Changes in physical and psychological distress and quality of life were explored in patients with hepatocellular carcinoma receiving one course of transarterial chemoembolization treatment, and significant pre- and postdischarge factors related to changes in physical and mental domains of quality of life over a period of 2 months were identified.
Keywords: Carcinoma, Chemoembolization, Fatigue, Hepatocellular, Quality of Life
Learning Objectives:
After completing this course, the reader will be able to:
List the top 10 ranked symptoms after discharge suffered by patients with hepatocellular carcinoma (HCC) receiving transarterial chemoembolization (TACE) treatment.
Identify the significant factors in the associations between quality of life (QOL) and demographic factors and clinical factors over a period of 2 months in patients with HCC receiving TACE.
Design individualized education programs for newly diagnosed and recurrent HCC patients in order to maintain better QOL after treatment.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Objective.
To (a) explore changes in physical and psychological distress and quality of life (QOL) and (b) identify the significant pre- and postdischarge factors related to changes in physical and mental domains of QOL over a period of 2 months in patients with hepatocellular carcinoma receiving one course of transarterial chemoembolization (TACE) treatment.
Methods.
A longitudinal prospective design was used, with participants recruited from a teaching hospital in Northern Taiwan. Data were collected three times: within 3 days prior to discharge (T0) and at the fourth (T1) and eighth (T2) weeks after discharge. A set of structured questionnaires was used to assess participants' QOL, symptom distress, anxiety, and depression. Changes in QOL and associated factors were examined using generalized estimating equations.
Results.
Eighty-nine patients were included in this study. Fatigue was reported to be the most distressful symptom after treatment. Overall QOL improved monthly after discharge. Change in physical QOL 2 months after TACE treatment was associated with age, diagnosis status, level of symptom distress, and depression after discharge. Change in mental QOL was significantly associated with gender, diagnosis status, and anxiety and depression after discharge.
Conclusions.
Health care providers should pay special attention to patients of older age, those who are male, and those who have higher levels of depression and anxiety after discharge. Designing personalized education programs before discharge for patients with newly diagnosed cancer versus those who have recurrent disease is suggested to help patients maintain a better QOL after discharge.
Introduction
Liver cancer, the third most common cause of cancer-related death, is an increasingly common health care problem in the world [1]. In Taiwan, it has been identified as a critical health care problem because of its rating as the second leading cause of death in 2009, with 7,759 patients dying from liver cancer [2]. Hepatocellular carcinoma (HCC) is the most common liver cancer; however, only 10%–20% of cases are detected early. Around 40% of patients undergo transarterial chemoembolization (TACE), percutaneous ethanol injection (PEI), or radiofrequency ablation (RFA) [3, 4].
Compared with RFA and PEI, which are sometimes administered with curative treatments, TACE is recognized as a palliative treatment for unresectable HCC patients with large or multifocal tumors. Evidence has shown that TACE can improve the 3-year survival rate from 10% to 40%–50%, with a median survival duration of 16–20 months [5]. The TACE procedure includes the injection of an anticancer drug (e.g., doxorubicin) emulsified with lipiodol into the hepatic artery, followed by arterial embolization with gelatin sponge particles or powder in order to block the blood supply of tumor cells [6, 7]. Because HCC is hypervascular, multifocal, and highly recurrent, TACE is typically repeated at fixed intervals for a planned number of courses or until death occurs [7]. In general, patients receiving TACE have an average of 3–4 days' hospitalization and then are followed up at an outpatient clinic 2 weeks after discharge. In Taiwan, the treatment effect of TACE is generally evaluated 2 months after each course in order to decide whether or not patients should receive another course.
Although evidence has shown that TACE can improve patients' 3-year survival rate [5], postembolization syndrome (e.g., transient fever, abdominal pain, nausea, and elevated alanine aminotransferase) and side effects related to chemotherapy (e.g., fatigue, nausea, vomiting) are the most often reported complications [5, 8–11]. These symptoms could affect patients' psychological health and overall quality of life (QOL) after discharge [11–13] and might further decrease patients' treatment compliance, leading them to potentially refuse to receive another course of treatment.
