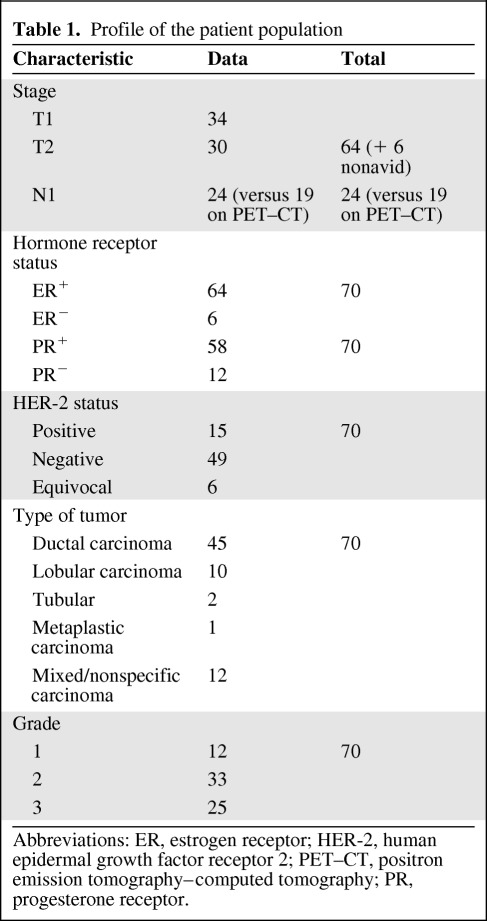
Table 1.
Profile of the patient population
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; PET–CT, positron emission tomography–computed tomography; PR, progesterone receptor.
Ethics board approval and informed consent was obtained.
PET–CT Imaging
Following 6 hours of fasting prior to examination (ensuring a blood glucose level <10 mmol/L in all patients), images were acquired 1 hour after injecting 200 MBq of 18F-FDG using a dedicated combined PET–64-detector-CT (VCT-XT Discovery; GE-Healthcare Technology, Waukesha, WI). A CT scan was performed (for attenuation correction) using 64-mm × 3.75-mm detectors, a pitch of 1.5, and a 5-mm collimation (140 kVp and 80 mA in 0.8 seconds) from vertex to mid-thigh. Oral but not i.v. CT contrast medium was administered. Maintaining the patient position, an 18F-FDG-PET scan was performed that covered an area identical to that covered by the CT scan. All PET acquisitions were carried out in two-dimensional mode (8 minutes per bed position, compensating for the reduced administered activity, minimizing radiation exposure). Transaxial emission images of 3.27 mm thickness (pixel size, 3.9 mm) were reconstructed using ordered subsets expectation maximization with two iterations and 28 subsets. The axial field of view was 148.75 mm, resulting in 47 slices per bed position. The images were reconstructed as a 128 × 128 matrix.
PET–CT Image Interpretation
PET–CT images were displayed on an Advantage work station (GE Healthcare Technology, Waukesha, WI). On the dual-screen work station, the PET and CT datasets were both displayed as separate but coregistered images and also displayed using color (PET) images fused with CT. All readers had access to all these datasets. All the PET–CT images were interpreted by two readers (independently of each other). A third reader adjudicated any discrepancies. All readers had ≥5 years of specific experience. The only clinical data provided to the readers was primary breast carcinoma. The readers were unaware of the other imaging and histological findings. Both PET and CT images were reviewed by all readers. The PET–CT reporting and acquisitions used current European cancer guideline criteria and standardizations [27].
Histology
Histology was performed as per routine clinical management using the surgically excised specimens (mastectomy in 20 patients and wide local excision in 50 patients).
Statistical Analysis
The population size was determined as 70 using a proportion of p = 90% and a precision of 6% (in our estimation), including an additional 10%, allowing for incomplete data. Receiver operating characteristic (ROC) curve analysis was performed to identify the ideal standardized uptake value (SUV) threshold for detecting axillary node metastasis. κ statistics were used to assess interobserver variability in the staging of early breast cancer with PET–CT. Sensitivity and specificity were formally calculated (Table 2) for axillary node metastasis calculation. All statistics were performed using SPSS 2011 software (IBM; Armonk, NY).
