The impact of a video designed to overcome attitudinal barriers to clinical trial participation among African American cancer patients is assessed.
Keywords: African American accrual, Clinical trials, Cancer disparities, Culturally targeted intervention
Abstract
Purpose.
Barriers to clinical trial participation among African American cancer patients are well characterized in the literature. Attitudinal barriers encompassing fear, distrust, and concerns about ethical misconduct are also well documented. To increase trial accrual, these attitudes must be adequately addressed, yet there remains a lack of targeted interventions toward this end. We developed a 15-minute culturally targeted video designed to impact six specific attitudes of African American cancer patients toward therapeutic trials. We conducted a pilot study to test in the first such intervention to increase intention to enroll.
Patients and Methods.
The primary study outcome was self-reported likelihood to participate in a therapeutic trial. Using a mixed methods approach, we developed the Attitudes and Intention to Enroll in Therapeutic Clinical Trials (AIET) instrument, a 30-item questionnaire measuring six attitudinal barriers to African American trial participation. We enrolled 108 eligible active treatment patients at a large urban cancer institute. McNemar's test for matched pairs was used to assess changes in attitudes and likelihood to enroll in a clinical trial at baseline and immediately after the video. Pre- and post-video AIET summative scores were analyzed by paired t-test for each attitudinal barrier.
Results.
Patients' likelihood of enrolling in a clinical trial significantly increased post-video with 36% of the sample showing positive changes in intention [McNemar's χ2 = 33.39, p < .001]. Paired t-tests showed significant changes in all six attitudinal barriers measured via AIET summative scores from pre- to post-video.
Conclusion.
These data suggest utility of our video for increasing African American participation in clinical trials.
Introduction
The accrual of diverse patients to clinical trials remains one of the biggest challenges to advancing cancer treatment and improving outcomes [1–3]. African Americans (AA) continue to display the lowest 5-year cancer survival rates compared with all other ethnic groups and experience mortality rates approximately 25% higher than whites [2,4,5]. Of all U.S. adults diagnosed with cancer, an estimated 5%–10% will actually participate in a therapeutic clinical trial [6–9] and a significantly smaller proportion of those are eligible AA [8,10,11]. A greater burden of disease among AA necessitates their participation in clinical trials, as only sufficient representation will determine whether treatments are equally efficacious [9,11–14].
The extant literature is replete with established barriers to trial participation for AAs, some of which impede even the most willing patient [7,15–18]. Trial design factors such as eligibility criteria, provider level factors [19–22], and socio-cultural factors all constitute well-documented barriers [23–25]. Increasing evidence supports the role of patient level barriers such as cultural differences in attitudes toward cancer and its treatment in driving outcomes [20,26–28]. The literature identifies several attitudinal barriers to AA clinical trial participation, many of which are rooted in historical events such as the US Public Health Services Syphilis Study [16,20,23,29]. Specifically, concerns about the ethical misconduct of investigators, or poor treatment for being a minority or being economically disadvantaged, constitute documented attitudinal barriers [30–32]. Attitudinal barriers also include a fear and distrust of the medical establishment [20,29], worry about a loss of autonomy [13,20,33], and a general lack of awareness about clinical trials among AA cancer patients [1,17,33].
Recognition of the specific attitudes of AA patients and their relationship with intention to enroll is critical to ameliorating disparities in trial participation [34]. Facilitating communication about clinical trials between the patients and their providers is also particularly important. A study among a sample of general respondents explored relationships between trust and a patient's expressed intentions to enroll in clinical trials [35]. Although this sample was not ethnically focused, patients who were more trusting of their doctors and believed their doctors would treat them with respect and fairness were more willing to communicate about clinical trial enrollment. Within a health education and health communication context, interventions have been shown to be more effective when they are culturally appropriate for the population they serve [36]. We believe a short video that effectively addresses specific attitudes as a way to increase willingness to participate may help to achieve this. As no such tool exists, to our knowledge, we developed one and conducted a pilot study to assess its impact in an intervention with AA cancer patients.
