The duration of hospitalization, biochemical patterns, medication usage, morbidity, and procedure-related mortality of patients who underwent hepatic artery embolization for metastatic neuroendocrine tumors are examined.
Keywords: Liver metastasis, Neuroendocrine, Embolization, Practice improvement
Learning Objectives:
After completing this course, the reader will be able to:
Identify the components of the “postembolization syndrome”: elevated liver function tests, right upper quadrant pain, nausea and vomiting, and fever.
Distinguish the postembolization syndrome from rare complications of embolization that would merit an extended hospitalization.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
There is scant evidence to guide the management of patients after hepatic artery embolization (HAE). We examined length of stay (LOS), laboratory patterns, medication usage, morbidity, and mortality of patients hospitalized after HAE for metastatic neuroendocrine tumors.
Methods.
Data were abstracted retrospectively from electronic medical records on LOS, liver function tests (LFTs), i.v. antibiotics, analgesia, peak temperature, bacteremia, hepatic abscess formation, carcinoid crisis, and metastatic burden on cross-sectional imaging.
Results.
In 2005–2009, 72 patients underwent 174 HAEs for carcinoid and islet cell tumors. The median LOS was 4 days (range, 1–8 days). There was no correlation between peak LFTs and tumor burden. Declines in LFTs were not uniform before hospital discharge; 25%, 37%, 30%, 53%, and 67% of patients were discharged before their respective aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total and direct bilirubin levels began to decline, with no readmissions for acute hepatic failure. The median i.v. analgesia dose was 60 mg oral morphine equivalents (range, 3–1,961 mg). Pre-HAE i.v. antibiotics were administered in 99% of cases; post-HAE fever occurred in 37% of patients, with no documented bacteremia. One patient developed a hepatic abscess after HAE. There were two carcinoid crises. The single in-hospital death was associated with air in the portal veins.
Conclusions.
The duration and intensity of in-hospital care following HAE should be managed on an individual basis. A downward trend in LFTs is not required before discharge. Modest use of i.v. analgesia suggests that many patients could exclusively receive oral analgesics. Given the rarity of serious complications, hospital stays could be shortened, thereby reducing costs and nosocomial risks.
Background
Neuroendocrine malignancies are a heterogeneous group of neoplasms classified by their grade (variably determined by assessing the mitotic rate, Ki-67 proliferative index, and presence or absence of necrosis) as well as by their anatomic location [1–3]. The nomenclature by which well-differentiated neuroendocrine tumors (NETs) of the luminal digestive tract are designated “carcinoids” has increasingly been criticized in favor of more precise, histology-based terminology. Nonetheless, the degree of symptomatology from these tumors depends not only on their location within the gastrointestinal tract but also on the extent of disease, and the term “carcinoid syndrome)” is still used to describe a complex of signs and symptoms—diarrhea, flushing, bronchospasm, and right-sided heart failure through fibrosis of the tricuspid and pulmonic valves—that strongly suggests metastasis to the hepatic parenchyma, because the liver normally inactivates the secretory products released by tumors into the enterohepatic circulation [4]. In addition to bothersome symptoms that can worsen patients' quality of life, carcinoid syndrome is associated with a poorer prognosis [5], and hepatic involvement by carcinoid is the most common disease-related cause of death [6]. Surgical resection of metastatic liver lesions is frequently complicated or even precluded by multifocal or bilobar disease [7].
Somatostatin analogs like octreotide are often beneficial for symptomatic patients and can also exert an antiproliferative effect on the underlying tumor [8], but the disease tends to become refractory to this hormonal blockade. Accordingly, patients with hepatic neuroendocrine metastases are commonly offered liver-directed therapy to control tumor progression and palliate carcinoid syndrome [9]. Because liver metastases derive most of their blood supply from the hepatic artery, local devascularization offers a targeted approach that takes advantage of neoplastic hypervascularity, especially because healthy hepatocytes derive most of their blood supply from the portal vein [10]. Hepatic arterial embolization (HAE) is currently used in patients with symptoms that are difficult to control with somatostatin analog therapy, radiographic evidence of progressive disease, and/or a large tumor burden such that any further progression may compromise hepatic function. The NETs of the luminal digestive tract are often considered together with pancreatic endocrine neoplasms as gastroenteropancreatic NETs and, indeed, similar principles and management techniques are applied to patients with metastatic pancreatic islet cell tumors, which are also recognized for their vascularity and vulnerability to embolization.