Despite these significant problems, health care providers mainly focus on controlling physical distress rather than patient QOL following discharge in clinical settings. Patient QOL has been identified as a significant prognostic factor in predicting survival outcome in HCC patients [14]. It is a complicated and dynamic multidimensional construct. Factors related to QOL may change over time and vary based on symptom distress, disease-related factors, and psychological distress (e.g., anxiety and depression) [15–22]. Therefore, the factors prior to and after discharge need to be examined simultaneously with patient QOL to help us better understand changes in QOL and design individualized education programs for patients with HCC after receiving treatment.
To the best of our knowledge, only one previous study has reported fatigue patterns within 1 week following TACE [11], and only one study has examined changes in QOL 4 months after receiving TACE [13], but there is no study conducted over a period of 2 months after TACE in which patients return to the clinic to evaluate medical treatment outcomes. Worsened QOL after a course of TACE might affect the willingness of patients to receive another course. Thus, it is important to identify factors related to QOL in order to identify potential risk factors after discharge. Health care providers could offer more educational interventions for high-risk populations in order to improve QOL.
Therefore, the aims of the present study were to: (a) explore changes in physical and psychological distress and QOL over a period of 2 months after receiving one course of TACE and (b) identify significant pre- and postdischarge factors related to changes in the physical and mental domains of QOL over a period of 2 months in patients with HCC receiving TACE.
Methods
Study Design
A longitudinal correlational design with purposive sampling was used in this study. Data were collected at three time periods, including three days before discharge (T0), the fourth week after discharge (T1), and the eighth week after discharge (T2).
Participants
Participants were recruited from the medical ward of a leading medical center in northern Taiwan. Individuals eligible for this study (a) had been diagnosed with HCC according to the American Association for the Study of Liver Disease criteria [5] and were informed of the diagnosis, (b) were receiving TACE, (c) were adult (≥20 years old) patients, (d) were able to communicate verbally, and (e) were willing to sign a consent form after receiving a detailed explanation of the study purpose and procedures. Those patients who had arranged to receive their next medical treatment within 2 months were excluded from this study.
Measures
The Chinese versions of the Symptom Distress Scale (SDS), the Hospital Anxiety and Depression Scale (HADS), the Short Form-12 Health Survey (SF-12), and a background information form were used to assess patients' symptom distress, anxiety, depression, QOL, demographic data, and clinical characteristics.
The Chinese version of the SDS, modified by Lai [23], is a 19-item scale derived from the original SDS [24]. It is a Likert-type scale ranging from 1 (no distress at all) to 5 (as much distress as possible). The higher the score, the greater the level of symptom distress. Symptom distress after completion of TACE was assessed using the SDS, with Cronbach's α in the range of 0.69–0.82 across the three data collection times in this study.
The HADS is a 14-item measure used to assess patient anxiety and depression [25]. Each item is scored at 0–3, with two subscales of seven items, so that each subscale's total score is in the range of 0–21. Higher scores represent higher levels of anxiety and depression. A total score >11 means that the patient is suffering from anxiety or depression. The HADS has been used with cancer patients with good reliability in Taiwan [26, 27]. Cronbach's α coefficients for the HADS-A (anxiety) and HADS-D (depression) in this study were in the range of 0.90–0.91 and 0.86–0.92 across the three data collection times, respectively.
The SF-12 is a 12-item generic measure of health status developed from the widely used SF-36 [28]. The second version of the SF-12 (SF-12 v2) can yield scores for eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. It also provides overall summaries of the physical and mental components. After reversal and recalibration, the scores can be transformed to a 0–100 scale and then to norm-based scores, with higher scores indicating better health. The Chinese version of SF-12 v2 was used successfully in a previous study [29]. Cronbach's α for the SF-12 was in the range of 0.82–0.86 across the three data collection times in this study.
A background information form was designed for this study covering demographic and clinical characteristics. Demographic characteristics included gender, age, employment status, marital status, education, and religious affiliation. Clinical characteristics included Child-Pugh class, Barcelona Clinic Liver Cancer (BCLC) stage, length of time since diagnosis (in months), hepatitis B virus (HBV) carrier status, anti–hepatitis C virus (HCV) status, time of medical treatment related to liver cancer, number of TACE treatments, dosage of doxorubicin, current diagnosis status, and functional status. Functional status was measured using the Karnofsky performance status (KPS) score, an 11-point scale that uses 10-point intervals ranging from 100 (normal function) to 0 (death) [30]. It has been used in cancer-related studies in Taiwan [31, 32].