Methods
All aspects of the study were approved by an appointed Data Safety and Monitoring Board (DSMB) and the Georgetown/Medstar Health Institutional Review Board. The DSMB routinely reviewed study progress and procedures throughout all stages of protocol activity.
Intervention Design
A Community Advisory Group (CAG) was convened to guide and approve the video content and script development. The video consisted of unscripted narratives of African American patients discussing their experiences with clinical trials, their attitudes, and multitude factors influencing their decision-making following their cancer diagnosis. The video spotlighted Washington Cancer Institute (WCI) patients and their family members identified by research coordinators. Members of the clergy, WCI physicians, ethicists, and staff were also featured. A skilled producer and health videographer conducted on-camera interviews using open-ended questions developed by the research team related to the six attitudinal barriers. Patients recounted their feelings and any challenges related to each barrier, detailing their experiences and how their respective families grappled with them. The final content was selected by the CAG and the production team to ensure each barrier was thoroughly and fairly addressed by the patient narratives. A locally recognized news anchor served as narrator, and together with WCI physicians and staff, addressed any myths associated with the six barriers. Emphasis was on the facts about clinical trials, presented in lay language. The content also briefly described the US Public Health Services Syphilis Study in an attempt to break down the related barriers of misinformation and myth surrounding the events at Tuskegee. The resulting 15-minute video was the intervention tool for this study.
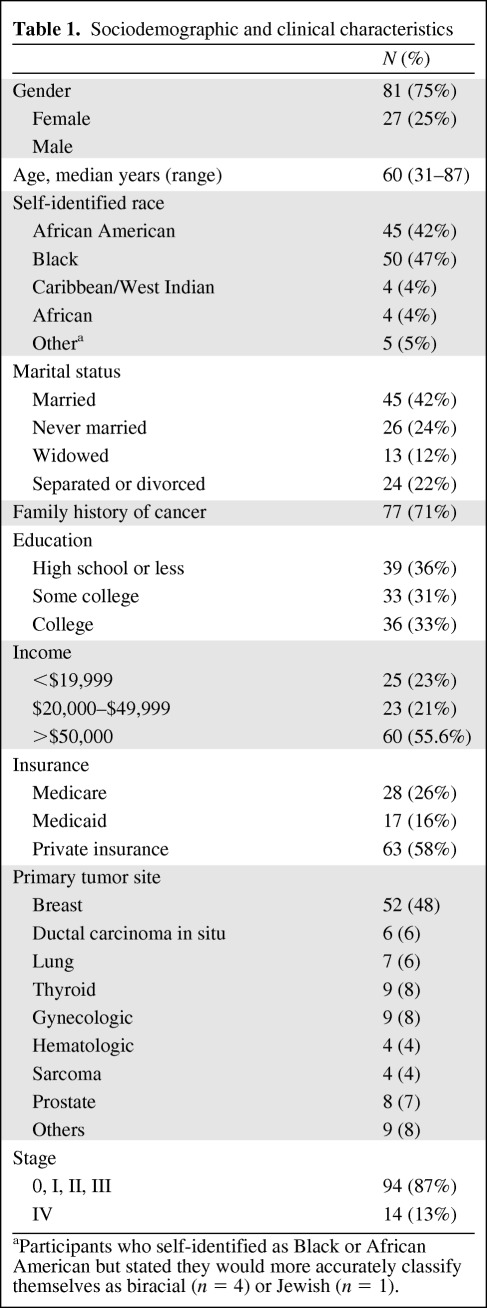
Eligible patients were identified by physicians, nurse navigators, and clinical research coordinators at the WCI who reviewed medical records to confirm the patient's eligibility. The study sample consisted of 108 male and female cancer patients in active treatment between October 2010 and February 2011. Patients self-identified race and were age 21 years or older (Table 1). Anticipated cancer treatment was to be given at WCI and all patients were potentially eligible for a therapeutic clinical trial. Only those who were able to understand and give informed consent were invited to participate. Patients who had previously signed a consent form to participate in any type of research study and therefore had some experience with clinical research were ineligible. Any apparent physical distress or an altered mental status precluding the ability to give informed consent and/or complete study procedures was also basis for exclusion.
