The feasibility and toxicity of concurrent intensity-modulated radiotherapy and carboplatin plus paclitaxel for the treatment of locally advanced head and neck cancer were examined along with the outcomes of treatment.
Keywords: Squamous cell carcinoma of the head and neck, Radiotherapy, Intensity modulated, Paclitaxel, Carboplatin
Abstract
Background.
Intensity-modulated radiation therapy (IMRT) and alternative chemotherapy regimens strive to maintain efficacy while minimizing toxicity in locally advanced head and neck cancer (LAHNC) treatment. Our experience with concurrent IMRT and taxane-based chemotherapy is presented.
Methods.
A retrospective review of 150 consecutive patients with LAHNC treated with IMRT and concurrent taxane-based chemotherapy with curative intent was performed. The IMRT fractionation regimen consisted of 69.3 Gy to gross disease (2.1 Gy/fraction) and 56.1 Gy to prophylactic nodal sites (1.7 Gy/fraction). Weekly paclitaxel (30 mg/m2) and carboplatin (area under the concentration–time curve [AUC], 1) were given concurrently to all patients, and 69% received weekly induction with paclitaxel (60 mg/m2) and carboplatin (AUC, 2).
Results.
Over 90% of patients received the prescribed radiation dose. Ninety-six percent completed five or more cycles of concurrent chemotherapy, with similar tolerability for induction chemotherapy. A percutaneous endoscopic gastrostomy (PEG) tube was required in 80 patients, with 10 maintaining PEG use >18 months. Acute grade 4 mucositis and dermatitis developed in 2.0% and 4.0% of patients, respectively. No patient experienced nadir sepsis, grade ≥3 late xerostomia, or significant nephropathy or gastrointestinal toxicity. Median follow-up was 30 months. The 3-year locoregional control rate was 83.2% with disease-free survival and overall survival rates of 78.8% and 76.5%, respectively.
Conclusion.
Rates of acute and late toxicities were low, with excellent radiation dose delivery and impressive tumor control at 3 years, suggesting that concurrent carboplatin and paclitaxel with IMRT is a reasonable therapeutic option for the curative treatment of LAHNC.
Introduction
Head and neck cancer, which is primarily of squamous cell origin, was most recently estimated to account for 3% of newly diagnosed cancers in adults in the U.S. and ∼4% worldwide [1, 2]. The majority of patients present with locally advanced disease, creating a therapeutic challenge because the overall survival (OS) outcome for stage III–IV patients is poor, with treatment failures predominantly resulting from locoregional recurrences. A recent meta-analysis demonstrated that concurrent chemotherapy and locoregional therapy (primarily radiation) led to better 5-year OS and local failure rates, by 6.5% and 9.3%, respectively, than with locoregional therapy alone [3]. However, this comes at the expense of greater toxicity. Standard concurrent chemotherapy consists of higher-dose cisplatin every 3 weeks, and this regimen is associated with ototoxicity and grade 3–4 hematologic, mucosal, and gastrointestinal (GI) toxicities [4–6]. Various induction chemotherapy regimens have also been studied to further improve outcomes for patients with locally advanced head and neck cancer (LAHNC), though their impact on locoregional control and OS rates is controversial and they provide only modestly lower rates of distant metastasis [3, 5, 7].
Alternatives to higher-dose cisplatin have been explored in an attempt to reduce toxicity while maintaining efficacy. Carboplatin, an agent with structural and functional similarity to cisplatin, is associated with lower rates of nephro-, neuro-, and ototoxicity [8]. For LAHNC, carboplatin has been combined with paclitaxel to enhance overall radiosensitization without significant overlapping toxicities [9]. Though no study to date has prospectively compared higher-dose cisplatin alone with a taxane-based regimen, concurrent and neoadjuvant chemotherapy with combined platinum and taxane agents is well tolerated and feasible, with many studies demonstrating comparable rates of locoregional control and OS [10]. In one study, combined weekly carboplatin (100 mg/m2) and paclitaxel (45 mg/m2) given concurrently with radiation resulted in low rates of grade 4 toxicity and a 75% complete response rate, with a 3-year OS rate of 61% among this group of patients [9]. When combined taxane- and platinum-based induction chemotherapy was added to a similar concurrent regimen, a large majority of patients were able to complete both prescribed treatments. After induction chemotherapy, 89% of patients experienced a complete or partial response and the 3-year OS rate was 67% [11]. Similar results with taxane-based agents were seen in other studies [10, 12].
Intensity-modulated radiotherapy (IMRT) is also increasingly being employed as a means to further minimize toxicity through better normal-tissue sparing. With this technique, a highly conformal dose can be delivered to the tumor, allowing for dose escalation and differential doses per fraction to gross and subclinical disease [13, 14]. Despite the potential for inadequate tumor coverage with conformality, retrospective studies show that marginal recurrences are uncommon [15–17]. Furthermore, no difference in the 2-year locoregional progression-free survival rate or OS rate was found in a recent prospective, randomized study comparing rates of toxicity between three-dimensional (3-D) conformal radiotherapy and IMRT [18].