Ethics Approval
The study protocol and questionnaires were reviewed and approved by the institutional review board of the National Taiwan University Hospital (200908011R). All participants were provided with information regarding the study purpose and signed consent forms before data were collected.
Data Collection
Data were collected from October 2009 to December 2010. In total, 104 patients met the inclusion criteria, but 15 patients refused to participate in this study at baseline because of communication problems (n = 4), physical distress (n = 7), family refusal (n = 1), or unknown reasons (n = 3). The dropout rate was 13.5% (n = 12) as a result of loss to follow-up (n = 6), unexpected readmission (n = 4), busy working (n = 1), and symptom distress (n = 1).
Statistical Analysis
Data were entered into and analyzed using SPSS, version 15.0 (SPSS Inc., Chicago IL). Descriptive statistics were used to analyze the demographic and clinical characteristics and changes in measured variables (i.e., symptom distress, anxiety, depression, and the subdomains of QOL). Correlations between QOL and other measured variables (e.g., demographics, clinical characteristics, symptom distress, and psychological distress) were examined and those that were associated with QOL (p < .05) were entered into the generalized estimating equation (GEE). Two GEEs for the two subdomains of QOL (physical and mental) were used to examine the factors that were significant to QOL. The GEE, an extension of the generalized linear model, was developed by Zeger and Liang [33] and has the statistical power to deal with both normal and non-normal distributions for repeated measures [34].
Results
Patient Characteristics
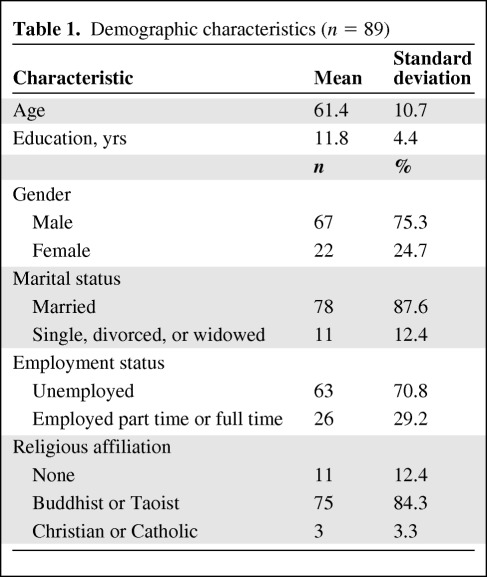
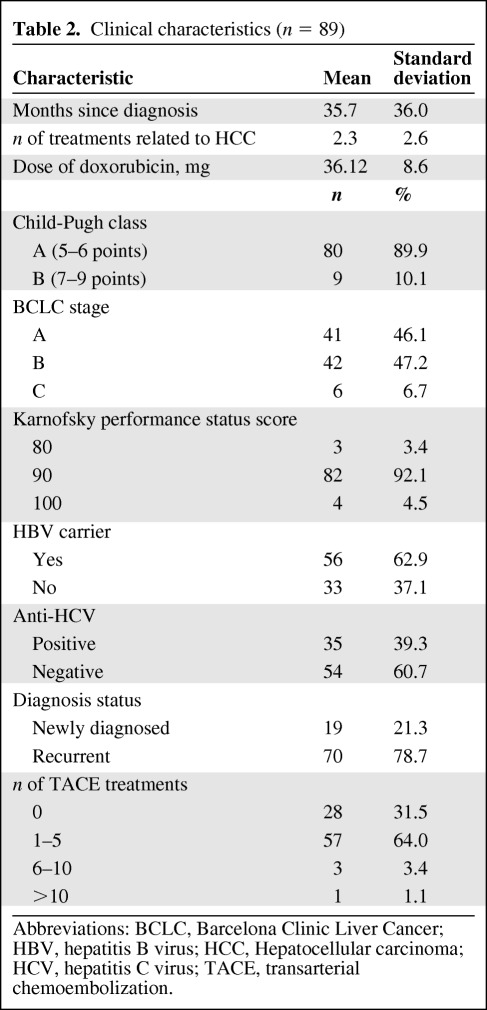
Demographic characteristics and disease-related information for the 89 participants are presented in Tables 1 and 2. In this study, 75.3% (n = 67) of the participants were male, with ages in the range of 31–80 years (mean, 61.4; standard deviation [SD], 10.7). The majority were unemployed (70.8%) and married (87.6%), and the most common religious affiliation was Buddhist or Taoist (84.3%, n = 75). The average education level was 11.8 years (SD, 4.4). Most participants were BCLC stage B (47.2%) with Child-Pugh class A (89.9%) liver disease. The average time since being diagnosed with HCC was 35.7 months (SD, 36.0), with a range of 1–149 months. Most of the participants (96.6%) had good functional status (KPS score ≧90). Doxorubicin was used in this study with a mean dosage of 36.12 mg (SD, 8.6). More than 60% of the participants were infected with HBV and 39.3% had HCV infection.