Table 1.
Sociodemographic and clinical characteristics
aParticipants who self-identified as Black or African American but stated they would more accurately classify themselves as biracial (n = 4) or Jewish (n = 1).
To evaluate whether the video impacted attitudes and intention to enroll in therapeutic clinical trials, each eligible patient was invited to commit to up to 1 hour to complete study procedures. Individual interviews were conducted by a racially congruent study coordinator who also explained to participants what a therapeutic trial was. This description was also provided in the video. Each individual session was held in the study coordinator's office where the video was also set up for viewing. Participants completed a questionnaire (pre-test) verbally administered by the coordinator before viewing the intervention video. The questionnaire measured patients' attitudes on six barriers and a single item of self-reported likelihood to enroll in a trial. The same questionnaire (post-test) was administered immediately after viewing the video. The patient's verbal responses were recorded by the coordinator on a paper version of the questionnaire and later double-keyed into a secure electronic database.
Study Measures and Outcome Variables
Participants' intention to enroll in a clinical trial was the primary study outcome. A single item on the questionnaire was used to assess the participant's hypothetical willingness or intention to participate in a clinical trial by asking, “At this moment, is it likely that you would participate in a clinical trial?” The “Yes ” or “No ” response option was the binary-dependent variable used for analyses.
Standard demographic data including age, gender, and ethnicity were collected (Table 1). The six attitudinal barriers to trial participation were the secondary endpoints. These were fear and distrust of the medical establishment, concern about the ethical conduct of investigators, fear of losing one's rights by signing a research informed consent document, worry that investigators will treat poor or minority patients unfairly, and a lack of knowledge and awareness of clinical trials.
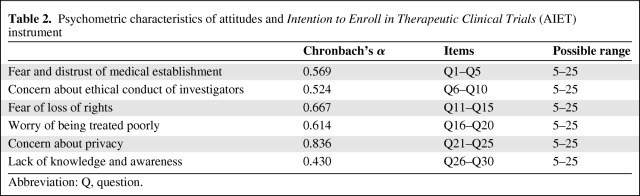
No previously validated instrument existed to measure the specific attitudes identified for study. A new assessment tool, measuring Attitudes and Intention to Enroll in therapeutic clinical Trials (AIET) in cancer patients was developed. Items were all adapted from existing scales that measured concepts similar, but not identical, to those assessed in this study.
Each of six attitudinal barriers measured represented a subscale on the AIET consisting of five items, each assessed on a 5-point Likert-type scale. Responses were scored 1 = Strongly Disagree; 2 = Somewhat Disagree; 3 = Not sure/Neither; 4 = Somewhat Agree; 5 = Strongly Agree. The five-item responses for each attitudinal barrier were summed to produce a cumulative score with a possible range from 5 to 25. Two items were reverse-scored as appropriate. Appendix A in the supplemental online data presents the questionnaire with all items, full response options, and corresponding scoring for each barrier.
The AIET was reviewed by two study consultants and five University of Maryland behavioral scientists with relevant expertise for face and content validity. The questionnaire was also pilot-tested with a cancer survivors support group whose comments were audio-recorded and used to edit the questionnaire accordingly. Finally, the CAG reviewed the questionnaire to evaluate clarity, level of understanding, and general acceptability for use in the context of this study.
Statistical Analyses
In stage 1 basic descriptive statistics assessed the distribution of socio-demographic and clinical characteristics in the study population. In stage 2 the psychometric properties of the new instrument were assessed using Cronbach's α to determine the internal consistency of the subscales measuring each attitude. An α of 0.70 or above is considered indicative of acceptable internal consistency [37]. In stage 3 the intervention was evaluated. McNemar's test for paired data was used to assess the change in intention to enroll in a trial before and after viewing the video. The impact of the video on study participants' attitudes was assessed using paired t-tests of the difference in summative scores of each attitude pre- and post-video for each barrier. Each possible predictor for the change in intention to enroll in a clinical trial among those indicating they would not participate in a trial prior to viewing the video was evaluated in separate logistic regression models adjusted for age and gender. Two-tailed p < .05 was considered statistically significant. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC).