For LAHNC, the primary benefit of IMRT is the potential for parotid gland sparing, and numerous studies have demonstrated lower rates of xerostomia with IMRT [18–21]. A randomized, prospective study comparing IMRT with conventional radiation for early-stage nasopharyngeal carcinoma further confirmed that IMRT led to significantly better stimulated parotid salivary flow [22]. IMRT may also have a benefit in reducing the rate of long-term dysphagia by minimizing high-dose radiation to the larynx and pharyngeal constrictor muscles [23]. Prospective studies have shown that reducing the volume of these structures receiving high-dose radiation with IMRT leads to lower objective and subjective measures of late dysphagia [23–25]. Furthermore, chemotherapy with concurrent IMRT prospectively planned to minimize the dose to the swallowing structures resulted in acceptable 3-year rates of disease-free survival (DFS) and locoregional control, with no reported failures within or near the spared swallowing structures [25].
Though concurrent IMRT and carboplatin plus paclitaxel is increasingly being used for the treatment of LAHNC, comprehensive data on the tolerability and efficacy of this combination are limited. In this retrospective study, we examined our experience with this combination in 150 consecutive patients with LAHNC. The objectives of this study were to assess the feasibility and toxicity of this regimen as well as the outcomes of treatment. We specifically sought to determine (a) the ability of individuals to complete the prescribed treatment, (b) the incidence and relative rate of toxicities, (c) the effect of this regimen on locoregional control, and (d) the DFS and OS rates associated with this treatment.
Methods
Design and Patients
We performed a retrospective chart review of consecutive patients who received definitive concurrent chemoradiotherapy for LAHNC between December 2002 and October 2007 at Vanderbilt University Medical Center or the Vanderbilt-Ingram Cancer Center at Franklin. Patients with chemotherapy administered at the Veterans Administration Hospital in Nashville or Williamson County Medical Center were also included provided that their radiotherapy was performed at a Vanderbilt-affiliated facility. Eligibility included biopsy-proven American Joint Commission on Cancer stage III–IVB squamous cell carcinoma of the head and neck for which curative surgical resection was not indicated or recommended by a comprehensive multidisciplinary head and neck tumor board. Acceptable subsites included the nasopharynx, oropharynx, paranasal sinuses, oral cavity, hypopharynx, and larynx. Patients with unknown primary were also eligible if their disease was localized. Patients were at least 18 years old with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2. Exclusion criteria included prior surgical resection (other than biopsy), chemotherapy, or radiotherapy for head and neck cancer, distant metastatic disease at diagnosis, and concurrent active malignancy. Prior to starting radiotherapy, all patients underwent dental evaluation with appropriate preventative treatments as needed. This study was approved by the institutional review board of Vanderbilt University Medical Center.
Radiation Treatment Planning
Radiation was delivered using IMRT. Patients underwent computed tomography (CT)-based simulation with immobilization of the head and neck in the supine position with a custom Aquaplast mask (Aquaplast, Wycoff, NJ). The primary tumor and grossly involved lymph nodes were contoured based on physical examination findings and imaging studies. Prior neck CT with contrast, magnetic resonance imaging, and/or positron emission tomography (PET) scans (when available) were fused to the simulation CT to assist in tumor delineation. A margin of 1.0–1.5 cm was added to the gross tumor volume (GTV) to account for areas of potential subclinical disease and daily setup error. Involved and prophylactic lymph node levels were contoured based on the Radiation Therapy Oncology Group consensus guidelines and routinely included bilateral level II–IV lymph nodes [26]. If involved at presentation, level I and V lymph nodes were also included in the nodal volume, and these nodal groups were covered prophylactically for subsites at higher risk for subclinical disease in these locations. For patients with unknown primary, comprehensive nodal (bilateral levels II–IV) and pharyngeal (naso- and oropharynx) irradiation was performed. Surrounding critical structures, particularly the spinal cord, brainstem, larynx (for nonlaryngeal tumors), and parotid glands, were also outlined along with at-risk and avoidance structures to assist in treatment planning. Treatment planning was performed using Eclipse planning software (Varian Medical Systems, Palo Alto, CA).
Radiation Dose and Delivery
A single differential fractionation regimen was employed. The prescribed dose to the GTV plus margin (as described above) and involved lymph node levels was 2.1 Gy/fraction to 69.3 Gy. The dose prescribed to the prophylactic nodal levels was 1.7 Gy/fraction to 56.1 Gy. All patients were treated using 6 MV photons with seven to nine radiation fields using a stop-and-shoot method with sliding window technique. Treatment was given once a day for five consecutive days each week.