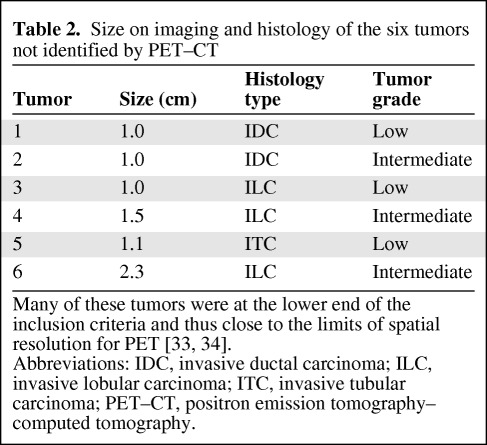
Table 2.
Size on imaging and histology of the six tumors not identified by PET–CT
Many of these tumors were at the lower end of the inclusion criteria and thus close to the limits of spatial resolution for PET [33, 34].
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ITC, invasive tubular carcinoma; PET–CT, positron emission tomography–computed tomography.
Results
Primary Tumor
The primary tumor was identified in 64 of 70 patients. The mean maximum SUV (SUVmax) in the identified lesions was 6.4 ± 5.6. Of the six unidentified lesions, surgical pathological data revealed two invasive ductal carcinomas, one invasive tubular carcinoma, and three invasive lobular carcinomas (Table 2).
Multifocal Disease
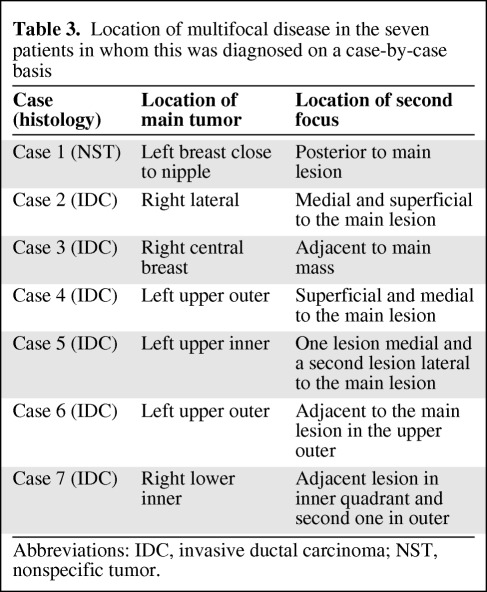
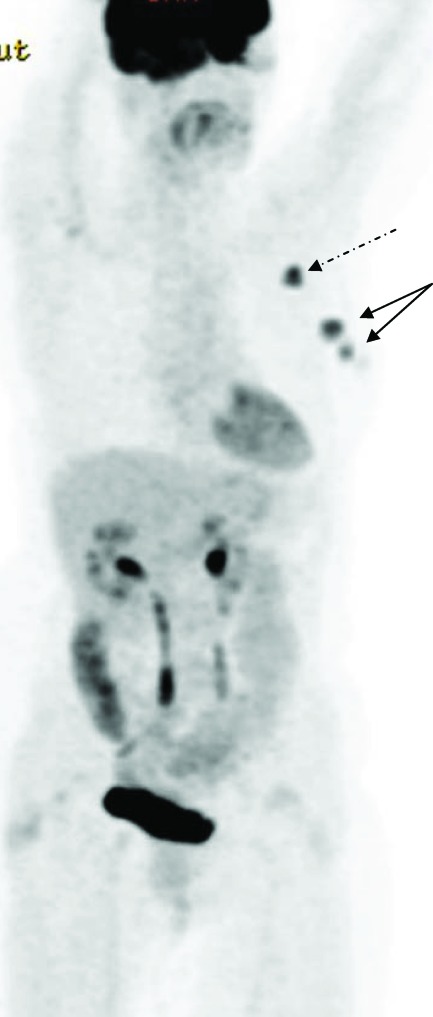
Undiagnosed multifocal disease was shown on PET–CT in seven of 70 patients (Table 3 and Fig. 1). This was not identified by mammography or ultrasound. Multifocal disease was confirmed by histopathology in all cases.
