The differences among 17 independent studies of the association between CYP2D6 polymorphism and tamoxifen treatment outcome were explored to identify factors that may have contributed to the discrepant findings.
Keywords: Tamoxifen, Cytochrome P450 CYP2D6, Pharmacogenetics, Review
Learning Objectives:
After completing this course, the reader will be able to:
Describe the significant heterogeneity among the published studies on the link between CYP2D6 genotype and tamoxifen treatment efficacy.
Explain the role of CYP2D6 metabolism in the conversion of tamoxifen to its active metabolite, endoxifen, and the potential importance of CYP2D6 polymorphisms to this process.
Discuss the role that insufficient genotyping, CYP2D6 inhibition, and tamoxifen combination treatment may have had in the inconsistent findings of past pharmacogenetic studies.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Tamoxifen is an effective antiestrogen used in the treatment of hormone receptor–positive breast cancer. Bioconversion of tamoxifen to endoxifen, its most abundant active metabolite, is primarily dependent on the activity of cytochrome P450 2D6 (CYP2D6), which is highly polymorphic. Over 20 published studies have reported on the potential association between CYP2D6 polymorphism and tamoxifen treatment outcome, with highly inconsistent results. The purpose of this review is to explore differences among 17 independent studies to identify factors that may have contributed to the discrepant findings. This report discusses six putative factors that are grouped into two categories: (a) clinical management criteria: hormone receptor classification, menopausal status, and tamoxifen combination therapy; (b) pharmacologic criteria: genotyping comprehensiveness, CYP2D6 inhibitor coadministration, and tamoxifen adherence. Comparison of these factors between the positive and negative studies suggests that tamoxifen combination therapy, genotyping comprehensiveness, and CYP2D6 inhibitor coadministration may account for some of the contradictory results. Future association studies on the link between CYP2D6 genotype and tamoxifen treatment efficacy should account for combination therapy and CYP2D6 inhibition, and interrogate as many CYP2D6 alleles as possible.
Introduction
Use of the antiestrogen tamoxifen for 5 years in hormone receptor (HR)+ breast cancer is associated with a nearly 50% lower recurrence rate and provides an overall survival benefit [1]. However, ∼20%—30% of women relapse despite the full 5 years of therapy [2]. Another class of endocrine therapy, the aromatase inhibitors, is slightly more effective than tamoxifen alone in the postmenopausal setting but is not appropriate for premenopausal patients [3, 4]. Aromatase inhibitors are not available or are prohibitively expensive in many parts of the world, with tamoxifen therefore being the primary choice for endocrine therapy for most patients.
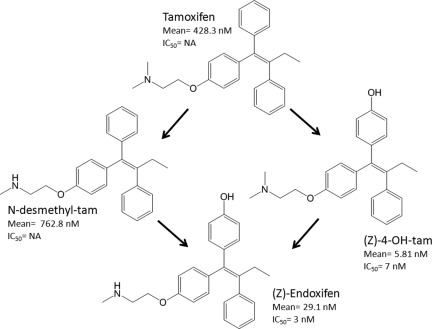
The parent tamoxifen molecule has two primary metabolites: 4-hydroxy-tamoxifen and N-desmethyl-tamoxifen. Each metabolite can be bioconverted to (Z)-4-hydroxy-N-desmethyl-tamoxifen, commonly referred to as endoxifen (Fig. 1). Comprehensive analysis of tamoxifen and 22 of its metabolites confirms that endoxifen is the most abundant active metabolite of tamoxifen [5, 6]. Bioactivation of tamoxifen to endoxifen is mediated by a multitude of cytochrome P450 (CYP) enzymes, with CYP2D6 being central to metabolic activation [7, 8]. CYP2D6 is a highly polymorphic enzyme with >80 annotated isoforms [9]. These isozymes range in activity from splice variants with no metabolic capability to gene duplications that possess activity 10- to 30-fold greater than that of the wild-type enzyme [10]. Knowledge of CYP2D6 genotype enables classification as a poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM), or ultrarapid metabolizer (UM), indicating the extent of drug metabolism [11].
Figure 1.
Tamoxifen metabolism. The metabolism of tamoxifen to endoxifen is heavily dependent on cytochrome P450 2D6 activity. The mean steady-state plasma concentrations and 50% inhibitory concentration (IC50) values for each molecule [6] suggest that endoxifen is the active metabolite responsible for tamoxifen efficacy.
Abbreviations: 4-OH-tam, 4-hydroxy-tamoxifen; N-desmethyl-tam, N-desmethyl-tamoxifen; NA, not available.
More than 20 reports investigating whether or not CYP2D6 genotype influences the efficacy of tamoxifen treatment have been published. The conclusions of these studies range from a possible longer disease-free survival interval [12] to a substantially shorter recurrence-free survival time [13] for patients carrying CYP2D6 genotypes conferring diminished tamoxifen metabolism. These conflicting findings have led to confusion among clinicians and regulatory bodies regarding whether or not CYP2D6 genotyping should be performed, and from a clinical standpoint, whether or not genotype-guided tamoxifen therapy should be pursued. The inconsistency in study results is likely attributable to heterogeneity in study designs and patient populations.
This review examines six factors that may have influenced the conclusions of CYP2D6–tamoxifen association studies. These factors are grouped into two categories: (a) clinical management criteria: HR classification, menopausal status, and tamoxifen combination therapy; (b) pharmacologic criteria: genotyping comprehensiveness, CYP2D6 inhibitor coadministration, and tamoxifen adherence. This process will inform some general interpretive rules around the CYP2D6–tamoxifen literature.