Dose–Volume Analysis of Treatment Plans
Dose–volume histograms for each treatment plan were evaluated prior to starting radiotherapy and were required to meet specific constraints. Dose to ≥95% of the target volume was required to be within ±5% of the prescribed dose. Dose to ≥95% of the prophylactically treated nodal volume was required to be within +8% to −5% of the prescribed dose. The maximum dose to the spinal cord and brain was required to be <4,500 cGy and the dose to 50% of each parotid gland was required to be <2,000 cGy. For nonlaryngeal tumors, treatment plans attempted to achieve a dose ≤2,000 cGy to 50% of the laryngeal volume and a maximal dose to the pharyngeal constrictors <5,000 cGy to minimize toxicity.
Chemotherapy
Induction chemotherapy was used at the discretion of the treating medical oncologist and was recommended for patients with a bulky tumor, nodal (N) stage N2 or N3 disease, or prominent level IV or V lymph nodes. It consisted of weekly paclitaxel (60 mg/m2) and carboplatin to an area under the concentration–time curve (AUC) of 2 for a total of 9 weeks, based on a conversion of our prior induction regimen [11] to weekly dosing. Concurrent chemotherapy consisted of weekly paclitaxel (30 mg/m2) and carboplatin (AUC, 1) given with ∼7 weeks of external-beam radiation. Chemotherapy was held for an absolute neutrophil count <1,000, platelet count <100,000, grade 4 mucositis, and grade 3 dermatitis.
Percutaneous Endoscopic Gastrostomy Tube Placement
Percutaneous endoscopic gastrostomy (PEG) tubes were not routinely placed prior to starting therapy, except when a patient demonstrated considerable weight loss (generally >10% body weight) from tumor-induced dysphagia. Patients underwent PEG tube placement after starting treatment when they demonstrated the inability to maintain adequate oral intake secondary to mucositis-associated pain or dysphagia.
Assessment of Complications
Acute and late toxicities were graded according the ECOG Common Toxicity Criteria. Acute radiation toxicities were graded weekly by the treating radiation oncologist during treatment and late toxicities were graded at scheduled radiation oncology follow-up visits. Acute chemotherapy toxicities were assessed retrospectively.
Follow-Up
Patients had follow-up examinations with fiberoptic endoscopy every 6–8 weeks for the first year, every 3 months for the second year, and every 4–6 months thereafter until 5 years. Post-treatment CT was required at 6–12 weeks and at 1 year, though generally CT was also performed at regular intervals up to 2 years post-treatment or if clinically indicated. Thyroid-stimulating hormone levels were obtained every 6 months after treatment. PET or panendoscopy was not performed except for suspicion of disease by clinical assessment or CT. Any suspicious clinical or radiographic lesion(s) required biopsy confirmation before establishing treatment failure. Neck dissections were performed for clinical or radiographic evidence of persistent or recurrent nodal disease.
Statistical Methods
The OS duration was defined as the time from treatment completion to death from any cause. The DFS interval was defined as the time from treatment to any type of recurrence. Local recurrence was defined as recurrence at the site of primary disease and locoregional recurrence was defined as recurrence at the primary or a nodal site. OS, DFS, local recurrence-free survival, locoregional recurrence-free survival, and distant disease-free survival times were estimated using the Kaplan–Meier statistical method. Univariate analysis with log-rank tests was used to identify significant prognostic variables for DFS time. Multivariate analysis was performed using the Cox proportional hazards model. p-values <.05 were considered statistically significant.
Results
Patient Characteristics
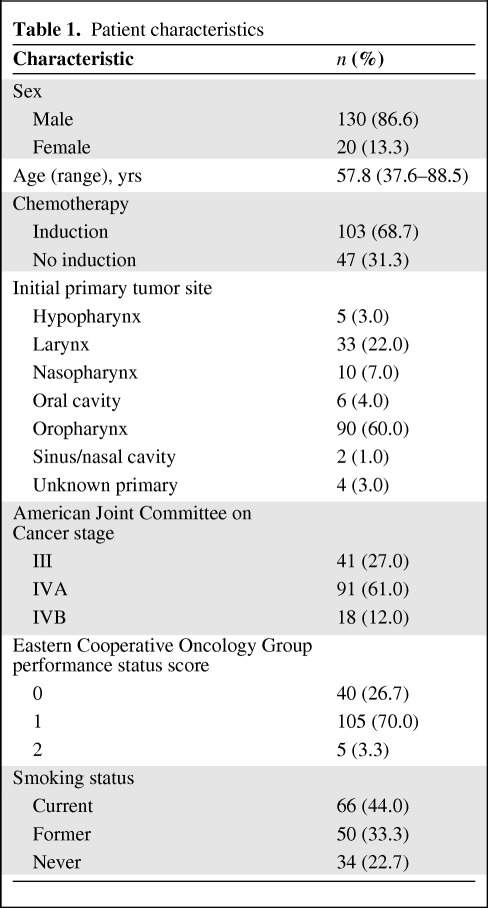
One hundred fifty consecutive patients who received concurrent chemoradiation with IMRT and weekly carboplatin plus paclitaxel between December 2002 and October 2007 were analyzed for this study. Patient characteristics are shown in Table 1 and supplemental online Table S1. The median age was 58.2 years (range, 37.6–88.5 years), with mean and median follow-up durations of 32 months and 30 months, respectively (range, 6–69 months for surviving patients). The majority of patients were male (86.6%) with stage IVA disease (61%) and primary oropharyngeal tumors (60%). Sixty-six percent had N2 or N3 disease, and 69% of patients also underwent induction chemotherapy.