Results
A total of 161 patients were approached, 17 refused participation (10.5%), and 33 stated they did not have enough time, were unable to arrange transportation to enable them to stay after their appointment, or did not want to make accompanying family members wait. The 111 patients remaining completed the intervention and both assessments. After completing study procedures, three participants were later confirmed to be part of familial registries or previously signed a consent form for another research study. They were deemed ineligible and excluded, leaving 108 patients eligible for analysis.
Stage 1 analysis included baseline demographic characteristics of these study participants and the clinical characteristics of their respective cancers (Table 1) as presented.
In stage 2 Cronbach's α for each subscale ranged from 0.430 to 0.836 (Table 2). Only the subscale measuring concern about loss of privacy proved to be psychometrically sound by the α = 0.70 standard. A detailed item-by-item analysis for pre- and post-video intervention is presented in Appendix B in the supplemental online data.
Table 2.
Psychometric characteristics of attitudes and Intention to Enroll in Therapeutic Clinical Trials (AIET) instrument
Abbreviation: Q, question.
Primary Outcome
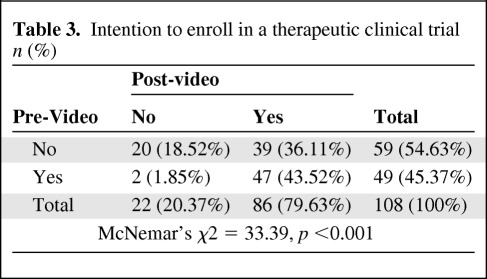
The proportion of participants expressing likelihood to enroll in a trial significantly increased after viewing the video: 45.4% (49 out of 108) pre-video and 79.6% (86 out of 108) post-video (McNemar's χ2 = 33.39, p < .001). Table 3 shows the direction of change of self-reported intention to enroll in a clinical trial prior to and immediately following the video intervention for study participants. Forty-nine participants (45.4%) indicated they would likely enroll in a clinical trial at baseline, all but two of whom maintained this after watching the video. Fifty-nine participants (54.6%) reported they would decline enrollment in a trial prior to watching the video. Of these, 66% changed their mind after viewing the video, stating they would enroll. Overall, 36% of the original sample had a positive change in intention after viewing the video.
Table 3.
Intention to enroll in a therapeutic clinical trial n (%)
Secondary Outcome
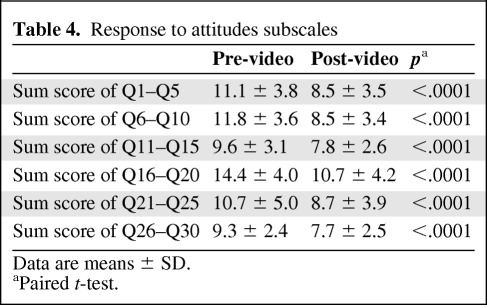
For each of the attitudinal barriers, there was a significant pre-test/post-test difference, with a change in post-test responses in a shift from negative to positive attitudes toward clinical trial participation (all p-values < .0001) (Table 4).
Table 4.
Response to attitudes subscales
Data are means ± SD.
aPaired t-test.