Table 1.
Demographic characteristics (n = 89)
Table 2.
Clinical characteristics (n = 89)
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCC, Hepatocellular carcinoma; HCV, hepatitis C virus; TACE, transarterial chemoembolization.
Change in Physical and Psychological Distress and QOL Across the Three Time Periods
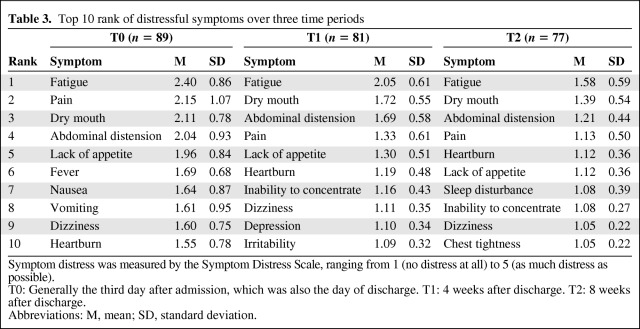
The overall levels of symptom distress across the three time periods were mild, with item mean scores in the range of 1.58 before discharge to 1.1 at 8 weeks after treatment. The top 10 most distressful symptoms were ranked for the three time periods and are shown in Table 3. Fatigue was the number one symptom distress from discharge to 8 weeks after treatment. Abdominal pain was second before discharge (T0) but was fourth after 4 (T1) and after 8 (T2) weeks of treatment. Dry mouth and abdominal distension were second and third at T1 and T2.
Table 3.
Top 10 rank of distressful symptoms over three time periods
Symptom distress was measured by the Symptom Distress Scale, ranging from 1 (no distress at all) to 5 (as much distress as possible).
T0: Generally the third day after admission, which was also the day of discharge. T1: 4 weeks after discharge. T2: 8 weeks after discharge.
Abbreviations: M, mean; SD, standard deviation.
Fever, nausea, and vomiting were among the top 10 ranked symptom suffered by participants at T0, but were not in the top 10 after discharge. In contrast, heartburn, sleep disturbance, and chest tightness became more distressful from T1 to T2.
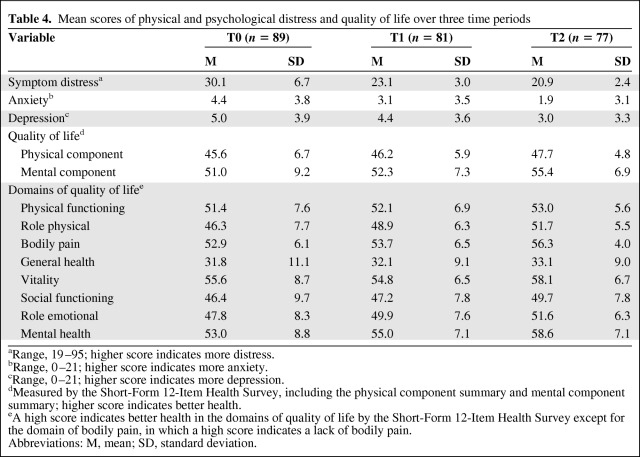
Changes in physical and psychological distress and QOL were measured three times and are shown in Table 4. Changes in the mean scores for physical and psychological distress all decreased across the three time periods and QOL improved over the 8 weeks after one course of TACE treatment. Scores for the physical components of QOL were lower than scores for the mental components of QOL across the three time periods. Further analyzing the eight domains of QOL, the mean scores all increased across the three time periods except for vitality, which slightly decreased at 1 month after discharge (T1). This indicates that most domains of QOL improved after discharge, but vitality did not improve until 2 months after discharge.