Table 1.
Patient characteristics
Feasibility
Of the 103 patients receiving induction chemotherapy, only two patients (1.3% of total) received fewer than six of nine cycles. All 150 patients underwent concurrent chemoradiation with weekly paclitaxel plus carboplatin and IMRT. The vast majority of patients (96%) were able to complete at least five cycles of concurrent chemotherapy without any significant delays. Six patients (4%) completed four cycles or fewer of concurrent chemotherapy, and only eight patients (5%) required delays in radiation treatment >5 days. Approximately 93% of patients were able to receive the full prescribed dose of 6,930 cGy.
PEG tubes were placed prior to initiating therapy in 10 patients (7%). During treatment, PEG tubes were placed in 80 patients (53%). A slight majority of this group (44 patients) experienced a >10% weight loss. Only two patients (1%) underwent PEG placement after completing therapy. Fifty-eight patients (39%) were able to maintain their weight during and after treatment and never required PEG placement. The mean and median percentages of weight loss for all 150 patients were ∼7%.
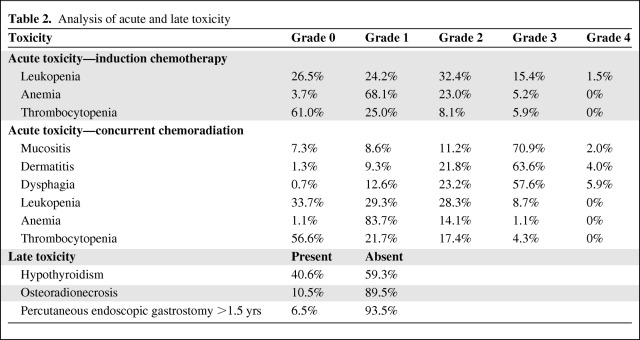
Toxicity
One patient died 3 days after concurrent chemoradiotherapy as a result of an unknown cause and another discontinued treatment early because of grade 2 mucositis and dysphagia. A third patient was unable to complete treatment because of grade 4 mucositis and dermatitis. There were no reported cases of nadir sepsis or significant nephropathy or GI toxicity. Rates of common acute and late toxicities are listed in Table 2. A majority of patients experienced grade 3 mucositis, dermatitis, and/or dysphagia. However, rates of acute grade 4 toxicity were minimal and developed in ≤5% of patients. The most common late toxicity was radiation-induced hypothyroidism, which occurred in ∼40% of patients, with a median time of onset of 14 months after completion of treatment (range, 4–47 months). Most patients had minimal or no late xerostomia, with only 6.7% developing grade 2 xerostomia. There were no instances of grade 3 or 4 xerostomia. Hematologic toxicity for concurrent chemotherapy was minimal. Fourteen patients (9.3%) required a PEG tube for ≥12 months as a result of persistent dysphagia and/or odynophagia. These patients were divided evenly among larynx (n = 5), base of tongue (n = 4), and tonsil (n = 5) with regard to the site of their primary lesion. Only 10 patients (6.7%) required a PEG tube for ≥18 months.
Table 2.
Analysis of acute and late toxicity
Induction chemotherapy with carboplatin and paclitaxel was also well tolerated. Toxicity related to induction chemotherapy was not as consistently scored as toxicity during concurrent chemoradiation; however, hematologic toxicity was reasonable, with only 1.5% of patients developing grade 4 leukopenia and no case of grade 4 anemia or thrombocytopenia. The most common toxicities requiring treatment breaks involved neutropenia and thrombocytopenia.