Table 3.
Location of multifocal disease in the seven patients in whom this was diagnosed on a case-by-case basis
Abbreviations: IDC, invasive ductal carcinoma; NST, nonspecific tumor.
Figure 1.
18F-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography of a patient with newly diagnosed primary breast cancer. The maximum intensity projection image shows multifocal disease in the left breast (solid arrows). Avid left axillary nodal uptake is also identified (dashed arrow). No distant FDG-avid disease is identified.
Axillary Lymph Node Staging
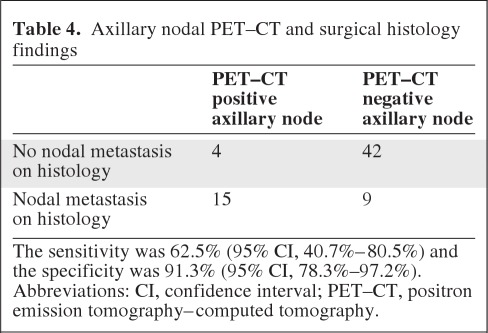
All patients were staged using histology as the gold standard. PET–CT identified avid axillary lymph nodes in 19 of 70 patients, compared with 24 of 70 confirmed on histology. There were four patients who were axillary node positive on PET but had no axillary disease at surgery (Table 4). In total, 18 nodes were obtained at surgery and histologically analyzed. The maximum short axis diameter (in mm) in the nodes of these four patients on CT were 6.4, <5, 11.2, and 15.6.
Table 4.
Axillary nodal PET–CT and surgical histology findings
The sensitivity was 62.5% (95% CI, 40.7%–80.5%) and the specificity was 91.3% (95% CI, 78.3%–97.2%).
Abbreviations: CI, confidence interval; PET–CT, positron emission tomography–computed tomography.
ROC curve analysis revealed an SUVmax threshold of 1.4 to maximize the best compromise of sensitivity and specificity.
Extra-Axillary FDG-Avid Foci
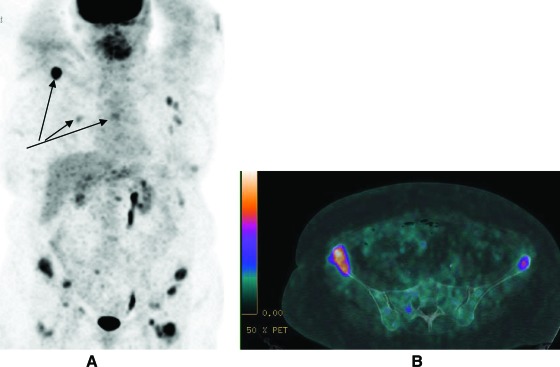
Metastatic lesions were reported in five patients. After imaging and clinical assessment, metastatic disease was found in two of 70 patients. One of these patients, aged 75 years with a 2.2-cm intermediate-grade lobular estrogen receptor–positive carcinoma, had widespread avid skeletal disease, which was also identified on CT (Fig. 2). The most avid skeletal lesion had an SUVmax of 19.5. The second patient, aged 54 years with a 3.5-cm intermediate-grade ductal estrogen receptor–positive carcinoma, had nodal disease above and below the diaphragm with lymph node, liver, and lung nodule (the largest was 7 mm and avid) involvement. The most avid distant lesion in that patient had an SUVmax of 6.0.
Figure 2.