Studies Included in Review
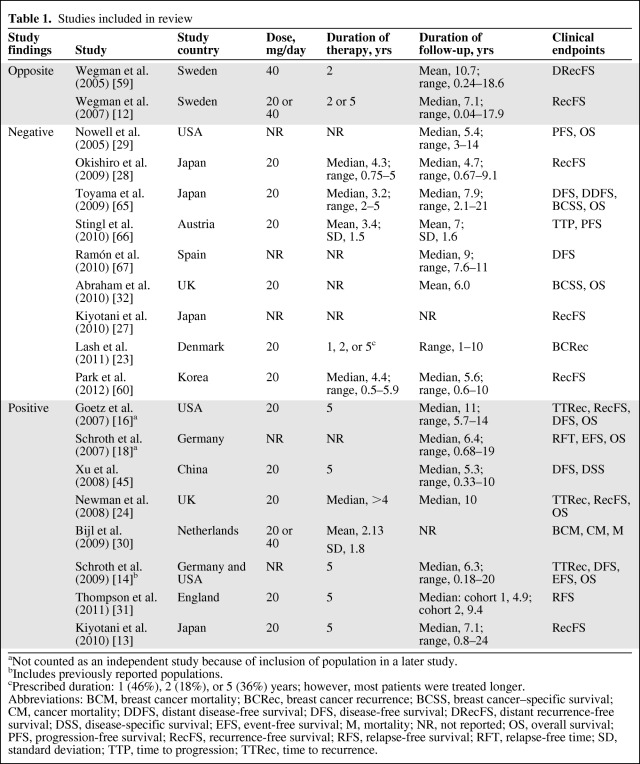
A PubMed search was conducted using combinations of the following search terms: CYP2D6, tamoxifen, pharmacogenetic, and pharmacogenomic. All published studies that investigated the association of CYP2D6 genotype with the effectiveness of tamoxifen therapy in the treatment of nonmetastatic breast cancer were eligible for inclusion in this review. As of August 2011, 22 studies on the association of CYP2D6 genotype and tamoxifen efficacy in nonmetastatic breast cancer treatment were published; however, in a number of cases, populations were reused in multiple publications. In these cases, only the most recent, thorough study was included in this analysis and the others were excluded. In the first instance, the study from Schroth et al. [14] was included because it had combined and added to the populations used in previous studies by Goetz et al. [15–17] and Schroth et al. [18]. Similarly, Kiyotani et al. [13] reused a population from a previous study [19]. After removing this redundancy, there were a total of 17 independent studies published, six of which found an association between PM CYP2D6 genotypes and inferior tamoxifen efficacy in nonmetastatic breast cancer treatment (positive), nine that found no relationship (negative), and two that reported the reverse association (opposite). All 17 studies included in the review are listed in Table 1 by classification (positive, negative, or opposite), with the country in which they were conducted, tamoxifen dose and duration, duration of follow-up, and clinical endpoints analyzed. For the purposes of comparison in this report, the nine negative and two opposite studies are combined into a single (negative) group.
Table 1.
Studies included in review
aNot counted as an independent study because of inclusion of population in a later study.
bIncludes previously reported populations.
cPrescribed duration: 1 (46%), 2 (18%), or 5 (36%) years; however, most patients were treated longer.
Abbreviations: BCM, breast cancer mortality; BCRec, breast cancer recurrence; BCSS, breast cancer–specific survival; CM, cancer mortality; DDFS, distant disease-free survival; DFS, disease-free survival; DRecFS, distant recurrence-free survival; DSS, disease-specific survival; EFS, event-free survival; M, mortality; NR, not reported; OS, overall survival; PFS, progression-free survival; RecFS, recurrence-free survival; RFS, relapse-free survival; RFT, relapse-free time; SD, standard deviation; TTP, time to progression; TTRec, time to recurrence.
Clinical Management Criteria
Retrospective studies are inherently confined to the population that was enrolled in the parent study or that existed in the clinical database from which it sampled. There are a variety of differences among the patient cohorts in these studies, including: patient enrollment method; percentage of total cohort available for pharmacogenetic testing; tumor stage and receptor status; patient race, age, and menopausal status; tamoxifen indication, dose, and duration; auxiliary treatment; follow-up duration; and measure of treatment efficacy. The relationship between CYP2D6 genotype and tamoxifen efficacy may only exist in a subset of patients; therefore, any of these factors may be critical in determining whether or not the relationship, if it exists, could be detected in a given study. Three factors were selected to illustrate putative explanations for the discrepant reports: HR classification, menopausal status, and tamoxifen combination therapy.
HR Classification
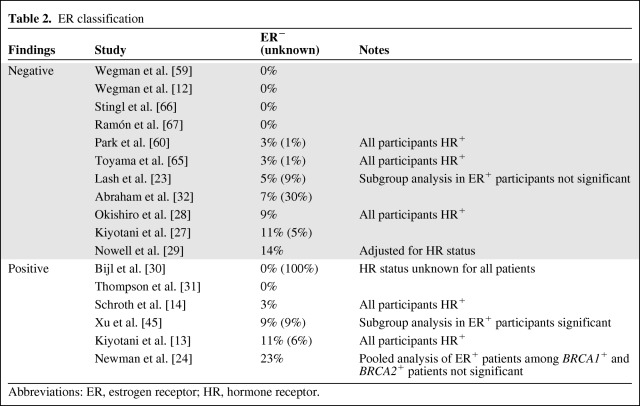
Tamoxifen efficacy is limited to estrogen-dependent tumors, which express the estrogen receptor (ER) and/or progesterone receptor (PR) [1, 20]. However, some of the studies included ER−PR− patients, who do not benefit from tamoxifen, and therefore would not have differential benefit based on CYP2D6 activity. It has been suggested that a lack of centralized testing of HR status can lead to misclassification of participants within a study [21], and this was confirmed retrospectively [22]. Instead of conjecture on possible misclassifications, it may be informative to go through the positive and negative studies to identify which ones could have been influenced by the inclusion of HR− tumors.