Table 4.
Mean scores of physical and psychological distress and quality of life over three time periods
aRange, 19–95; higher score indicates more distress.
bRange, 0–21; higher score indicates more anxiety.
cRange, 0–21; higher score indicates more depression.
dMeasured by the Short-Form 12-Item Health Survey, including the physical component summary and mental component summary; higher score indicates better health.
eA high score indicates better health in the domains of quality of life by the Short-Form 12-Item Health Survey except for the domain of bodily pain, in which a high score indicates a lack of bodily pain.
Abbreviations: M, mean; SD, standard deviation.
Significant Factors Related to Physical and Mental Components of QOL
The associations between QOL and demographic factors (age, gender, employment status, marital status, education, and religious affiliation) and clinical factors (functional status, Child-Pugh class, BCLC stage, length of time since diagnosis, HBV carrier status, anti-HCV status, status of diagnosis, and time of medical treatment related to liver cancer) were examined using correlation statistics (i.e., Spearman's correlation and the Mann-Whitney U-test). Significant factors identified were age, gender, years of education, functional status, status of diagnosis (newly diagnosed versus recurrent), BCLC stage (A, B, or C), and time of medical treatment related to HCC.
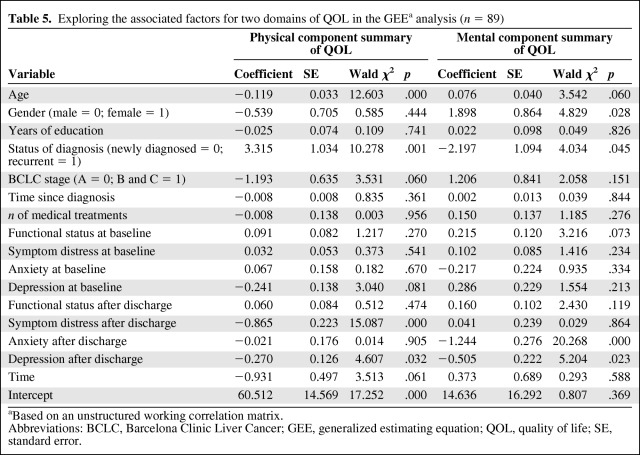
The associated demographic and clinical characteristics and physical and psychological factors were entered into the GEE models along with the two domains of QOL (physical and mental). The results are shown in Table 5. In order to identify whether or not functional status, symptom distress, anxiety, and depression prior discharge were associated with change in QOL after discharge, the levels at baseline and after discharge were entered into the GEE models.
Table 5.
Exploring the associated factors for two domains of QOL in the GEEa analysis (n = 89)
Based on an unstructured working correlation matrix.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; GEE, generalized estimating equation; QOL, quality of life; SE, standard error.
Age (β = −0.119; p < .0001), status of diagnosis (recurrent = 1, newly diagnosed = 0) (β = 3.315; p = .001), symptom distress after discharge (β = −0.865; p < .0001), and depression after discharge (β = −0.270; p = .032) were the significant factors associated with the physical component of QOL. This means that those who were older, were newly diagnosed as cancer patients, and had higher levels of symptom distress and depression after discharge had worse physical QOL. Gender (female = 1, male = 0) (β = 1.898; p = .028), status of diagnosis (recurrent = 1, newly diagnosed = 0) (β = −2.197; p = .045), anxiety after discharge (β = −1.244; p < .0001), and depression after discharge (β = −0.505; p = .023) were significant factors associated with the mental component of QOL. This means that being male, having recurrent disease, and having a higher level of anxiety and depression after discharge resulted in worse mental QOL.