Treatment Outcome and Patterns of Failure
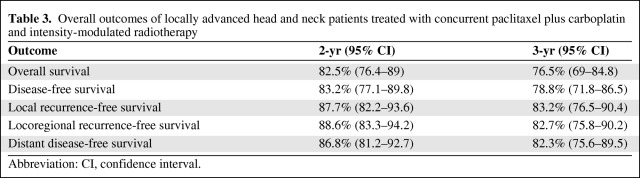
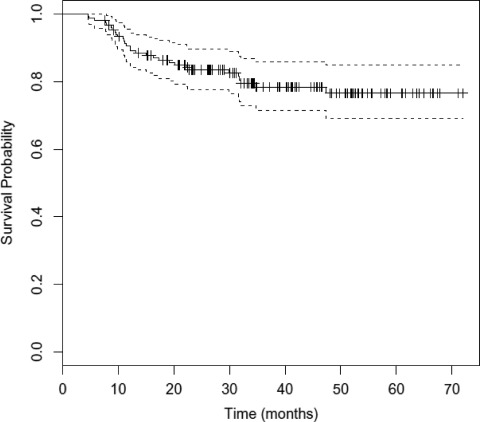
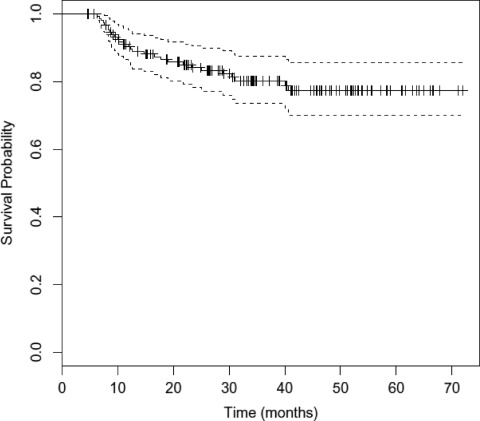
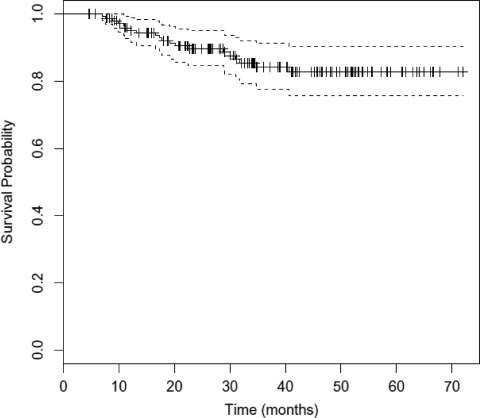
The overall outcomes of treatment are shown in Table 3. The median OS duration has not been reached. At 3 years, the DFS rate was 78.8% (95% confidence interval [CI], 71.8–86.5) and the OS rate was 76.5% (95% CI, 69.0–84.8) (Figs. 1 and 2). The local, locoregional, and distant metastasis-free survival rates at 3 years were similar, at 83.2%, 82.7%, and 82.3%, respectively (Fig. 3 and supplemental online Figs. S1 and S2). At the time of last analysis, 16 patients (10.7%) experienced local recurrence and 10 patients (6.7%) experienced nodal recurrence. The median time to failure was 11.5 months (range, 2.5–39 months), with nine patients developing failure within 6 months as a result of persistent disease. Twenty-four patients underwent a neck dissection, and this was performed for clinical and/or radiographic evidence of persistent disease (67% of patients), with the remainder performed for recurrent disease. Residual tumor was present in 46% of patients (n = 11). Fourteen patients (9.3%) developed biopsy-confirmed distant metastatic disease. The median time to distant failure was 5.5 months (range, 2–37 months), with two patients having concomitant residual locoregional disease. Of the patients who developed distant failure, 71.4% (n = 10) had undergone induction, and those 10 patients accounted for 9.7% of the entire group receiving induction chemotherapy. These outcomes and patterns of failure with IMRT are comparable with our previous experience with similar taxane-based induction and concurrent chemotherapy with 3-D conformal radiation [11, 12].
Table 3.
Overall outcomes of locally advanced head and neck patients treated with concurrent paclitaxel plus carboplatin and intensity-modulated radiotherapy
Abbreviation: CI, confidence interval.
Figure 1.
Kaplan–Meier estimates of overall survival time with 95% confidence interval.
Figure 2.
Kaplan–Meier estimates of disease-free survival time with 95% confidence interval.
Figure 3.
Kaplan–Meier estimates of locoregional recurrence-free survival time with 95% confidence interval.
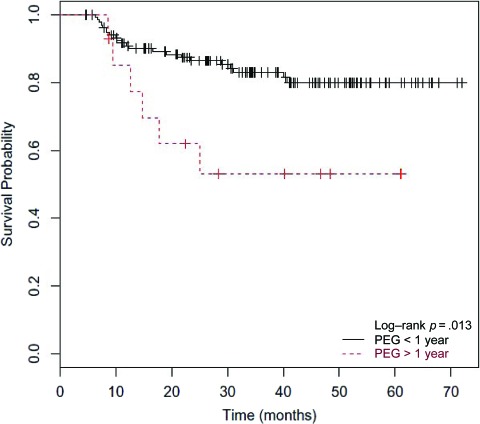
Univariate analysis showed a correlation between the DFS time and whether or not a patient required a PEG tube for >1 year, with long-term PEG requirement correlating with a significantly shorter survival time (p = .013) (Fig. 4). Among patients with long-term PEG use, the average percentage weight loss during treatment was comparable with that of the remaining cohort (∼7% in both groups), and persistent local disease was only present in one patient, demonstrating adequate maintenance of nutrition with its use. The DFS interval was not dependent on stage, site of primary tumor, age, amount of weight loss during treatment, or whether or not a patient underwent induction chemotherapy. Multivariate analysis, however, demonstrated a marginally significant correlation between induction chemotherapy treatment and age (p = .057), with a trend toward a shorter DFS time for patients undergoing induction chemotherapy with increasing age.