Differences in reporting methods complicate this comparison. Table 2 lists the percentage of patients from each study who are ER− and denotes which studies specified that all participants were HR+. Contrary to what was expected, a slightly higher proportion of positive studies included ER− patients. Excluding studies in which all patients were known to be HR+ and the study with no known receptor status, 66% of the positive studies (two of three) and 50% of the negative studies (four of eight) included ER− patients. In the four negative studies, the percentage of patients who were ER− was low (5%–14%), so it is unlikely that this contributed to the negative findings, particularly in the study from Lash et al. [23], in which a subanalysis in ER+ patients was also negative. The study with the largest percentage of ER− patients overall (23%), from Newman et al. [24], reported a positive finding in the BRCA2 mutation cohort (91% ER+) but not in the BRCA1 mutation cohort (57.5% ER+); however, a pooled analysis of all ER+ participants was not statistically significant.
Table 2.
ER classification
Abbreviations: ER, estrogen receptor; HR, hormone receptor.
The inclusion of HR− individuals does not explain negative study findings or differentiate between positive and negative studies. Nevertheless, given what is known about tamoxifen's mechanism and efficacy, it is important that future studies confine their population to only HR+ patients, in whom tamoxifen is effective at preventing cancer recurrence, and consider covariate adjustment for the ER+PR− and ER−PR+ subgroups, because a recent publication suggests that, given the ER status of a tumor, PR status is not an important predictor of tamoxifen efficacy [1]. Future research examining adjustment for different intrinsic subtypes of breast cancer within ER+ tumors (i.e., luminal A versus luminal B) would also be illuminating in developing further predictive models for tamoxifen efficacy.
Menopausal Status
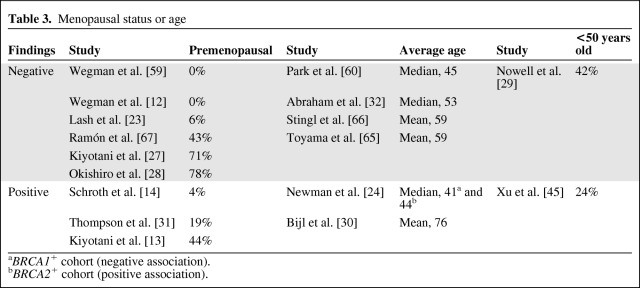
There has been speculation that the association of CYP2D6 and tamoxifen efficacy may be confined to premenopausal patients. In postmenopausal women, tamoxifen and N-desmethyl-tamoxifen together occupy >99.9% of the available ERs, suggesting that variation in endoxifen concentration would have little role in blocking estrogen signaling [25]. However, endoxifen may be critical to saturate the ER in premenopausal women, in whom tamoxifen and N-desmethyl-tamoxifen are estimated to occupy only 90%–95% of the available receptors [26]. If receptor occupancy, competition with estradiol, or some other mechanism causes endoxifen to be critical only in premenopausal women, then only studies that include primarily premenopausal patients would detect a CYP2D6–tamoxifen association.
Each of the 17 studies reported some measure of menopausal status or age, though differences in reporting methods make direct comparison among all studies impossible (Table 3). Looking at either the percentage of participants who were premenopausal, the average age of the patients, or the percentage of patients aged <50 years, there is little evidence that negative study results can be attributed to the enrollment of postmenopausal patients. On the contrary, studies with the highest percentages of premenopausal patients [27, 28] and patients aged <50 years [29] were negative whereas the study with the highest average age was positive [30]. Future studies should consider adjusting for menopausal status in case there is a differential effect of tamoxifen in these populations; however, based on a comparison of the available studies, there does not seem to be a substantial influence of menopausal status on the possible association between CYP2D6 genotype and tamoxifen efficacy.
Table 3.
Menopausal status or age
aBRCA1+ cohort (negative association).
bBRCA2+ cohort (positive association).