To the best of our knowledge, although randomized clinical trials have been performed to prove the utility of HAE for hepatocellular carcinoma [11] and metastatic colorectal cancer [12, 13], there is no comparable evidence to guide its use in liver-directed treatment of NETs. Rather, the experience from embolizing other tumor types has been extrapolated to neuroendocrine histologies, wherein bland embolization can be employed as a nonoperative management strategy alongside consideration of hepatic artery chemoembolization, radioembolization, hepatic artery infusion (HAI), and thermal ablative therapies (radiofrequency ablation, microwave ablation, and cryoablation) [14]. Despite the popularity of HAE as a cytoreductive alternative to surgery, the clinical course of NET patients immediately after bland embolization has not been meticulously described and their postprocedural management is not standardized among institutions. Following HAE, it has been common practice at the Mayo Clinic and some other institutions, including the Moffitt Cancer Center [7], for patients to remain hospitalized until their liver function tests (LFTs) have peaked and begun their downward trend. However, there is no published literature to validate this practice. Elevations in LFTs are expected and may not reflect perturbation of underlying hepatic function to a degree that would necessitate inpatient care. As such, a post-HAE patient may incur an unnecessarily lengthy hospital stay, with all the nosocomial risks and expenses thereof.
Consistent with the principles of quality improvement in patient care surrounding a therapeutic intervention, we were interested in analyzing both the process and the outcome of embolization procedures. The specific purpose of our study was to examine the duration of hospitalization, biochemical patterns, medication usage, morbidity, and procedure-related mortality of patients at our institution who underwent HAE for metastatic NETs. With this information, we intended to assess the safety of the procedure and improve peri-HAE management in a systematic manner that could be implemented at our institution and at other facilities offering this intervention.
Methods
We queried the admission records at the Rochester Methodist Hospital at Mayo Clinic in Rochester, Minnesota to capture hospitalizations on the medical oncology service for January 1, 2005 to December 31, 2009 with the Current Procedural Terminology (CPT) code 37204 (transcatheter occlusion or embolization). The search yielded 239 admissions linked to this procedure code, of which 174 were bland embolization of NETs. The electronic medical record associated with each hospitalization was then reviewed by physicians to extract the following information: patient age; patient gender; pathology of the target lesion(s); date of diagnosis with a NET; date of embolization; number of prior embolizations; type of embolization infusate; length of stay (days); preprocedure, peak, and time-of-discharge levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, and direct bilirubin; time (days) to peak value of AST, ALT, alkaline phosphatase, total bilirubin, and direct bilirubin; use and duration of i.v. analgesia; total i.v. analgesia dose in oral morphine equivalents (using an opioid conversion calculator to convert from i.v. analgesics to an equivalent dose in milligrams of sustained-release morphine sulfate tablets [15]); use and duration of i.v. antiemetics; volume of parenteral hydration; use of preprocedural i.v. antibiotics; maximum temperature during hospitalization and time to peak temperature (days); ordering of blood cultures and culture positivity when temperature measured ≥38.3°C; use of pre- and postprocedural octreotide; incidence of carcinoid crisis, defined as postprocedural hypotension (systolic blood pressure ≤90 mmHg) requiring medical intervention; percent change in glomerular function rate postprocedure; rate and reason of readmission within 30 days of embolization; and mortality at 30 days. QREADS clinical imaging software (v5.1.10, Mayo Clinic) was used to view the radiographic study (either computed tomography or magnetic resonance imaging) that most closely preceded each embolization, and, to estimate tumor burden, two-dimensional measurements were made post hoc of the largest lesion targeted by embolization. Postembolization radiographic studies were also reviewed and used to confirm any clinical presentations consistent with hepatic abscess formation.