Discussion
This is the first study to identify pre- and postdischarge factors associated with changes in QOL among patients with HCC after receiving one course of TACE. Fatigue was the most distressing symptom up to 2 months after treatment in this study. Consistent with previous studies, changes in QOL were associated with age [35], gender [36], symptom distress [36, 37], tumor recurrence [35, 38], and anxiety and depression [37]. Our study, after further analyzing the association between significant factors and the domains of QOL, found that the status of the diagnosis (newly diagnosed or recurrent) and depression after discharge were significant factors associated with both physical and mental QOL.
Fatigue is the symptom most reported by cancer patients, including those who have completed active treatment [39]. It is also commonly reported by patients with HCC [11, 40]. In addition, our study found that the mean score for vitality in the domains of QOL decreased 1 month after discharge. A higher level of fatigue could lower the sense of hope [41], and it could affect patient QOL, which is correlated with functional status and the survival rate [42]. However, there are no care programs related to managing fatigue and performing physical activity to maintain good functional status for patients with HCC after they have received treatments in a clinical setting. Previous studies pointed out that cancer patients do not report their fatigue because they are afraid of distracting physicians from treating the disease [43, 44]. We, therefore, suggest that fatigue should routinely be assessed in the follow-up visits after treatment, and that health care providers should educate patients on how to manage fatigue. Suggestions for managing fatigue include performing optimal physical activity to maintain vitality and functional status based on different levels of fatigue [45]. This may help patients with HCC maintain a better level of QOL after treatment.
Importantly, the current disease status (newly diagnosed or recurrent) was the main disease factor associated with a change in QOL in this study. Participants with recurrent disease had a better physical QOL but a worse mental QOL than those with a new diagnosis receiving their first course of TACE. Furthermore, previous studies indicated that tumor recurrence negatively impacts post-treatment total QOL [35, 38], but if patients underwent more than two courses of TACE, they were likely to perceive better mental health [13]. This inconsistency might be a result of differences in study design and methods of data analysis. The studies by Wang and colleagues have examined the correlation between the rate of recurrence and overall QOL [35], but they did not further analyze the subdomains of QOL [35, 38]. However, in our study, we compared the QOL of those who had been newly diagnosed with liver cancer receiving their first course of TACE with the QOL of those who had recurrent disease. A previous study [13] that examined changes in QOL from the first course of TACE to 12 months after treatment pointed out that vitality scores worsened after the first chemoembolization. On the other hand, patients with multiple treatments might have had more adequate control of their physical condition after receiving TACE. These might be the reasons why newly diagnosed patients undergoing their first course of TACE in this study had worse physical QOL than those with recurrent disease who had received several courses of TACE. In addition, symptom distress and depression after discharge were important factors associated with physical QOL in this study. This supports the idea that patients faced more challenges with their initial course of TACE and might be unfamiliar with the consequences or symptom distress after discharge; they may also be experiencing a depressive mood because of their recent cancer diagnosis. In contrast, patients with recurrent disease had a worse mental QOL, which might mean that they felt uncertainty related to their disease [36] and a lack of control.
These results suggest that health care providers should pay special attention to those with a new diagnosis and who have received their first course of TACE in terms of their physical QOL. Prior to discharge, health care providers might offer sufficient and effective education programs for managing symptom distress and emotional coping after discharge. For those with recurrent disease, education related to managing the anxiety and depression caused by the recurrence could be helpful in improving mental QOL.
Furthermore, the level of depression after discharge was found to play an important role in affecting both physical and mental QOL from discharge to 2 months after discharge. Anxiety after discharge was also an important factor associated with mental QOL. Previous studies have reported that depression was correlated with QOL [36, 37], but they did not further analyze the associations between these factors and the domains of QOL. The positive correlation between symptom distress and depression over the three time periods (r = 0.54–0.74; p < .0001) in this study supports the idea that there is an association between the physical and psychological domains. In general, patients with HCC who were eligible to receive TACE had a good functional status, and they could function in the general population without any complications. However, high recurrence rates and repeated treatments might impact their mood. Around 76% of patients had recurrent disease in this study. Patients with HCC have a 5-year survival rate in the range of 20%–50% [4, 12, 46] and a high tumor recurrence rate (70% in 5 years) [9] after receiving nonsurgical treatments. The majority of patients are male (71.3%) and have lower levels of mental QOL, as found in this study. In general, men are unwilling to express their feelings within the Chinese culture. Therefore, psychological assessments should be routinely used in outpatient settings and those with a higher level of psychological distress (anxiety and depression) should be referred for further care.