Tamoxifen Combination Therapy
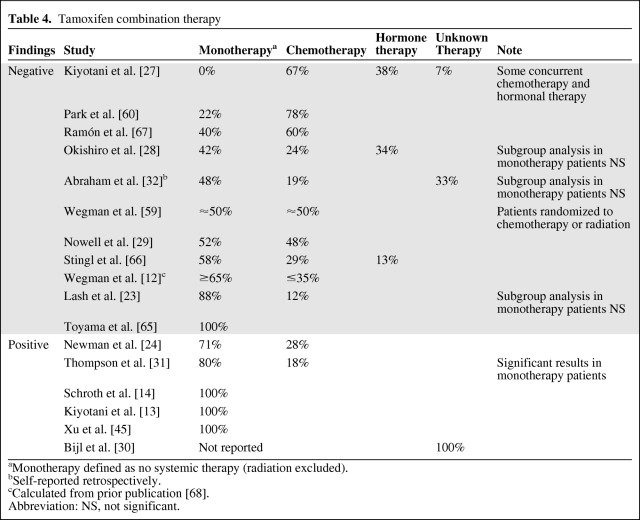
Kiyotani et al. [27] published a study suggesting that the inclusion of patients on combination therapy could have contributed to the negative findings of previous analyses. They classified patients who received any additional therapy, including radiation, as combined therapy. For our comparison, patients not receiving additional systemic therapy, excluding radiation, were classified as receiving tamoxifen monotherapy. Updating the original comparison of Kiyotani et al. [27], the use of additional therapy seems to differ between negative and positive studies (Table 4). Of the negative studies, only one used a cohort that received tamoxifen monotherapy (one of 11, 9%), compared with three of the five positive studies (60%). The study from Bijl et al. [30], which has no information on additional treatment, was excluded from this comparison. In fact, the nine studies with the lowest percentages of patients on monotherapy were all negative, whereas five of the seven studies with >70% of patients on monotherapy were positive. Additionally, one recent study showed a stronger association in the tamoxifen monotherapy subgroup [31]. However, the lack of association in the tamoxifen monotherapy subgroup in some recent negative studies [23, 28, 32] indicates that this single factor does not fully explain the conflicting findings. Nevertheless, it does seem that the influence of CYP2D6 genotype on tamoxifen efficacy may be confounded by additional therapy, and only patients on tamoxifen monotherapy should be included in future studies of this potential pharmacogenetic association.
Table 4.
Tamoxifen combination therapy
aMonotherapy defined as no systemic therapy (radiation excluded).
bSelf-reported retrospectively.
cCalculated from prior publication [68].
Abbreviation: NS, not significant.
Pharmacologic Criteria
The theoretical association between CYP2D6 genotype and tamoxifen efficacy relies on two assumptions. The first is that the CYP2D6 genotype modulates endoxifen formation and can be used as a proxy for endoxifen exposure. This has been consistently demonstrated by many different groups [13, 33–35], with CYP2D6 genotype explaining up to 40% of the variability in endoxifen steady-state concentrations [6]. The second assumption, that exposure to endoxifen dictates response, was not formally tested until a recent report from Madlensky et al. [36] demonstrated that individuals in the lowest endoxifen concentration group experienced an inferior benefit from tamoxifen therapy. Taken together, the data strongly suggest that CYP2D6 genotype, through its influence on endoxifen biotransformation, could be an important predictor of tamoxifen efficacy.
Additional research suggests that endoxifen exposure can be modulated by a host of factors beyond CYP2D6 intrinsic function, leading to the development of elaborate phenotypic classification systems [37]. The coadministration of CYP2D6 inhibitors and the adherence to tamoxifen therapy influence the endoxifen concentration, and potentially tamoxifen efficacy [38, 39]. Thus, comparing the genotyping comprehensiveness, inhibitor coadministration, and tamoxifen adherence in the positive and negative studies may explain some of the inconsistency in the results of the 17 studies.
Genotyping Comprehensiveness
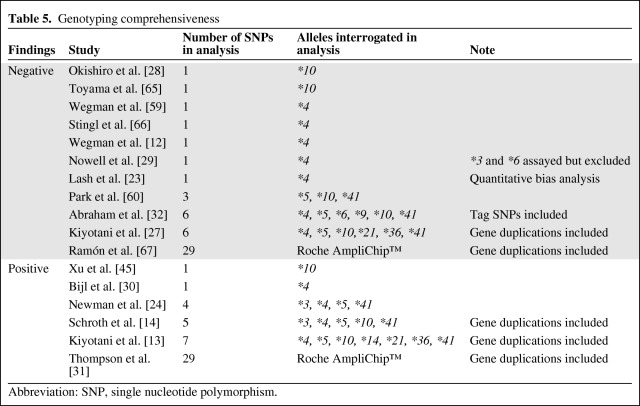
The original studies of CYP2D6 genotype and tamoxifen efficacy focused exclusively on the CYP2D6*4 allele, a splicing defect that produces an enzyme with no metabolic capacity. Additional alleles that affected metabolic activity are known, including the null activity CYP2D6*3, CYP2D6*5, and CYP2D6*6 alleles, and reduced activity CYP2D6*10 and CYP2D6*41 alleles. For a more detailed review of the polymorphisms and their effect on activity see Zanger et al. [11]. The current gold standard for comprehensive CYP2D6 genotyping is the U.S. Food and Drug Administration–approved AmpliChip™ CYP450 Test (Roche Diagnostics, Indianapolis, IN), which interrogates 29 polymorphisms and copy number variants, enabling detection and categorization of 33 alleles as null, diminished, normal, or high activity [40].
The negative results of some studies may be attributed to insufficiently comprehensive genotyping, as demonstrated by Schroth et al. [41]. That paper compared the hazard ratio, study power, and percentage of individuals who would be classified as PM, IM, or EM in situations of varying numbers of genotyped alleles. Using the data from their clinical cohort, expanding the allelic coverage from only the CYP2D6*4 allele to inclusion of all alleles on the AmpliChip™, increased the estimated hazard ratio from 1.33 (p = .58) to 2.87 (p = .006), with a corresponding increase in study power from 7.8% to 63.2%. Interestingly, although the percentage of PM patients had an absolute increase of only 2.8% (5.5% to 8.3%), there were dramatic changes in the percentage of IM (32.7% to 54.1%) and EM (61.8% to 37.6%) patients, suggesting that most studies misclassify many IMs as EMs. This clearly demonstrates that insufficiently comprehensive genotyping leads to patient misclassification and diminishes the ability to detect a genotype–outcome association.