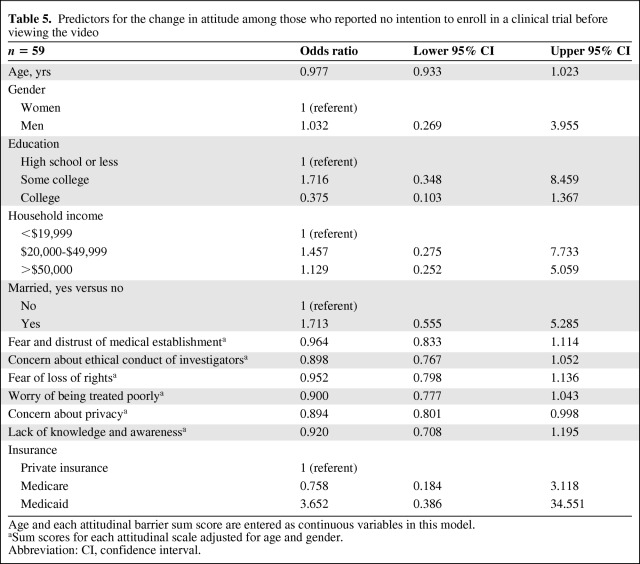
Separate logistic regression models for each variable adjusted for age and gender were performed (Table 5). Patients with higher sum scores for the attitudinal barrier measuring the concern about loss of privacy were less likely to enroll. This was statistically significant [odds ratio (OR) = 0.894; 95% confidence interval (CI): 0.801–0.998]. Those with a college education were far less likely to express intention to enroll than those with high school education or less (OR = 0.375; 95% CI: 0.103–1.367). Married patients were more likely to express intention to enroll than unmarried patients (OR = 1.713; 95% CI: 0.555–5.285). Overall, patients with higher sum scores on each of the attitude variables were less likely to report an intention to enroll, when age and gender were adjusted (Table 5).
Table 5.
Predictors for the change in attitude among those who reported no intention to enroll in a clinical trial before viewing the video
Age and each attitudinal barrier sum score are entered as continuous variables in this model.
aSum scores for each attitudinal scale adjusted for age and gender.
Abbreviation: CI, confidence interval.
Discussion
The results of this pilot study suggest our video intervention increases the intention and likelihood of African American patients to enroll in cancer trials. Consensus recommendations state that addressing cancer health disparities requires the inclusion of underrepresented populations in cancer clinical trials and proven efficacious strategies to do so [3,14]. We developed and evaluated a tool that could effectively address attitudinal barriers that decrease African American cancer patients' intention to participate in trials.
Health behavior theory states intention is the direct antecedent of actual behavior [38], thus changing a patient's intention or willingness to participate in a trial is a crucial step. One third of our sample expressed no intention to participate in a trial at baseline, but changed their mind after the video intervention, stating they would likely enroll in a clinical trial.
Previous studies have created patient education videos to educate patients about cancer-related topics [6,31,39–43]. A randomized trial of a video mailed to patients before a physical exam showed no difference in its ability to increase colorectal cancer screening rates between intervention and control groups [41]. This video was not culturally targeted. A systematic review showed the video to be an effective medium for patient education, emphasizing the benefit of video format for role modeling [42]. These studies were not limited to AA cancer patients or to address cancer clinical trials. The use of real patients conveying their personal, unscripted narratives provides a unique and impactful perspective to our intervention medium. Narratives have been shown to be of particular value in AA populations in a study of mammography use and cancer-related beliefs [44,45]. Our study is the first to specifically address the attitudinal barriers to clinical trial participation faced by AAs and directly evaluate their intention to enroll.
Methods used to recruit underrepresented populations into cancer prevention and treatment trials have been systematically reviewed with the conclusion there is still limited evidence of effective strategies [46]. Among existing studies, few evaluated efficacy of their recruitment strategies and even fewer evaluated strategies targeting African Americans [19]. Specific recruitment strategies including media campaigns and church-based project sessions with enhanced recruitment letters and telephone calls have resulted in improved accrual [18]. These strategies did not specifically address attitudes and intentions of ethnic minorities. However, given the proven efficacy of their general approaches, it is conceivable that our video could be easily incorporated to such strategies given its impact on an individual level.
Our results contribute to the literature in several important ways. First, although our sample size is modest, it did include patients with over 12 different primary tumor types, suggesting the ability to generalize the effectiveness of this video and its potential utility to increase willingness to participate across all cancer types and varied therapeutic trials.