Results
In 2005–2009, 72 unique patients (57% male) aged 30–80 years (median, 60 years) underwent 174 bland HAEs for carcinoid (n = 112) and islet cell (n = 62) tumors. There were no formal selection criteria, but patients were chosen for embolization on an individual basis through multidisciplinary discussion between providers in medical oncology and interventional radiology. The median time from NET diagnosis to first embolization was 1.6 years (range, 1 week to 12.4 years). The median number of hospitalizations for HAE in each unique patient was two (range, 1–8). Contour™ (250- to 355-μm particles of polyvinyl alcohol; Boston Scientific, Marlborough, MA) was the infusate in 149 (86%) procedures, with the remaining 25 (14%) embolizations accomplished with Bead Block (300- to 500-μm particles of polyvinyl alcohol; Biocompatibles, Farnham, U.K.). Patient demographics are summarized in Table 1.
Table 1.
Patient demographics and tumor characteristics
The median hospital stay was 4 days (range, 1–8 days). The time to peak LFTs varied with each parameter of hepatic structure and function (Fig. 1). AST and ALT both had a median peak at 3 days (range, 1–5 days) whereas alkaline phosphatase had a median peak at 2 days (range, 1–11 days). Total bilirubin had a median peak at 3 days (range, 1–7 days) as did direct bilirubin (range, 1–8 days).
Figure 1.
Percentage of patients reaching peak liver function tests by days after embolization.
However, the documented decline in LFTs was not uniform before hospital discharge with cessation of routine LFT monitoring. Twenty-five percent, 37%, 30%, 53%, and 67% of patients were discharged before their respective AST, ALT, alkaline phosphatase, total bilirubin, and direct bilirubin levels had begun to decline, with no readmissions for acute hepatic failure. Among patients undergoing serial embolizations over more than one hospitalization, the height of the AST peak declined with successive procedures (r = 0.93), but no such correlation was found with other LFTs. There was no correlation between the height of peak LFTs and the size of the metastatic burden as estimated on pre-HAE imaging.
One hundred percent of patients received patient-controlled i.v. analgesia, and the median total dose was 60 mg oral morphine equivalents (range, 3–1,961 mg). The median duration of i.v. analgesia was 2 days (range, 1–6 days). The median duration of i.v. antiemetics was 1 day (range, 0–5 days). The median i.v. hydration for the entire hospital stay was 3.6 L of normal saline or lactated Ringer's (range, 0.5–16.5 L).
The glomerular filtration rate (GFR) decreased between admission and discharge in 51 (29%) of 174 cases. Among patients whose renal function worsened by dismissal, the median decrease in GFR was 15% (range, 7%–37%). In two cases, the GFR declined to <30 mL/minute per 1.73 m2 during hospitalization, and in one of the two cases, the GFR remained below this threshold at the time of dismissal, with no documentation of later recovery in renal function, although the patient did not become dialysis dependent. In 66 (38%) of 174 cases, renal function was completely stable through hospitalization, and in 57 (33%) of 174 cases, the GFR improved between admission and dismissal. The volume of contrast dye administered with each HAE was not recorded, and necessarily varied between procedures, but the average dye load was estimated at 100 mL. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) as postprocedural analgesia was avoided.
Pre-HAE i.v. antibiotics were delivered in 99% of cases, and post-HAE fever (temperature ≥38.3°C during hospitalization) occurred in 37% of cases; among febrile patients, there was 71% compliance in obtaining blood cultures and no documented bacteremia. In one case, a hepatic abscess formed 1 month after HAE, 3 weeks after an afebrile postprocedural hospital course; the patient had not previously had pancreaticobiliary surgery or stenting.
Octreotide was used preprocedurally in 40 of the 112 carcinoid cases and in 28 of the 62 islet cell cases. There were two carcinoid crises, both occurring within 48 hours of HAE. In one of the two carcinoid crises, octreotide had not been given preprocedurally.