18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) of a patient with newly diagnosed primary breast cancer. The maximum intensity projection image (A) shows widespread avid disease including the right scapula, right 6th rib and the T6 vertebra (see arrows). The fused PET–CT image (B) shows that focal avidity in the pelvis coregisters to the bony pelvis.
In the remaining three patients, the lesions were subsequently believed to be benign. In one of those patients, biopsy of a moderately avid pleural nodule revealed a fibroma. In the second patient (treated with trastuzumab), who had a mildly avid lung nodule on PET, follow-up CT showed no identifiable lung nodules and the patient remained disease free 25 months after the PET–CT study. In the last patient, with a moderately avid paratracheal lymph node on PET–CT imaging, clinical follow-up for 31 months showed no evidence of disease recurrence off treatment.
The mean clinical follow-up period was 22.7 ± 12.6 months. To date there is no evidence of false-negative metastatic disease.
Interobserver Variability
The κ statistic for tumor, node, and metastasis reporting were 0.91, 0.83, and 0.86, respectively.
Discussion
Original, prospective 18F-FDG-PET–CT data from 70 patients with primary early breast carcinoma are presented. Over 95% of the invasive ductal carcinomas were identified on PET–CT, but only one third of lobular carcinomas were identified. In this study, distant PET findings that were reported as metastatic were found in 7% of patients and previously undetected multifocal disease was identified in 9% of patients. Patient breast cancer management was directly affected in nine (13%) patients—two patients with distant metastases and seven patients with multifocal disease. Our findings from early breast cancer mirror the results of 18F-FDG-PET–CT scanning in patients with more advanced primary breast cancer, for which PET–CT was found to impact patient management in 42% [19] and 18% [20] of cases. Given the useful biological correlates of FDG-PET in primary breast cancer [28], there is a need for further assessment of FDG-PET–CT for primary breast cancer.
In this study, 18F-FDG avidity was identified above and below the diaphragm as well as in the skeleton. Extralocoregional metastases alter patient management and provide additional prognostic information. The findings of skeletal metastatic disease also significantly alter the management pathway and again impact prognosis, and thereby aid targeted individualized treatment for the patient [29]. In both these cases, systemic therapy is indicated. In one case in this study, a pleural abnormality detected on PET required additional ultrasound and biopsy to determine the etiology; these showed a benign pleural tumor, illustrating the effort needed to obtain the correct diagnosis. The findings of unidentified multifocal breast disease in seven patients also impacted patient management; these patients require mastectomy instead of wide local excisions. MRI and mammography are useful to diagnose multifocal disease, but their diagnostic accuracy is limited [30]. MRI has nonetheless been shown to be useful in the management of breast cancer with the potential of reducing unnecessary surgery [31, 32].
The performance of 18F-FDG-PET is limited in diagnosing axillary disease because of its failure to detect micrometastases [33]. Both the size of the lesion and the intensity of tracer uptake are important determinants for lesion detectability with PET. Phantom studies show that lesions <5 mm are not reliably detected on PET [33]. Thus, it is not surprising that micrometastases (<2 mm) are not detected, and indeed a recent technology assessment showed that the smallest axillary node detected was 3 mm [34]. One can improve the sensitivity of axillary nodal metastasis detection by reducing the SUV threshold, but this can negatively impact specificity. Therefore, low-grade 18F-FDG nodal uptake may simply represent inflammatory (reactive) changes and thus give rise to false-positive findings. Our findings confirm the presence of both false-negative and false-positive lymph nodes, which is consistent with a large multicenter study using stand-alone PET [34]. The sensitivity and specificity in our study were higher, and this may reflect the contribution of the CT component of PET–CT over PET-only scanners. Compared with a recent health technology assessment, our data showed similar accuracy in diagnosing axillary disease, with high specificity but limited sensitivity [34]. Although PET–CT is limited for axillary staging, a recent study showed that PET/CT may extend the use of sentinel lymph node biopsy over more invasive methods [35].