Second, approximately one third of participants in this pilot study had a high school education or lower. Our intervention suggests a practical channel for delivering clinical trials information and an ideal format to present it regardless of literacy level. Our use of an interviewer-administered questionnaire and a video format are two factors that appeared to address barriers to comprehension that may be posed by low literacy. This represents an important way to reach a subset of AA who may be less willing to participate in trials for lack of knowledge and awareness.
The extra time needed to gain individual and community acceptance of clinical trials among minorities is a known prerequisite to effect their participation in clinical trials [21]. Our video as part of this overall approach could lend itself well to addressing AA underrepresentation on both an individual and community level. We believe this to be an important and effective way to address myths and strongly held attitudes toward trials, particularly among African American patients in various settings.
Although attitudinal barriers have been well characterized through survey studies [47,48], there appears to be no consensus on the relative influence or interrelationship of these barriers. Our preliminary findings suggest that among those who had a positive change in intention to enroll, the concern about loss of privacy may be the predictor of intention. This subset of the sample (n = 59), however, represents a number too small to assess the full impact and predictive value of each individual attitude. The subscale measuring concern about loss of privacy was the only psychometrically sound subscale on the AIET. This may explain the finding that concern about privacy was the only significant predictor. Further development of the AIET is required to substantiate this.
We note some study limitations. Study participants were at various stages of treatment and at varying time points after diagnosis. This may have impacted both their attitudes toward therapeutic trials and intention to enroll. We have limited ability to determine the direction of the relationships hypothesized and/or infer causality of impact on attitudes based solely on the video intervention. More work is needed to determine whether intention to enroll influences attitudes or whether the attitudinal barriers affect intention to enroll.
The overall sample size was small, resulting in limited power to detect nuanced differences in attitudes among this pilot population. Furthermore, a self-selection bias may exist, given the individuals who agreed to participate and complete the survey were likely to be less resistant to research in general. Participants were thus predisposed to be more willing to participate in a clinical trial, biasing the responses to items on the survey. Furthermore, participation in this study required patients undergo an informed consent process, potentially influencing responses on any questionnaire items pertaining to consent. Given the nature of the subject matter, the potential for an overall social desirability bias in response to the AIET items is also acknowledged.
The six barriers measured represented related, but distinct, dimensions of attitude. As such, the AIET does not yet constitute a fully developed multidimensional scale, rather a novel and important contribution for further development where no other instrument exists. Although the reliability of several of the scales on the AIET is low, it should be noted that these attitudes represent emerging constructs, the dimensions of which are challenging to measure with such few items. We present these data as a pilot effort for researchers to use and expand upon in future refinements of the AIET instrument.
With an anticipated increase in cancer incidence for minority populations far greater than the corresponding increase for Caucasians [27], it is imperative that the inherent selection bias and objectivity of clinical trial design be addressed [15]. Simple, effective, patient-level interventions that can be rapidly and widely disseminated offer promise in increasing AA participation in clinical trials. Toward this end, we propose the potential utility of our evaluated, culturally targeted video. Our findings provide an important foundation and direction for increasing intention to enroll among AA and the much-needed development of metrics for use in this arena. The next step is to replicate this study using a randomized multicenter trial design with a larger patient sample.
See www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors thank Dr. Barbara V. Howard, Dr. Nawar Shara, and Dr. Mihri Mete of Medstar Health Research Institute and Dr. Katrina Debnam of University of Maryland, College Park, for their help with manuscript development. The authors also thank Carol M. Rosenbaum productions for video development and production. This research is supported by National Center on Minority Health and Health Disparities: 1RC1MD004185-01.
This manuscript was presented in part at the ASCO Annual 2011 Meeting, Chicago (poster presentation) and the ASCO Breast Cancer Symposium 2011, San Francisco (poster presentation and poster discussion session).