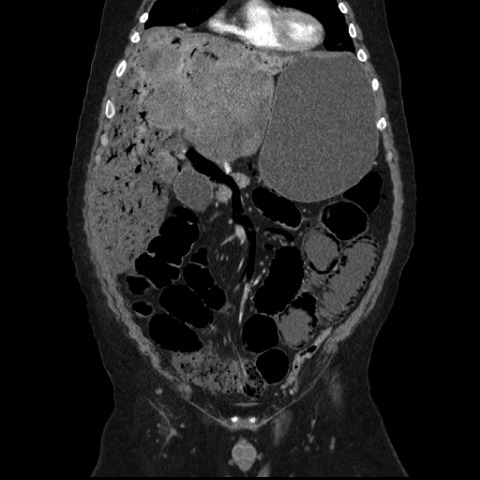
With regard to in-hospital complications, there were nine intensive care unit transfers (one for myocardial infarction, one for stress-induced transient apical ballooning syndrome [Takotsubo cardiomyopathy [16]], and seven for hypertensive crises requiring i.v. vasodilators) and three groin hematomas. There was one in-hospital death associated with air in the portal venous system, a very rare complication after HAE [17] (Fig. 2). One of 174 hospitalizations was associated with death within 1 month postprocedurally, for a 30-day mortality rate of 0.5% per HAE-related hospital stay and one death in 72 patients (1.4%).
Figure 2.
Coronal computed tomography scan of the abdomen/pelvis showing air in the portal venous system and pneumatosis intestinalis following hepatic artery embolization.
Of 174 cases, there were 14 (8%) readmissions within 30 days (for reasons other than the next sequential HAE): four for uncontrolled nausea or vomiting, three for fever of unknown origin, two for intractable diarrhea, two for acute renal failure (neither consistent with hepatorenal syndrome), two for pain management, and one for hypercalcemia. Eleven of these admissions were clearly prompted by postembolization sequelae. Complications are summarized in Table 2.
Table 2.
Complications of hepatic artery embolization
Discussion
Although surgical resection remains the standard of care for neuroendocrine metastases to the liver when either cure or >90% cytoreduction can be achieved, HAE is an important treatment option for patients with inoperable disease [10]. Inducing tumor ischemia through selective arterial occlusion is an attractive strategy with which to diminish metastases and improve quality of life for patients who are not surgical candidates. However, the procedure does carry risks, and postembolization syndrome in particular requires thoughtful management. Although precise definitions of the syndrome vary in the literature [18–22], the well-recognized constellation of expected symptoms and signs after HAE include elevated LFTs, right upper quadrant pain, nausea and vomiting, and fever.
Transaminases may not normalize for ≥2 weeks after embolization [7, 18], so it is not reasonable to hospitalize patients until LFTs entirely return to baseline. The time to peak values in liver enzymes has been reported as 48–72 hours after embolization in other studies [5, 7], which is consistent with our own median findings. Hepatic failure is among the most feared postembolization complications, but the risk for procedure-induced fulminant hepatic necrosis can be attenuated by careful patient selection (i.e., those in whom <75% of the normal liver is replaced by tumor [18]) and by the interventional radiologists' embolization of smaller vascular territories; rates of hepatic failure have diminished over time with a general shift in practice away from common hepatic artery occlusion or simultaneous bilobar treatment toward sequential, lateralized embolization of the left or right hepatic artery [7, 23]. In practice, the interventional radiologist often approaches the liver on a segmental basis, so the vascular tree is occluded beyond the bifurcation of the hepatic artery. We did not record any occurrences of fulminant hepatic failure, and the preponderance of small-particle infusates in our series indicates that vascular targets were usually more distal [19].