Generally, conventional imaging modalities such as whole-body CT and liver ultrasound are not routinely used in asymptomatic breast cancer patients with stage 1 or stage 2 disease [7, 36]. Given that the quoted incidence of detectable metastatic disease at the time of breast cancer diagnosis is ∼4% [36, 37], then the rationale for such a strategy may be justified. However, the detection of anatomical changes using conventional cross-sectional imaging is less sensitive than the picomolar sensitivity of PET [3]. Moreover, whole-body PET–CT gives the potential of synergistic diagnostic performance by combining the spatial resolution of multidetector CT data with the metabolic sensitivity of PET. Our findings in patients with early breast cancer, along with PET–CT findings in patients with more advanced primary disease [19, 20], confirm that, in many cases, there is potential to change patient management.
In order to evaluate the usefulness of PET–CT for early breast cancer management, many factors need to be assessed. First, the true incidence of 18F-FDG-PET–CT–detectable disease in various stages of primary breast cancer needs to be determined and systematically measured. Then, how this detection alters patient management, survival, and quality of life should be assessed. The anxiety caused by detecting foci of avidity that are later found to be benign should not be overlooked. It should also be appreciated that PET–CT staging may also give important prognostic information, for example, 18F-FDG uptake has been shown to predict survival and angiogenesis in lung cancer patients [38–40]. This type of additional information is needed, but is limited in breast cancer. Second, the cost to patients of undergoing PET–CT needs to be assessed, such as radiation exposure and inconvenience. Finally, the cost of performing 18F-FDG-PET–CT to the health service provider needs to be accurately calculated. These factors are complex, and much more data are needed than presently exist to perform a health technology assessment. There would appear to be need for a prospective, randomized, controlled study such as the recent COMICE (comparative effectiveness of MRI in breast cancer) study that evaluated MRI staging [30].
Studies of the type undertaken here have inherent limitations. Breast cancer is a heterogeneous disease [1]. In this respect, we were able to obtain a relatively homogeneous cohort in terms of early disease, histology, and lack of presurgical neoadjuvant chemotherapy or radiotherapy. A larger patient population would have been helpful, but this would have significantly increased the recruitment and follow-up periods. It should be appreciated that it is much easier to confirm the presence of metastatic disease than to exclude it. Therefore, a longer follow-up period would have been ideal, but this should be balanced against the need to obtain data that are lacking in the current literature.
Conclusion
We present original prospective data on the use of 18F-FDG-PET–CT in the staging of early breast cancer. In keeping with the limited literature on advanced primary breast cancer, PET–CT was shown to impact cancer management in 16% of patients with early primary breast carcinoma. Given that the current recommendations for primary breast cancer do not routinely advocate the use of PET–CT for staging, our findings highlight the recent call for a randomized, controlled trial to further investigate the role of PET–CT in breast cancer staging [4].
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Dr. Manuel Rodriguez-Justo for histopathological support. We thank Dr. John Dickson for his academic physics assistance and Nick Papathanasiou for his statistical input.