In comparing the genotyping comprehensiveness between the positive and negative studies, there is a clear trend toward a greater likelihood of a positive finding with expanded allelic coverage (Table 5). Of the studies that interrogated a single variant, only 22% (two of nine) were positive. This percentage is higher, at 50%, for studies that assayed three to seven variants (three of six) or used the AmpliChip™ (one of two). In agreement with the findings of Schroth et al. [41], Thompson et al. [31] reported that their positive association would not have been detectable if they had exclusively genotyped the CYP2D6*4 allele.
Table 5.
Genotyping comprehensiveness
Abbreviation: SNP, single nucleotide polymorphism.
There is strong evidence that inadequate allelic coverage can diminish study power through patient misclassification. The negative findings in many, but not all, of these 17 studies may be attributed to insufficiently comprehensive genotyping. In the future, all studies should use a genotyping technology that is at least as comprehensive as the widely available AmpliChip™. Additional research should be undertaken to discover and characterize additional CYP2D6 polymorphisms that influence tamoxifen-to-endoxifen bioconversion.
CYP2D6 Inhibitor Coadministration
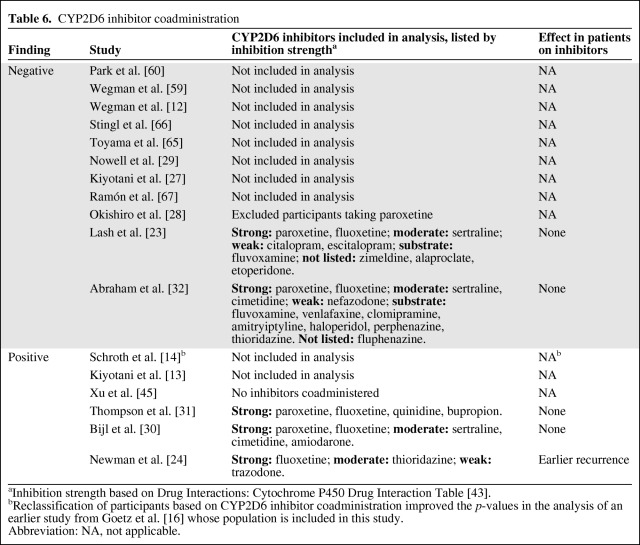
Selective serotonin reuptake inhibitor (SSRI) antidepressants diminish the severity and occurrence of hot flashes, one of the most common adverse effects of tamoxifen [42]. Most SSRIs inhibit CYP2D6 [43], and coadministration with tamoxifen leads to lower concentrations of endoxifen [34, 44], an effect that may be most pronounced with the strong inhibitor paroxetine [38]. In an effort to clarify the relative importance of genetics and CYP2D6 inhibition, a recent study used information on CYP2D6 genotype and inhibitor coadministration to develop a phenotype score to explain variability in the endoxifen/N-desmethyl-tamoxifen ratio. Coadministration of a CYP2D6 inhibitor explained 38%–53% of the variability whereas the genetic information provided no additional explanatory value [37].
If concomitant administration of CYP2D6 inhibitors diminishes endoxifen production, as demonstrated by previous data, then it is likely to abrogate tamoxifen efficacy as well. Therefore, it may be revealing to investigate which of the positive and negative pharmacogenetic studies accounted for CYP2D6 inhibition. One positive and one negative study excluded patients taking paroxetine or other strong CYP2D6 inhibitors [28, 45]. Of the remaining studies, a larger percentage of the positive studies (three of five, 60%) than the negative studies (two of 10, 20%) reported an assessment of inhibitor use in their analysis (Table 6). Additionally, the positive studies did a superior job of limiting their analysis to strong CYP2D6 inhibitors. The most commonly administered SSRI in the Dutch cohort used in the negative study from Lash et al. [23] is the weak inhibitor/noninhibitor citalopram, which may not interfere with endoxifen formation [46]. Similarly, the list of study medications classified as inhibitors by Abraham et al. [32] includes many medications that are merely substrates, severely limiting the relevance of this analysis.
Table 6.
CYP2D6 inhibitor coadministration
aInhibition strength based on Drug Interactions: Cytochrome P450 Drug Interaction Table [43].
bReclassification of participants based on CYP2D6 inhibitor coadministration improved the p-values in the analysis of an earlier study from Goetz et al. [16] whose population is included in this study.
Abbreviation: NA, not applicable.
Among the three positive studies that accounted for inhibitor use, only the study by Newman et al. [24] reported a significant effect on tamoxifen treatment outcomes. It should also be noted that one of the earlier studies from Goetz et al. [16], which was excluded because of population overlap with Schroth et al. [14], did include CYP2D6 inhibitors and reported better p-values after reclassification of patients based on inhibitor use.
The evidence presented in this review suggests that inhibitor coadministration may be influencing the results of pharmacogenetic studies. The theoretical concern of coadministration of strong CYP2D6 inhibitors during tamoxifen therapy has gained acceptance in clinical treatment and it would be ideal if future pharmacogenetic studies excluded or accounted for concomitant CYP2D6 inhibitor treatment.
Tamoxifen Adherence
Patient nonadherence to treatment is a common issue in outpatient drug therapy. Taking ≥80% of the doses prescribed is a frequently used threshold for adherence. Using this threshold, only 77% of patients are adherent after 1 year of tamoxifen. This number gradually declines to 50% by year 4 [47], and differences in the propensity to discontinue tamoxifen treatment by race, age, and disease have been reported [48]. Further complicating the inclusion of adherence data is the tendency of patients to exaggerate when self-reporting, which has been demonstrated specifically for hormonal agents [49]. Importantly, poor adherence has been linked to a poor breast cancer event-free time [50] and survival time [51]. Thus, poor tamoxifen adherence leads to suboptimal treatment efficacy, but it is very difficult to account for compliance in clinical studies, which may be biasing these retrospective pharmacogenetic analyses.