Abdominal pain localized to the right upper quadrant after embolization has been putatively attributed to inflammatory stretching of the fibrous capsule that envelops the liver, and i.v. opioids are commonly used as initial analgesia. In our series, if LFT trends had not been used as a discharge criterion, then pain management would have become the rate-limiting step to dismissal. The median duration of i.v. analgesia in our patient group was 2 days. The median number of oral morphine equivalents used during an entire admission corresponded to 60 mg of morphine sulfate sustained-release tablets taken in aggregate for a patient's hospital stay. This dosage suggests that analgesia could be provided effectively in readily available strengths of oral medications, as soon as the patient can tolerate swallowing pills.
Nausea and vomiting after embolization can arise from the emetogenic potential of pain or from adynamic ileus, which may be compounded by concurrent opioid use. On average, ileus, if it occurs at all, lasts up to 36 hours after the procedure [18], and it is obviously appropriate to support patients with i.v. fluids when they cannot maintain adequate hydration by mouth. As a surrogate marker for tolerance of oral intake, the median duration of i.v. antiemetic use in our study was 1 day, so, among the limiting symptoms, nausea and vomiting seem to pose less of a delay to discharge from the hospital than pain. As a result, it is difficult to conclude that inadequate control of nausea and vomiting contributed to the observed 29% rate of decline in the GFR during post-HAE hospitalization. Again, the majority of patients demonstrated either stable or improved kidney function during their hospital stay. However, there may be a subset of patients who would benefit from greater i.v. hydration, identified either by clinical signs of hypovolemia, by a history of profuse diarrhea related to carcinoid syndrome, or, perhaps, by the interventional radiologist in procedures in which a greater-than-average amount of contrast dye, that is, >100 mL, is used. Clearly, known nephrotoxic agents should be avoided if at all possible in the postembolization period, and we suggest against the use of NSAIDs as analgesia for this reason.
Fever in the absence of documented bacteremia is likely a result of tumor necrosis and an inflammatory response from the surrounding hepatic parenchyma [18]. Uniform administration of antibiotics prior to the procedure has markedly reduced the risk for septic complications [18]. Our study cannot demonstrate that preprocedural antibiotics are unnecessary because, despite the consistent lack of positive blood cultures, the specimens from febrile patients could have been sterilized precisely because of antibiotic exposure, perhaps demonstrating the efficacy of antimicrobial prophylaxis in preventing bloodstream infections. Embolization can induce massive cystic degeneration in the liver, but this tissue breakdown rarely results in abscess formation [24], as seen in our study after very high compliance with a preprocedural antibiotic protocol. The one documented case of postprocedural abscess formation in our cohort occurred weeks after a hospitalization of 6 days' duration without any recorded fever; the culprit organism was a strain of Klebsiella pneumoniae whose susceptibility studies later demonstrated sensitivity to the same levofloxacin given to the patient prophylactically, so it is not clear that any further measures could have been taken to prevent this abscess.
The occurrence of carcinoid crisis in a patient who did not receive octreotide before embolization highlights the procedure's potential to liberate vasoactive neuroendocrine substances contained within the target tumor and shows the importance of administering pre-embolization somatostatin analogs to carcinoid patients. The fact that a carcinoid crisis occurred in one other patient despite preemptive use of octreotide illustrates the need for vigilance and the availability of somatostatin analogs on an as-needed basis for up to 48 hours after the procedure. Furthermore, it is possible that our definition of carcinoid crisis as hypotension was too narrow during the initial review of periembolization hemodynamic parameters, in that some of the observed postprocedural hypertensive episodes may have signified catecholamine release from the target lesions and should also have been considered carcinoid crises [7]. Takotsubo cardiomyopathy, observed in one case, has been linked pathophysiologically to catecholamine excess [16], and it may have a similar etiologic explanation stemming from neurohormonal release by a carcinoid tumor that is necrosing after embolization. However, it is also not possible within our dataset to distinguish hyperadrenergic responses to the HAE itself, particularly in the context of postprocedural pain.
Other limitations of our study include its retrospective collection of clinicopathologic data as well as the use of a specific CPT code to establish the cohort, which would necessarily have failed to identify patients whose procedures were improperly coded or whose hospitalizations were not electronically linked to this intervention.