Figure 4.
Kaplan-Meier estimates of disease-free survival time by percutaneous endoscopic gastrostomy (PEG) status. Correlation between the disease-free survival time and PEG status was evaluated by univariate analysis. Patients were characterized as either requiring PEG tube placement for >1 year (red dashed line) or requiring PEG tube placement for <1 year or not at all (solid black line).
Discussion
Attempts to maintain better outcomes with concurrent chemoradiation while minimizing toxicity in the treatment of LAHNC have involved modifications of both concurrent chemotherapy and delivery of radiation. Here, we report our experience with IMRT concurrent with carboplatin and paclitaxel. This regimen was found to be well tolerated and feasible, with 93% of patients receiving the full prescribed radiation dose and 95% able to complete combined therapy without requiring significant breaks. Though most patients (57%–70%) developed grade 3 radiation-induced acute toxicity, the rate of grade 4 radiation-induced acute toxicity was minimal with this regimen (2%–5.9%). These rates of acute toxicity were similar to those from previously reported concurrent chemoradiotherapy studies using conventional fractionation [6, 27–29]. Treatment outcomes with regard OS, DFS, and failure rates were also promising and comparable with those from other institutions using IMRT [17, 30], and particularly those institutions reporting outcomes with concurrent chemotherapy and IMRT [31, 32].
As anticipated with IMRT, the rates of late toxicity were relatively modest in our cohort and were in agreement with other studies [31–33]. The vast majority of patients experienced grade 0–1 late xerostomia (93%), with the remainder experiencing grade 2 toxicity. No patient experienced grade ≥3 xerostomia with a median follow-up of 30 months. The relatively lower rates of grade 2 xerostomia than in other published reports with concurrent chemotherapy plus IMRT are likely a result of our greater heterogeneity with regard to primary disease site [31, 32]. Grade 3–4 late dysphagia was relatively uncommon in our cohort, with 9.3% of patients requiring prolonged PEG placement for ≥1 year. However, those patients were found to have a significantly shorter DFS interval than individuals who did not require extended or any PEG use. This did not appear to be associated with a higher percentage weight loss, more instances of persistent disease, or overrepresentation of contributory comorbidities. Because the ability of IMRT to spare the swallowing structures can be limited by the need to adequately cover clinical and subclinical disease, we examined primary tumor location in patients who required prolonged PEG use. Thirty-six percent (n = 5) had laryngeal primary tumors, which tend to include critical swallowing structures, such as pharyngeal constrictors, in the high-dose treatment volume, thus increasing the chance for developing late dysphagia. The remaining 64% of patients requiring long-term PEG use had oropharyngeal primary tumors, and generalizations on the inclusion of swallowing structures in these treatment volumes are more difficult. We are actively reviewing the IMRT dose to the upper, middle, and lower pharyngeal constrictors, how this impacted PEG tube requirement, and if dose negatively impacted survival outcomes.
In our study, all patients received weekly carboplatin and paclitaxel concurrent with radiation. Though higher-dose cisplatin has historically been used in most intergroup studies, numerous institutions have alternatively used concurrent carboplatin and paclitaxel because of its radiosensitizing properties and more favorable toxicity profile. Single-institution phase II trials using conventional radiotherapy had shown that concurrent carboplatin plus paclitaxel resulted in a ≥75% clinical complete response rate, with a 3-year OS rate of ∼60% among these responders [9, 34]. Toxicity associated with this regimen appeared to correlate primarily with the dose of paclitaxel. Weekly paclitaxel at 60 mg/m2 with concurrent carboplatin (AUC, 1) and radiation resulted in grade 4 mucositis in 30% of patients, with only 47% completing chemotherapy at full doses and 18% unable to complete radiation as prescribed [34]. On the other hand, 40–45 mg/m2 of weekly paclitaxel with carboplatin (100 mg/m2) and radiation was associated with no grade 4 mucositis, and 98% of patients were able to complete the prescribed therapy [9]. In our population, we treated with paclitaxel (30 mg/m2) and carboplatin (AUC, 1) weekly and experienced only a 2% rate of grade 4 mucositis and a 5.9% rate of grade 4 dysphagia, and 96% of patients were able to complete five to seven cycles of chemotherapy. These results are comparable with our prior experience with concurrent carboplatin plus paclitaxel (30 mg/m2) and 3-D conformal radiotherapy [11]. In addition to modest rates of grade 4 toxicity and high compliance, treatment outcomes were actually better than in these earlier carboplatin–paclitaxel studies, with our 3-year survival rate at 76.5% (compared with 50%–60%) [9, 34]. Considering our similar patient characteristics, the reason for this better survival outcome is not entirely clear, though the lower rate of treatment completion in the higher-dose paclitaxel group and subsequent greater rate of locoregional failure are likely contributors [34]. Regardless, our treatment outcomes are comparable with those of more recent studies using IMRT with concurrent chemotherapy [31, 32].