Unfortunately, only one of these 17 studies considered drug adherence. Thompson et al. [31] used prescription data to calculate that 14% of their patients were <80% adherent. Reassigning these individuals to the decreased metabolizer group and rerunning their primary analysis increased their estimated hazard ratio from 2.57 to 3.02. This suggests that ignoring adherence may be diluting the estimation of effect size in the other 16 studies, though this conclusion is based on very limited data. Nevertheless, it could be an important factor that has not been addressed in most retrospective studies, which should be included, when possible, in the analysis of any future studies.
There may also be interplay among these factors, making it difficult to deconvolute the role of each. Discontinuation of tamoxifen therapy is more common in EMs [52] and in women who are coadministered CYP2D6 inhibitors [50], presumably to treat hot flashes. These findings suggest that EMs may experience greater hot flashes, but additional studies have been unable to detect this association [53–55]. The confounding interaction of adverse events, inhibitor coadministration, and tamoxifen adherence is difficult to untangle from the data presently available.
Other Factors and Studies
Other Unexplored Factors
This review selected only six of the myriad differences among these 17 studies. Other factors that have been suggested were not explored, such as the percentage of patients from the parent study that were included in the pharmacogenetic substudy [56]. Perhaps exploring the differences in study design or methods of patient enrollment across studies may be informative, or looking at differences in the racial composition of studies and the allele frequencies in those populations may partly explain the discrepant results, particularly in light of the recent evidence that individuals of African descent are twice as likely to carry a lower metabolism allele [57]. Another intriguing difference is the use of the larger 40-mg tamoxifen dose in some studies [30, 58], including both of the studies that reported an opposite association [12, 59]. Perhaps this higher dose can overcome the diminished bioactivation in IMs and PMs [55]. Finally, patients in many studies were allowed to cross over or switch from the tamoxifen to the comparator arm after the study. This was the case in the study from Park et al. [60], in which 26% of the patients crossed over to an aromatase inhibitor. Perhaps this therapeutic switch is in some way abrogating the association of genotype and efficacy. Additional differences such as disease stage, endpoint definition, and length of follow-up time may further assist in explaining the inconsistent findings of these studies.
Unpublished Studies Presented at the San Antonio Breast Cancer Symposium
At the 2010 San Antonio Breast Cancer Symposium (SABCS), pharmacogenetic subanalyses of two large, well-designed studies in postmenopausal patients—the Arimidex, Tamoxifen, Alone or in Combination (ATAC) and the Breast International Group (BIG) 1–98/International Breast Cancer Study Group (IBCSG) 18–98 studies—were presented. The results of those studies did not support the hypothesis that patients with low activity CYP2D6 genotypes had worse outcomes from tamoxifen therapy [53, 61]. However, those studies are in contradiction to the findings of the currently unpublished Austrian Breast and Colorectal Cancer Study Group Study 8 presented at the 2008 SABCS [62]. All three studies used large populations of HR+ postmenopausal patients treated with tamoxifen monotherapy. None of the studies employed the AmpliChip™ test or included gene duplication, so there is the potential for some patient misclassification; however, the lack of a trend in the negative studies suggests that more comprehensive genotyping would not have materially changed the findings. Of the three, only the ATAC study controlled for potent CYP2D6 inhibitors and none incorporated tamoxifen adherence in their presented analysis.
Metastatic Disease and Prevention
Four published studies have reported an association between CYP2D6 genotype and tamoxifen effectiveness in the metastatic [33, 58] and prevention [63, 64] settings. These studies answered different clinical questions from the studies discussed previously in this report, and their relevance to the above discussions is debatable. However, three studies reported a statistically significant association between tamoxifen effectiveness and metabolizer status based on CYP2D6 genotype. The positive prevention study, from Serrano et al. [63], used the AmpliChip™ test and found that, among tamoxifen-treated individuals, the PM genotype was found more commonly in cases than in controls (15% versus 1.5%; p = .035). However, a larger analysis of the National Surgical Adjuvant Breast and Bowel Project P1 and P2 trials from Goetz et al. [64] could not replicate these findings. The two studies in the metastatic setting, both positive, included exclusively HR+ patients, many of whom were on prior therapy for adjuvant or metastatic disease. The study from Lim et al. [33] genotyped only the CYP2D6*10 allele and did not report inhibitor use, whereas the study from Lammers et al. [58] genotyped six alleles and reported that patients taking CYP2D6 inhibitors had a shorter time to progression and overall survival time than patients not coadministered an inhibitor. In the future, the metastatic setting, with its smaller, shorter studies, may be the ideal setting in which to prospectively assess the influence of CYP2D6 genotype on tamoxifen efficacy.