Author Contributions
Conception/Design: Deliya R. Banda, Sandra M. Swain
Provision of study material or patients: Deliya R. Banda, Sandra M. Swain
Collection and/or assembly of data: Deliya R. Banda, Sandra M. Swain
Data analysis and interpretation: Deliya R. Banda, Alexander V. Libin, Hong Wang, Sandra M. Swain
Manuscript writing: Deliya R. Banda, Alexander V. Libin, Hong Wang, Sandra M. Swain
Final approval of manuscript: Deliya R. Banda, Alexander V. Libin, Hong Wang, Sandra M. Swain
References
- 1.Park ER, Weiss ES, Moy B. Recruiting and enrolling minority patients to cancer clinical trials. Commun Oncol. 2007 Apr;:254–257. [Google Scholar]
- 2.Harnessing Science: Advancing Care by Accelerating the Rate of Cancer Clinical Trial Participation Hearing before the Committee on Government Reform House of Representatives One Hundred Eight Congress Second Session; May 13, 2004; Washington, DC. Report No.: Serial No. 108–189. [Google Scholar]
- 3.Institute of Medicine. Washington DC: National Academies Press; 2010. Transforming Clinical Research in the United States: Challenges and Opportunities. [PubMed] [Google Scholar]
- 4.American Cancer Society. Atlanta: American Cancer Society; 2010. Cancer Facts & Figures for African Americans 2009–2010. [Google Scholar]
- 5.American Cancer Society. Atlanta: American Cancer Society; 2011. Cancer Facts & Figures for African Americans 2011–2012. [Google Scholar]
- 6.Du W, Mood D, Gadgeel S, et al. An educational video to increase clinical trials enrollment among breast cancer patients. Breast Cancer Res Treat. 2009;117:339–347. doi: 10.1007/s10549-009-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baquet CR, Ellison GL, Mishra SI. Analysis of Maryland cancer patient participation in national cancer institute-supported cancer treatment clinical trials. J Clin Oncol. 2008;26:3380–3386. doi: 10.1200/JCO.2007.14.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–270. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 9.Philadelphia: Coalition of Cancer Cooperative Groups; 2006. Baseline study of patient accrual onto publicly sponsored US cancer clinical trials: an analysis conducted for the Global Access Project of the National Patient Advocate Foundation. [Google Scholar]
- 10.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 11.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 12.McCaskill-Stevens W, Pinto H, Marcus AC, et al. Recruiting minority cancer patients into cancer clinical trials: a pilot project involving the Eastern Cooperative Oncology Group and the National Medical Association. J Clin Oncol. 1999;17:1029. doi: 10.1200/JCO.1999.17.3.1029. [DOI] [PubMed] [Google Scholar]
- 13.Felder TM, Pena G, Chapital BF. Disparities in cancer clinical trials: an analysis of comprehensive cancer control plans. Prev Chronic Dis. 2011;6:A1116. [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine. National Academy Press; 1999. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medicall underserved. [PubMed] [Google Scholar]
- 15.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. J Clin Oncol. 2004;22:730–734. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 16.Corbie-Smith G. The continuing legacy of the Tuskegee Syphilis Study: considerations for clinical investigation. Am J Med Sci. 1999;317:5–8. doi: 10.1097/00000441-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Mouton CP, Harris S, Rovi S, et al. Barriers to black women's participation in cancer clinical trials. J Natl Med Assoc. 1997;89:721–727. [PMC free article] [PubMed] [Google Scholar]
- 18.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 19.Comis RL, Miller JD, Colaizzi DD, et al. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Oncol Practice. 2009;5:50–56. doi: 10.1200/JOP.0922001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra A, Paul DP., III African American participation in clinical trials: recruitment difficulties and potential remedies. Hosp Top. 2003;81:33–38. doi: 10.1080/00185860309598020. [DOI] [PubMed] [Google Scholar]