Induction chemotherapy is increasingly being used as an adjunct to concurrent chemoradiation to further improve OS outcomes and decrease the rate of distant failure. To this end, induction chemotherapy has shown variable success in LAHNC patients, with some improvement in distant failure but no consistent differences in OS or locoregional control outcomes [3, 5, 7]. Recent studies suggest that the addition of a taxane improves outcomes. Specifically, in randomized, prospective studies, the addition of docetaxel to cisplatin plus fluorouracil (TPF) resulted in greater OS, response, and locoregional control rates than with cisplatin plus fluorouracil alone, with less treatment-limiting toxicity [35, 36]. The use of paclitaxel with carboplatin has been explored as a less-toxic alternative to TPF, and phase II trials have shown that this induction regimen leads to a complete or partial response in ≥79% of patients, with a rate of distant metastasis of ∼8%–12% and a 2-year OS rate of ∼75% when followed by concurrent chemoradiation [11, 37]. At our institution, high induction response rates with carboplatin and paclitaxel have previously been reported [38], and we observed a comparable distant failure rate (9.7%) and 2-year OS rate (82.5% for all patients) in the current cohort. Although a large majority of patients in our study developed distant failure despite induction chemotherapy (71.4% rate of distant failure), patients who underwent induction had more advanced disease or the presence of adverse features portending a higher risk for developing distant metastases, thus complicating the interpretation of these results. Ultimately, the absolute benefit of induction chemotherapy (carboplatin plus paclitaxel or other regimens) over aggressive concurrent chemoradiation alone will need to be determined in a prospective, randomized fashion, and these studies are currently ongoing.
As a retrospective analysis, this study is limited by our lack of a direct comparison with a standard-of-care regimen (i.e., higher-dose cisplatin and concurrent 3-D conformal radiation) and the lack of a formal evaluation protocol during treatment to comprehensively assess toxicity and tolerability, particularly with regard to chemotherapy. However, our treating radiation oncologists prospectively grade toxicity during and after treatment according to ECOG Common Toxicity Criteria, allowing for a significant level of standardization during and after concurrent therapy. Our study is further limited by the absence of human papilloma virus (HPV) determination, which was not standard practice at our institutions during the timeframe of this analysis. Nevertheless, the likelihood that an overrepresentation of HPV+ patients skewed outcome data is small considering that the univariate analysis showed no difference in terms of the DFS interval based on tumor site, even though 60% of the patients in our cohort had oropharyngeal tumors. Overall, comparison of our toxicity, feasibility, and outcomes with those of historic controls and recent retrospective and prospective trials using IMRT or concurrent carboplatin plus paclitaxel suggests that our results are in accordance with, if not better than, those from these studies.