On the other hand, the same electronic medical records provided single-source information on vital signs, medication usage, laboratory results, and radiographic studies to reassemble the hospital stay of each patient in tremendous detail. Moreover, we deliberately confined our study to patients with neuroendocrine pathologies to strengthen our conclusions about postprocedural outcomes for their particular tumor types. As a result, we can report one of the largest cohorts of nonchemotherapeutic HAE with homogeneous histology among the target lesions.
The low 30-day mortality rate among our patients is comparable with, or slightly better than, the rates reported in other postembolization outcome studies [5, 25]. It is also comparable with the 1% mortality rate seen in an analysis of HAI for the treatment of colorectal liver metastases in a study with a much larger sample size of 4,580 cases [26]. The fatal complication of portal venous gas has been previously observed after HAE [17], and its physiologic cause remains speculative at best, possibly resulting from the diffusion of gas from surrounding necrotic embolized tissue. In this case, the patient's postembolization course was marked by persistent nausea and abdominal pain that were refractory to standard interventions, and he would not have been dismissed from the hospital even if less stringent discharge criteria had been in place at that time.
Conclusions
The duration and intensity of in-hospital supportive care following HAE should be assessed on a patient-by-patient basis. A downward trend in LFTs is not validated as a requirement for discharge. The modest median use of i.v. analgesia suggests that many patients could be converted to exclusively oral analgesia as soon as they can tolerate oral intake, although individual needs for pain management will vary. Reducing the intensity and duration of opioid use may also improve bowel motility and hasten the resumption of a normal diet. Intravenous fluids are necessary until consistent oral hydration becomes possible but do not eliminate the possibility of postprocedural declines in renal function. Prophylactic administration of somatostatin analogs is appropriate in carcinoid patients. Given the scarcity of serious complications and a low 30-day mortality rate, many hospital stays could be shortened in favor of attentive outpatient care, improving clinical practice by reducing costs and nosocomial risks. In conclusion, we propose the algorithm shown in Figure 3 for postembolization management.
Figure 3.
Suggested algorithm for postembolization management.
Abbreviations: p.o., orally; prn, as needed.
Editor's Note: See pages 732–739 of this issue for the companion article, “Quality of Life and Its Associated Factors in Patients with Hepatocellular Carcinoma Receiving One Course of Transarterial Chemoembolization Treatment: A Longitudinal Study,” by Shiow-Ching Shun, Chien-Hung Chen, Jin-Chuan Sheu et al.
Acknowledgment
The abstract for this manuscript was included among the 2011 American Society of Clinical Oncology Annual Meeting abstracts.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Mark A. Lewis, Sylvia Jaramillo, Axel Grothey
Collection and/or assembly of data: Mark A. Lewis, Sylvia Jaramillo
Data analysis and interpretation: Mark A. Lewis, Sylvia Jaramillo, Axel Grothey
Manuscript writing: Mark A. Lewis, Sylvia Jaramillo, Lewis Roberts, Chad J. Fleming, Joseph Rubin, Axel Grothey
Final approval of manuscript: Mark A. Lewis, Sylvia Jaramillo, Lewis Roberts, Chad J. Fleming, Joseph Rubin, Axel Grothey
References
- 1.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 2.Rindi G, Klôppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2007;451:757–762. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 3.Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502–1522. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 5.Ho AS, Picus J, Darcy MD, et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188:1201–1207. doi: 10.2214/AJR.06.0933. [DOI] [PubMed] [Google Scholar]