- 21.Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. JAMA. 2011;306:1798–1799. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst. 2011;103:384–397. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 24.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 25.Branson RD, Davis J, Butler KL. African Americans' participation in clinical research: importance, barriers, and solutions. Am J Surg. 2007;193:32–39. doi: 10.1016/j.amjsurg.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Moore M, Desai J, Croxford M, et al. Patient comorbidities and behaviour once diagnosed are major contributors to disparities in cancer health outcomes. J Clin Oncol. 2010;28:e36–e37. doi: 10.1200/JCO.2009.25.2007. [DOI] [PubMed] [Google Scholar]
- 27.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 28.Morris AM, Rhoads KF, Stain SC, et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 29.Scharff D, Mathews K, Jackson P, et al. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freimuth VS, Quinn SC, Thomas SB, et al. African Americans' views on research and the Tuskegee Syphilis Study. Soc Sci Med. 2001;52:797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 31.Taylor KL, Davis JL, III, Turner RO, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006;15:2179–2188. doi: 10.1158/1055-9965.EPI-05-0417. [DOI] [PubMed] [Google Scholar]
- 32.Corbie-Smith G, Thomas SB, St GD. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 33.Comis RL, Miller JD, Aldige CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 34.Kagawa-Singer M, Valdez Dadia A, Yu MC, et al. Cancer, culture, and health disparities: time to chart a new course? CA Cancer J Clin. 2010;60:12–39. doi: 10.3322/caac.20051. [DOI] [PubMed] [Google Scholar]
- 35.McComas KA, Yang Z, Gay GK, et al. Individuals' willingness to talk to their doctors about clinical trial enrollment. J Health Communication. 2010;15:189–204. doi: 10.1080/10810730903528058. [DOI] [PubMed] [Google Scholar]
- 36.Kreuter MW, Lukwago SN, Bucholtz DC, et al. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 2003;30:133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 37.Nunnally JC. New York: McGraw Hill; 1967. Psychometric Theory. [Google Scholar]
- 38.Ajzen I. Action-control: From cognition to behavior. In: Beckman J, Kuhl J, editors. From intentions to actions: A theory of planned behavior. Heidelberg: Springer; 1985. pp. 11–39. [Google Scholar]
- 39.Du W, Mood D, Gadgeel S, et al. An educational video to increase clinical trials enrollment among lung cancer patients. J Thorac Oncol. 2008;3:23–29. doi: 10.1097/JTO.0b013e31815e8bb2. [DOI] [PubMed] [Google Scholar]
- 40.Hutchison C, Cowan C, McMahon T, et al. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. Br J Cancer. 2007;97:705–711. doi: 10.1038/sj.bjc.6603943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zapka JG, Lemon SC, Puleo E, et al. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141:683–692. doi: 10.7326/0003-4819-141-9-200411020-00009. [DOI] [PubMed] [Google Scholar]
- 42.Gagliano ME. A literature review on the efficacy of video in patient education. J Med Educ. 1988;63:785–792. doi: 10.1097/00001888-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Williams RM, Zincke NL, Turner RO, et al. Prostate cancer screening and shared decision-making preferences among African-American members of the Prince Hall Masons. Psychooncology. 2008;17:1006–1013. doi: 10.1002/pon.1318. [DOI] [PubMed] [Google Scholar]
- 44.Hinyard LJ, Kreuter MW. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ Behav. 2007;34:777–792. doi: 10.1177/1090198106291963. [DOI] [PubMed] [Google Scholar]
- 45.Kreuter MW, Holmes K, Alcaraz K, et al. Comparing narrative and informational videos to increase mammography in low-income African American women. Patient Educ Couns. 2010;81(Suppl):S6–S14. doi: 10.1016/j.pec.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai GY, Gary TL, Tilburt J, et al. Effectiveness of strategies to recruit underrepresented populations into cancer clinical trials. Clin Trials. 2006;3:133–141. doi: 10.1191/1740774506cn143oa. [DOI] [PubMed] [Google Scholar]
- 47.Comis RL, Aldige CR, Stoval E. A quantitative survey of public attitudes towards cancer clinical trials. Proc Am Soc Clin Oncol. 2000;19:451a. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 48.Lara PN, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23:9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]