The majority of the funding in this study was provided by the Breast Cancer Campaign, UK. UCLH/UCL receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centre's funding scheme.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Ashley M. Groves, Manu Shastry, Ruth M. Warren, Peter J. Ell, Mohammed R. Keshtgar
Collection and/or assembly of data: Ashley M. Groves, Manu Shastry, Tina Kelleher, Diane Whittaker, Marie Meagher, Ruth M. Warren, Peter J. Ell, Mohammed R. Keshtgar
Data analysis and interpretation: Ashley M. Groves, Manu Shastry, Simona Ben-Haim, Irfan Kayani, Anmol Malhotra, Timothy Davidson, Brian Holloway, Ruth M. Warren, Peter J. Ell, Mohammed R. Keshtgar
Manuscript writing: Ashley M. Groves, Manu Shastry, Simona Ben-Haim, Irfan Kayani, Anmol Malhotra, Timothy Davidson, Brian Holloway, Ruth M. Warren, Peter J. Ell, Mohammed R. Keshtgar
Final approval of manuscript: Ashley M. Groves, Manu Shastry, Simona Ben-Haim, Irfan Kayani, Anmol Malhotra, Timothy Davidson, Tina Kelleher, Diane Whittaker, Marie Meagher, Brian Holloway, Ruth M. Warren, Peter J. Ell, Mohammed R. Keshtgar
References
- 1.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer: A study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Groves AM, Win T, Haim SB, et al. Non-[18F] FDG PET in clinical oncology. Lancet Oncol. 2007;8:822–830. doi: 10.1016/S1470-2045(07)70274-7. [DOI] [PubMed] [Google Scholar]
- 4.Groheux D, Hindié E, Rubello D, et al. Should FDG PET/CT be used for the initial staging of breast cancer? Eur J Nucl Med Mol Imaging. 2009;36:1539–1542. doi: 10.1007/s00259-009-1159-0. [DOI] [PubMed] [Google Scholar]
- 5.Schirrmeister H, Kḧn T, Guhlmann A, et al. Fluorine-18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: Comparison with the standard staging procedures. Eur J Nucl Med. 2001;28:351–358. doi: 10.1007/s002590000448. [DOI] [PubMed] [Google Scholar]
- 6.Cermik TF, Mavi A, Basu S, et al. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging. 2008;35:475–483. doi: 10.1007/s00259-007-0580-5. [DOI] [PubMed] [Google Scholar]
- 7.Gerber B, Seitz E, Ml̈ler H, et al. Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat. 2003;82:29–37. doi: 10.1023/B:BREA.0000003917.05413.ac. [DOI] [PubMed] [Google Scholar]
- 8.Puglisi F, Follador A, Minisini AM, et al. Baseline staging tests after a new diagnosis of breast cancer: Further evidence of their limited indications. Ann Oncol. 2005;16:263–266. doi: 10.1093/annonc/mdi063. [DOI] [PubMed] [Google Scholar]
- 9.Belohlavek O. What is the role of FDG-PET in the initial staging of breast cancer? Eur J Nucl Med Mol Imaging. 2008;35:472–474. doi: 10.1007/s00259-007-0643-7. [DOI] [PubMed] [Google Scholar]
- 10.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 11.Syed R, Bomanji JB, Nagabhushan N, et al. Impact of combined (18)F-FDG PET/CT in head and neck tumours. Br J Cancer. 2005;92:1046–1050. doi: 10.1038/sj.bjc.6602464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsumi M, Cohade C, Mourtzikos KA, et al. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging. 2006;33:254–262. doi: 10.1007/s00259-005-1835-7. [DOI] [PubMed] [Google Scholar]
- 13.Groves AM, Beadsmoore CJ, Cheow HK, et al. Can 16-detector multislice CT exclude skeletal lesions during tumour staging? Implications for the cancer patient. Eur Radiol. 2006;16:1066–1073. doi: 10.1007/s00330-005-0042-z. [DOI] [PubMed] [Google Scholar]
- 14.Zangheri B, Messa C, Picchio M, et al. PET/CT and breast cancer. Eur J Nucl Med Mol Imaging. 2004;31(suppl 1):S135–S142. doi: 10.1007/s00259-004-1536-7. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 16.Duch J, Fuster D, Muñoz M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–1557. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 17.Dunnwald LK, Gralow JR, Ellis GK, et al. Tumor metabolism and blood flow changes by positron emission tomography: Relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 2008;26:4449–4457. doi: 10.1200/JCO.2007.15.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groves AM, Wishart GC, Shastry M, et al. Metabolic-flow relationships in primary breast cancer: Feasibility of combined PET/dynamic contrast-enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36:416–421. doi: 10.1007/s00259-008-0948-1. [DOI] [PubMed] [Google Scholar]
- 19.Fuster D, Duch J, Paredes P, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008;26:4746–4751. doi: 10.1200/JCO.2008.17.1496. [DOI] [PubMed] [Google Scholar]