The levels of symptom distress, anxiety, and depression after discharge play a more important role in the change in QOL after discharge than the levels prior to discharge (Table 4). Our study results indicate that the physical and psychological distress that participants experienced were dynamic and could change monthly after discharge. Hence, we cannot predict change in QOL based on their condition prior to discharge. Therefore, patients' symptom distress, anxiety, and depression should routinely be followed up after discharge.
Despite the importance of our findings, the study had a few limitations. Although we followed patients for 8 weeks after discharge, the long-term effects of one course of TACE on QOL are unknown. Therefore, this study's results can only be generalized to 2 months after discharge; a more long-term assessment of QOL needs to be conducted. Second, we did not examine the relationships among laboratory data (i.e., alanine aminotransferase and albumin), tumor size, and QOL in this study. Future studies are needed to further examine their relationships in this population over a longer period. Third, the sample size of this study might affect the variability in QOL and limited the number of variables entered into the GEE model, and some variables were excluded from the correlation analysis. Larger future studies will need to clarify the differences in QOL among those who are newly diagnosed and those who have received several treatments, considering postembolization syndrome, comorbidity, actual tumor burden, and chronic liver disease.
Conclusion
In conclusion, the findings of this study present changes in QOL and its associated factors prior to and after discharge in patients with HCC within 2 months of one course of TACE. HCC patients who are at the greatest risk for a lower QOL are those who are older, male, and have higher levels of depression and anxiety after discharge. Health care providers should offer different information to patients with a new diagnosis than to those with recurrent disease. Future intervention studies are needed to help patients with HCC who have undergone treatment to maintain their vitality, decrease fatigue, and manage their psychological distress in order to enhance QOL.
Editor's Note: See pages 725–731 of this issue for the companion article, “Hepatic Artery Embolization for Neuroendocrine Tumors: Postprocedural Management and Complications,” by Mark A. Lewis, Sylvia Jaramillo, Lewis Roberts et al.
Acknowledgments
The authors gratefully acknowledge the assistance of the patients who participated in this study and grant support from the National Science Council (NSC98–2314-B-002–103-MY3).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Chien-Hung Chen, Shiow-Ching Shun, Yeur-Hur Lai
Provision of study material or patients: Chien-Hung Chen, Shiow-Ching Shun, Jyh-Chin Yang, Jin-Chuan Sheu, Ja-Der Liang
Collection and/or assembly of data: Shiow-Ching Shun
Data analysis and interpretation: Shiow-Ching Shun, Yeur-Hur Lai
Manuscript writing: Shiow-Ching Shun, Yeur-Hur Lai
Final approval of manuscript: Chien-Hung Chen, Shiow-Ching Shun, Yeur-Hur Lai, Jyh-Chin Yang, Jin-Chuan Sheu, Ja-Der Liang
References
- 1.Bosch FX, Ribes J, Díaz M, et al. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127(suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health, Executive Yuan, Taipei, Taiwan. Annual Statistics, 2010 Edition. 2010. Sep 7, [Accessed March 30, 2012]. Available at http://www.doh.gov.tw/CHT2006/DM/DM2_2_p02.aspx?class_no=440&now_fod_list_no=11397&level_no=-1&doc_no=76512.