Conclusions and Recommendations
The biological rationale for the relationship between CYP2D6 genotype and tamoxifen effectiveness is tantalizingly plausible, yet continued research has been unable to determine whether or not the association exists, let alone whether or not it is clinically meaningful. In this report, six factors were selected to compare across eleven negative and six positive studies. The number of positive studies suggests that there is some patient group that differentially benefits from tamoxifen based on CYP2D6 activity. Any study that includes patients outside this group, or misclassifies patients within this group, is liable to underestimate the effect of genotype on outcome, potentially leading to a false-negative finding (type I error). Therefore, it is not surprising that no single factor can consistently differentiate all the positive studies from the negative ones. Based on this comparison, studies that enrolled patients on tamoxifen monotherapy, genotyped the CYP2D6 gene more comprehensively, and accounted for CYP2D6 inhibitor coadministration were more likely to have positive findings. On the other hand, patient menopausal status did not seem to influence the likelihood of a study returning a positive result. Surprisingly, it does not seem that the inclusion of patients with HR− tumors explained the contradictory findings, and there were too few studies that accounted for treatment adherence to make any inference. From this review it seems that the best setting in which to detect a difference in outcome from tamoxifen therapy would be a cohort of ER+ patients treated with tamoxifen monotherapy, using comprehensive genotyping with adjustment for CYP2D6 inhibitors and tamoxifen adherence. However, two of the largest studies to date that best fit these criteria, the ATAC and BIG 1–98 studies presented at the SABCS 2010, did not detect the expected association. Perhaps this is an indication that there is no true effect of CYP2D6 genotype on tamoxifen outcome, or that the effect is so modest as to make it not clinically relevant. At this time, CYP2D6 testing does not meet evidence for routine clinical use, but further studies, potentially using the criteria defined in this review, to delineate its role in tamoxifen therapy are warranted.
Acknowledgments
Daniel Hertz is an American Foundation for Pharmaceutical Education Pre-Doctoral Fellow in Clinical Pharmaceutical Science.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Daniel L. Hertz, Howard L. McLeod, William J. Irvin Jr
Collection and/or assembly of data: Daniel L. Hertz, William J. Irvin Jr
Data analysis and interpretation: Daniel L. Hertz, Howard L. McLeod, William J. Irvin Jr
Manuscript writing: Daniel L. Hertz, Howard L. McLeod, William J. Irvin Jr
Final approval of manuscript: Daniel L. Hertz, Howard L. McLeod, William J. Irvin Jr
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackshaw A, Roughton M, Forsyth S, et al. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol. 2011;29:1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- 3.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC, Collins MM, Rowsby L, et al. A monohdroxylated metabolite of tamoxifen with potent antiestrogenic activity. J Endocrinol. 1977;75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 6.Mr̈dter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 7.Desta Z, Ward BA, Soukhova NV, et al. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 8.Crewe HK, Notley LM, Wunsch RM, et al. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: Formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002;30:869–874. doi: 10.1124/dmd.30.8.869. [DOI] [PubMed] [Google Scholar]
- 9.The Human Cytochrome P450 (CYP) Allele Nomenclature Database: CYP2D6 Allele Nomenclature. [accessed March 1, 2011]. Available at http://www.cypalleles.ki.se/cyp2d6.htm.
- 10.Ingelman-Sundberg M, Sim SC, Gomez A, et al. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Zanger U, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedeberg's Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 12.Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9:R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyotani K, Mushiroda T, Imamura CK, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 16.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 17.Goetz MP, Suman VJ, Couch FJ, et al. Cytochrome P450 2D6 and homeobox 13/interleukin-17B receptor: Combining inherited and tumor gene markers for prediction of tamoxifen resistance. Clin Cancer Res. 2008;14:5864–5868. doi: 10.1158/1078-0432.CCR-08-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroth W, Antoniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187–5193. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 19.Kiyotani K, Mushiroda T, Sasa M, et al. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99:995–999. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 21.Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: The role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther. 2008;83:160–166. doi: 10.1038/sj.clpt.6100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins LC, Marotti JD, Baer HJ, et al. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100:218–221. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lash TL, Cronin-Fenton D, Ahern TP, et al. CYP2D6 inhibition and breast cancer recurrence in a population-based study in Denmark. J Natl Cancer Inst. 2011;103:489–500. doi: 10.1093/jnci/djr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman WG, Hadfield KD, Latif A, et al. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res. 2008;14:5913–5918. doi: 10.1158/1078-0432.CCR-07-5235. [DOI] [PubMed] [Google Scholar]
- 25.Dowsett M, Haynes BP. Hormonal effects of aromatase inhibitors: Focus on premenopausal effects and interaction with tamoxifen. J Steroid Biochem Mol Biol. 2003;86:255–263. doi: 10.1016/s0960-0760(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 26.Dowsett M. 2010 San Antonio Breast Cancer Symposium: Year in review—translational breast cancer research. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–10, 2010; San Antonio, TX. [Google Scholar]
- 27.Kiyotani K, Mushiroda T, Hosono N, et al. Lessons for pharmacogenomics studies: Association study between CYP2D6 genotype and tamoxifen response. Pharmacogenet Genomics. 2010;20:565–568. doi: 10.1097/FPC.0b013e32833af231. [DOI] [PubMed] [Google Scholar]
- 28.Okishiro M, Taguchi T, Jin Kim S, et al. Genetic polymorphisms of CYP2D6*10 and CYP2C19*2,*3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer. 2009;115:952–961. doi: 10.1002/cncr.24111. [DOI] [PubMed] [Google Scholar]
- 29.Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91:249–258. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 30.Bijl MJ, van Schaik RH, Lammers LA, et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat. 2009;118:125–130. doi: 10.1007/s10549-008-0272-2. [DOI] [PubMed] [Google Scholar]
- 31.Thompson A, Johnson A, Quinlan P, et al. Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011;125:279–287. doi: 10.1007/s10549-010-1139-x. [DOI] [PubMed] [Google Scholar]
- 32.Abraham JE, Maranian MJ, Driver KE, et al. CYP2D6 gene variants: Association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res. 2010;12:R64. doi: 10.1186/bcr2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim H, Ju Lee H, Seok Lee K, et al. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–3845. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 35.Gjerde J, Hauglid M, Breilid H, et al. Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol. 2008;19:56–61. doi: 10.1093/annonc/mdm434. [DOI] [PubMed] [Google Scholar]
- 36.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borges S, Desta Z, Jin Y, et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol. 2010;50:450–458. doi: 10.1177/0091270009359182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 39.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain KK. Applications of AmpliChip™ CYP450. Mol Diagn. 2005;9:119–127. doi: 10.2165/00066982-200509030-00002. [DOI] [PubMed] [Google Scholar]
- 41.Schroth W, Hamann U, Fasching PA, et al. CYP2D6 polymorphisms as predictors of outcome in breast cancer patients treated with tamoxifen: Expanded polymorphism coverage improves risk stratification. Clin Cancer Res. 2010;16:4468–4477. doi: 10.1158/1078-0432.CCR-10-0478. [DOI] [PubMed] [Google Scholar]
- 42.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 43.Flockhart DA Indiana University School of Medicine. Drug interactions: P450 Drug Interaction Table. [accessed July 6, 2011]. Available at http://medicine.iupui.edu/clinpharm/ddis/table.aspx.