- 20.Groheux D, Moretti JL, Baillet G, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008;71:695–704. doi: 10.1016/j.ijrobp.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Jager JJ, Keymeulen K, Beets-Tan RG, et al. FDG-PET-CT for staging of high-risk breast cancer patients reduces the number of further examinations: A pilot study. Acta Oncol. 2010;49:185–191. doi: 10.3109/02841860903440262. [DOI] [PubMed] [Google Scholar]
- 22.Port ER, Yeung H, Gonen M, et al. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography scanning affects surgical management in selected patients with high-risk, operable breast carcinoma. Ann Surg Oncol. 2006;13:677–684. doi: 10.1245/ASO.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Pugliese M, Shivaram G, Rogers J, et al. PET-CT imaging in the initial management of high-risk breast cancer patients: Who did it help? Breast J. 2009;15:554–556. doi: 10.1111/j.1524-4741.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 24.Heusner TA, Kuemmel S, Umutlu L, et al. Breast cancer staging in a single session: Whole-body PET/CT mammography. J Nucl Med. 2008;49:1215–1222. doi: 10.2967/jnumed.108.052050. [DOI] [PubMed] [Google Scholar]
- 25.Heusner TA, Kuemmel S, Koeninger A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging. 2010;37:1077–1086. doi: 10.1007/s00259-010-1399-z. [DOI] [PubMed] [Google Scholar]
- 26.Niikura N, Costelloe CM, Madewell JE, et al. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. The Oncologist. 2011;16:1111–1119. doi: 10.1634/theoncologist.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groves AM, Shastry M, Rodriguez-Justo M, et al. 18F-FDG PET and biomarkers for tumour angiogenesis in early breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:46–52. doi: 10.1007/s00259-010-1590-2. [DOI] [PubMed] [Google Scholar]
- 29.Guarneri V, Conte PF. The curability of breast cancer and the treatment of advanced disease. Eur J Nucl Med Mol Imaging. 2004;31(suppl 1):S149–S161. doi: 10.1007/s00259-004-1538-5. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet. 2010;375:563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 31.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 32.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: Should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59:290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 33.Wahl RL, Siegel BA, Coleman RE, et al. PET Study Group. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: A report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22:277–285. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 34.Cooper KL, Meng Y, Harnan S, et al. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: Systematic review and economic evaluation. Health Technol Assess. 2011;15:iii–iv. doi: 10.3310/hta15040. 1–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heusner TA, Kuemmel S, Hahn S, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–1550. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 36.Barrett T, Bowden DJ, Greenberg DC, et al. Radiological staging in breast cancer: Which asymptomatic patients to image and how. Br J Cancer. 2009;101:1522–1528. doi: 10.1038/sj.bjc.6605323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravaioli A, Passini G, Polselli A, et al. Staging of breast cancer: New recommended standard procedure. Breast Cancer Res Treat. 2002;72:53–60. doi: 10.1023/a:1014900600815. [DOI] [PubMed] [Google Scholar]
- 38.Guo J, Higashi K, Ueda Y, et al. Microvessel density: Correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med. 2006;47:419–425. [PubMed] [Google Scholar]
- 39.Agarwal M, Brahmanday G, Bajaj SK, et al. Revisiting the prognostic value of preoperative (18)F-fluoro-2-deoxyglucose ((18)F-FDG) positron emission tomography (PET) in early-stage (I & II) non-small cell lung cancers (NSCLC) Eur J Nucl Med Mol Imaging. 2010;37:691–698. doi: 10.1007/s00259-009-1291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair VS, Barnett PG, Ananth L, et al. Veterans Affairs Solitary Nodule Accuracy Project Cooperative Studies Group. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected, clinical stage IA non-small cell lung cancer. Chest. 2010;137:1150–1156. doi: 10.1378/chest.09-2356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.