Conclusion
Concurrent taxane-based chemoradiotherapy with IMRT is feasible and well tolerated, with acceptable toxicity and treatment outcomes comparable with those of historic controls [4, 5]. The addition of induction carboplatin and paclitaxel to this concurrent regimen was also found to be feasible, with effective dose delivery. Though the benefit of induction is difficult to ascertain retrospectively, the overall efficacy of combined treatment was comparable with our prior experiences with induction and concurrent taxane-based chemotherapy with conventional radiation [11, 12]. Furthermore, our combined regimen may provide a less-toxic alternative to aggressive three-drug induction chemotherapy followed by concurrent chemoradiation. Given the excellent treatment delivery and tolerability of our regimen with acceptable outcomes, we believe that concurrent taxane-based chemoradiotherapy with IMRT is a reasonable therapeutic option for the curative treatment of LAHNC.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Roberto Diaz, Barbara A. Murphy, Patrick Murphy, Anthony J. Cmelak
Provision of study material or patients: Barbara A. Murphy, Robert J. Sinard, James L. Netterville, Wendell G. Yarbrough, Anthony J. Cmelak
Collection and/or assembly of data: Gregory Vlacich, Roberto Diaz, Steven W. Thorpe, Wyndee Kirby, Patrick Murphy, Anthony J. Cmelak
Data analysis and interpretation: Gregory Vlacich, Roberto Diaz, Steven W. Thorpe, Bashar Shakhtour, Anthony J. Cmelak
Manuscript writing: Gregory Vlacich, Anthony J. Cmelak
Final approval of manuscript: Gregory Vlacich, Roberto Diaz, Steven W. Thorpe, Barbara A. Murphy, Robert J. Sinard, Wendell G. Yarbrough, Anthony J. Cmelak
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update of 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 6.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Adelstein DJ, LeBlanc M. Does induction chemotherapy have a role in the management of locoregionally advanced squamous cell head and neck cancer? J Clin Oncol. 2006;24:2624–2628. doi: 10.1200/JCO.2005.05.3629. [DOI] [PubMed] [Google Scholar]
- 8.Aisner J, Sinibaldi V, Eisenberger M. Carboplatin in the treatment of squamous cell head and neck cancers. Semin Oncol. 1992;19(suppl 2):60–65. [PubMed] [Google Scholar]
- 9.Suntharalingam M, Haas ML, Conley BA, et al. The use of carboplatin and paclitaxel with daily radiotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2000;47:49–56. doi: 10.1016/s0360-3016(00)00408-9. [DOI] [PubMed] [Google Scholar]
- 10.Schrijvers D, Vermorken JB. Taxanes in the treatment of head and neck cancer. Curr Opin Oncol. 2005;17:218–224. doi: 10.1097/01.cco.0000158735.91723.0e. [DOI] [PubMed] [Google Scholar]
- 11.Cmelak AJ, Murphy BA, Burkey B, et al. Taxane-based chemoirradiation for organ preservation with locally advanced head and neck cancer: Results of a phase II multi-institutional trial. Head Neck. 2007;29:315–324. doi: 10.1002/hed.20522. [DOI] [PubMed] [Google Scholar]
- 12.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III and IV squamous cell carcinomas of the larynx or oropharynx: Results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 13.Grégoire V, De Neve W, Eisbruch A, et al. Intensity-modulated radiation therapy for head and neck carcinoma. The Oncologist. 2007;12:555–564. doi: 10.1634/theoncologist.12-5-555. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Puri DR, Blanco AI, et al. Intensity-modulated radiation therapy in head and neck cancers: An update. Head Neck. 2007;29:387–400. doi: 10.1002/hed.20332. [DOI] [PubMed] [Google Scholar]
- 15.Chao KS, Ozyigit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312–321. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 16.Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46:1117–1126. doi: 10.1016/s0360-3016(99)00550-7. [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: Implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao KS, Majhail N, Huang CJ, et al. Intensity-modulated radiation therapy reduces late salivary toxicity without compromising tumor control in patients with oropharyngeal carcinoma: A comparison with conventional techniques. Radiother Oncol. 2001;61:275–280. doi: 10.1016/s0167-8140(01)00449-2. [DOI] [PubMed] [Google Scholar]
- 20.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 21.Henson BS, Inglehart MR, Eisbruch A, et al. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncol. 2001;37:84–93. doi: 10.1016/s1368-8375(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 22.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–1171. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 28.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 29.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: A prospective randomized trial. J Clin Oncol. 2000;18:1458–1464. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 30.Chao KS, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Huang K, Xia P, Chuang C, et al. Intensity-modulated chemoradiation for treatment of stage III and IV oropharyngeal carcinoma. Cancer. 2008;113:497–507. doi: 10.1002/cncr.23578. [DOI] [PubMed] [Google Scholar]
- 32.Saba NF, Edelman S, Tighiouart M, et al. Concurrent chemotherapy with intensity-modulated radiation therapy for locally advanced squamous cell carcinoma of the larynx and oropharynx: A retrospective single-institution analysis. Head Neck. 2009;31:1447–1455. doi: 10.1002/hed.21120. [DOI] [PubMed] [Google Scholar]
- 33.Lee NY, De Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:966–974. doi: 10.1016/j.ijrobp.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Chougule PB, Akhtar MS, Rathore R, et al. Concurrent chemoradiotherapy with weekly paclitaxel and carboplatin for locally advanced head and neck cancer: Long-term follow-up of a Brown University Oncology Group phase II study (HN-53) Head Neck. 2008;30:289–296. doi: 10.1002/hed.20700. [DOI] [PubMed] [Google Scholar]
- 35.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 36.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 37.Ready NE, Rathore R, Johnson TT, et al. Weekly paclitaxel and carboplatin induction chemotherapy followed by concurrent chemoradiotherapy in locally advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2012;35:6–12. doi: 10.1097/COC.0b013e3182019ee3. [DOI] [PubMed] [Google Scholar]
- 38.Dang TP, Murphy BA, Cmelak AJ, et al. Carboplatin and Taxol as induction therapy for locally advanced carcinoma of the head and neck. Proc Am Soc Clin Oncol. 1998;17:393a. abstract 1516. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.