- 44.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19:1423–1429. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 46.Indiana University School of Medicine. Drug Interactions With Tamoxifen: A Guide for Breast Cancer Patients and Physicians. [accessed July 6, 2011]. Available at http://medicine.iupui.edu/clinpharm/COBRA/Tamoxifen%20and%202D6v7.pdf.
- 47.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 48.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 50.Dezentjé VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 51.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rae JM, Sikora MJ, Henry NL, et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9:258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leyland-Jones B, Regan MM, Bouzyk M, et al. Outcome according to CYP2D6 genotype among postmenopausal women with endocrine-responsive early invasive breast cancer randomized in the BIG 1–98 trial [S1–8]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–10, 2010; San Antonio, TX. [Google Scholar]
- 54.Henry NL, Rae JM, Li L, et al. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117:571–575. doi: 10.1007/s10549-009-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irvin WJ, Jr, Walko CM, Weck KE, et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J Clin Oncol. 2011;29:3232–3239. doi: 10.1200/JCO.2010.31.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goetz MP. Pharmacogenetics of tamoxifen: Controversy, insights, and way forward. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–10, 2010; San Antonio, TX. [Google Scholar]
- 57.Irvin WJ, Jr, Weck KE, Walko CM, et al. Comprehensive CYP2D6 genotyping in a multiracial population shows differences in allele frequencies between races. J Clin Oncol. 2011;29:e11095. [Google Scholar]
- 58.Lammers LA, Mathijssen RH, van Gelder T, et al. The impact of CYP2D6-predicted phenotype on tamoxifen treatment outcome in patients with metastatic breast cancer. Br J Cancer. 2010;103:765–771. doi: 10.1038/sj.bjc.6605800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wegman P, Vainikka L, Stal O, et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7:R284–R290. doi: 10.1186/bcr993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park IH, Ro J, Park S, et al. Lack of any association between functionally significant CYP2D6 polymorphisms and clinical outcomes in early breast cancer patients receiving adjuvant tamoxifen treatment. Breast Cancer Res Treat. 2012;131:455–461. doi: 10.1007/s10549-011-1425-2. [DOI] [PubMed] [Google Scholar]
- 61.Rae JM, Drury S, Hayes DF, et al. Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endpoints in the ATAC trial [S1–7]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–10, 2010; San Antonio, TX. [Google Scholar]
- 62.Goetz M, Ames M, Gnant M, et al. Pharmacogenetic (CYP2D6) and gene expression profiles (HOXB13/IL17BR and molecular grade index) for prediction of adjuvant endocrine therapy benefit in the ABCSG8 trial. Presented at the 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. [Google Scholar]
- 63.Serrano D, Lazzeroni M, Zambon CF, et al. Efficacy of tamoxifen based on cytochrome P450 2D6, CYP2C19 and SULT1A1 genotype in the Italian Tamoxifen Prevention Trial. Pharmacogenomics J. 2011;11:100–107. doi: 10.1038/tpj.2010.17. [DOI] [PubMed] [Google Scholar]
- 64.Goetz MP, Schaid DJ, Wickerham DL, et al. Evaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: Results from the NSABP P-1 and P-2 clinical trials. Clin Cancer Res. 2011;17:6944–6951. doi: 10.1158/1078-0432.CCR-11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toyama T, Yamashita H, Sugiura H, et al. No association between CYP2D6*10 genotype and survival of node-negative Japanese breast cancer patients receiving adjuvant tamoxifen treatment. Jpn J Clin Oncol. 2009;39:651–656. doi: 10.1093/jjco/hyp076. [DOI] [PubMed] [Google Scholar]
- 66.Stingl JC, Parmar S, Huber-Wechselberger A, et al. Impact of CYP2D6*4 genotype on progression free survival in tamoxifen breast cancer treatment. Curr Med Res Opin. 2010;26:2535–2542. doi: 10.1185/03007995.2010.518304. [DOI] [PubMed] [Google Scholar]
- 67.Ramón y Cajal T, Altés A, Paré L, et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. 2010;119:33–38. doi: 10.1007/s10549-009-0328-y. [DOI] [PubMed] [Google Scholar]
- 68.Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst. 1996;88:1543–1549. doi: 10.1093/jnci/88.21.1543. [DOI] [PubMed] [Google Scholar]