- 6.Norton JA, Kerlan RK. Hepatic artery embolization for treatment of patients with metastatic carcinoid tumors: A commentary. Cancer J. 2003;9:241–243. doi: 10.1097/00130404-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg JR, Choi J, Cantor AB, et al. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 8.Delaunoit T, Rubin J, Neczyporenko F, et al. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumors. Mayo Clin Proc. 2005;80:502–506. doi: 10.4065/80.4.502. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83:2293–2301. [PubMed] [Google Scholar]
- 10.Atwell TD, Charboneau JW, Que FG, et al. Treatment of neuroendocrine cancer metastatic to the liver: The role of ablative techniques. Cardiovasc Intervent Radiol. 2005;28:409–421. doi: 10.1007/s00270-004-4082-6. [DOI] [PubMed] [Google Scholar]
- 11.Reidy DL, Schwartz JD. Therapy for unresectable hepatocellular carcinoma: Review of the randomized clinical trials-I: Hepatic arterial embolization and embolization-based therapies in unresectable hepatocellular carcinoma. Anticancer Drugs. 2004;15:427–437. doi: 10.1097/01.cad.0000127330.21686.26. [DOI] [PubMed] [Google Scholar]
- 12.Salman HS, Cynamon J, Jagust M, et al. Randomized phase II trial of embolization therapy versus chemoembolization therapy in previously treated patients with colorectal carcinoma metastatic to the liver. Clin Colorectal Cancer. 2002;2:173–179. doi: 10.3816/CCC.2002.n.022. [DOI] [PubMed] [Google Scholar]
- 13.Hunt TM, Flowerdew AD, Birch SJ, et al. Prospective randomized controlled trial of hepatic arterial embolization or infusion chemotherapy with 5-fluorouracil and degradable starch microspheres for colorectal liver metastases. Br J Surg. 1990;77:779–782. doi: 10.1002/bjs.1800770720. [DOI] [PubMed] [Google Scholar]
- 14.Lewis MA, Hubbard J. Multimodal liver-directed management of neuroendocrine hepatic metastases. Int J Hepatol. 2011:452343. doi: 10.4061/2011/452343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Management of cancer pain guideline overview. Agency for Health Care Policy and Research Rockville, Maryland. J Nat Med Assoc. 1994;86:571–573. 634. [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst RT, Prasad A, Askew JW, 3rd, et al. Takotsubo cardiomyopathy: A unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641–649. doi: 10.1016/j.jcmg.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy P, Adam A, Jackson J, et al. Computed tomography demonstration of portal venous gas after hepatic artery embolization. Br J Radiol. 1990;63:647–648. doi: 10.1259/0007-1285-63-752-647. [DOI] [PubMed] [Google Scholar]
- 18.Berger DH, Carrasco CH, Hohn DC, et al. Hepatic artery chemoembolization or embolization for primary and metastatic liver tumors: Post-treatment management and complications. J Surg Oncol. 1995;60:116–121. doi: 10.1002/jso.2930600210. [DOI] [PubMed] [Google Scholar]
- 19.Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189:541–547. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff T, Chavan A, Galanski M. Chemoembo-lization of hepatic metastases from intestinal neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2000;12:141–143. doi: 10.1097/00042737-200012020-00001. [DOI] [PubMed] [Google Scholar]
- 21.Meij V, Zuetenhorst JM, van Hillegersberg R, et al. Local treatment in unresectable hepatic metastases of carcinoid tumors: Experiences with hepatic artery embolization and radiofrequency ablation. World J Surg Oncol. 2005;3:75. doi: 10.1186/1477-7819-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slomka M, Radwan P. The evaluation of clinical results of hepatic artery embolization. Mater Med Pol. 1992;24:193–195. [PubMed] [Google Scholar]
- 23.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of pa-tients with metastatic carcinoid tumors: The M.D. Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Drougas JG, Anthony LB, Blair TK, et al. Hepatic artery chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg. 1998;175:408–412. doi: 10.1016/s0002-9610(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 25.Thornton RH, Covey A, Petre EN, et al. A comparison of outcomes from treating hepatocellular carcinoma by hepatic artery embolization in patients younger or older than 70 years. Cancer. 2009;115:5000–5006. doi: 10.1002/cncr.24556. [DOI] [PubMed] [Google Scholar]
- 26.Barnett KT, Malafa MP. Complications of hepatic artery infusion: a review of 4580 reported cases. Int J Gastrointest Cancer. 2001;30:147–160. doi: 10.1385/IJGC:30:3:147. [DOI] [PubMed] [Google Scholar]