- 3.Taiwan Cancer Registry. Taiwan Cancer Database: Liver Cancer. 2008 [Google Scholar]
- 4.Takayasu K, Arii S, Ikai I, et al. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: Propensity score analysis. AJR Am J Roentgenol. 2010;194:830–837. doi: 10.2214/AJR.09.3308. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera R, Nelson DR. Review article: The management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–476. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67–76. doi: 10.1007/s10534-002-0714-y. [DOI] [PubMed] [Google Scholar]
- 7.Trevisani F, De Notariis S, Rossi C, et al. Randomized control trials on chemoembolization for hepatocellular carcinoma: Is there room for new studies? J Clin Gastroenterol. 2001;32:383–389. doi: 10.1097/00004836-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Cahill BA. Management of patients who have undergone hepatic artery chemoembolization. Clin J Oncol Nurs. 2005;9:69–75. doi: 10.1188/05.CJON.69-75. [DOI] [PubMed] [Google Scholar]
- 9.Forner A, Hessheimer AJ, Isabel Real M, et al. Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2006;60:89–98. doi: 10.1016/j.critrevonc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 11.Shun SC, Lai YH, Jing TT, et al. Fatigue patterns and correlates in male liver cancer patients receiving transcatheter hepatic arterial chemoembolization. Support Care Cancer. 2005;13:311–317. doi: 10.1007/s00520-004-0740-0. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am J Surg. 2008;195:829–836. doi: 10.1016/j.amjsurg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Wible BC, Rilling WS, Drescher P, et al. Longitudinal quality of life assessment of patients with hepatocellular carcinoma after primary transarterial chemoembolization. J Vasc Interv Radiol. 2010;21:1024–1030. doi: 10.1016/j.jvir.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Bonnetain F, Paoletti X, Collette S, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: Results from two French clinical trials. Qual Life Res. 2008;17:831–843. doi: 10.1007/s11136-008-9365-y. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi G, Loguercio C, Sgarbi D, et al. Reduced quality of life of patients with hepatocellular carcinoma. Dig Liver Dis. 2003;35:46–54. doi: 10.1016/s1590-8658(02)00011-7. [DOI] [PubMed] [Google Scholar]
- 16.Dagnelie PC, Pijls-Johannesma MC, Lambin P, et al. Impact of fatigue on overall quality of life in lung and breast cancer patients selected for high-dose radiotherapy. Ann Oncol. 2007;18:940–944. doi: 10.1093/annonc/mdm057. [DOI] [PubMed] [Google Scholar]
- 17.Schipper H, Clinch J, McMurray A, et al. Measuring the quality of life of cancer patients: The Functional Living Index–Cancer: Development and validation. J Clin Oncol. 1984;2:472–483. doi: 10.1200/JCO.1984.2.5.472. [DOI] [PubMed] [Google Scholar]
- 18.Shang E, Weiss C, Post S, et al. The influence of early supplementation of parenteral nutrition on quality of life and body composition in patients with advanced cancer. J Parenter Enteral Nutr. 2006;30:222–230. [PubMed] [Google Scholar]
- 19.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: How are they related? Support Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 20.Earlam S, Glover C, Fordy C, et al. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14:171–175. doi: 10.1200/JCO.1996.14.1.171. [DOI] [PubMed] [Google Scholar]
- 21.Ruste̸n T, Moum T, Padilla G, et al. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30:234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Dirix P, Nuyts S, Vander Poorten V, et al. The influence of xerostomia after radiotherapy on quality of life: Results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16:171–179. doi: 10.1007/s00520-007-0300-5. [DOI] [PubMed] [Google Scholar]
- 23.Lai YH. Symptom distress and home care needs in patients receiving chemotherapy in an outpatients setting. J Nurs Res. 1998;6:279–289. In Chinese. [Google Scholar]
- 24.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen ML, Chang HK, Yeh CH. Anxiety and depression in Taiwanese cancer patients with and without pain. J Adv Nurs. 2000;32:944–951. [PubMed] [Google Scholar]
- 27.Lai YH, Guo SL, Keefe FJ, et al. Multidimensional Pain Inventory–Screening Chinese version (MPI-sC): Psychometric testing in terminal cancer patients in Taiwan. Support Care Cancer. 2009;17:1445–1453. doi: 10.1007/s00520-009-0597-3. [DOI] [PubMed] [Google Scholar]
- 28.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Lam CL, Tse EY, Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual Life Res. 2005;14:539–547. doi: 10.1007/s11136-004-0704-3. [DOI] [PubMed] [Google Scholar]
- 30.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Shun SC, Beck SL, Pett MA, et al. Psychometric testing of three Chinese fatigue instruments in Taiwan. J Pain Symptom Manage. 2006;32:155–167. doi: 10.1016/j.jpainsymman.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Lai YH, Chang JT, Keefe FJ, et al. Symptom distress, catastrophic thinking, and hope in nasopharyngeal carcinoma patients. Cancer Nurs. 2003;26:485–493. doi: 10.1097/00002820-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 34.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7:127–149. [Google Scholar]
- 35.Wang YB, Chen MH, Yan K, et al. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: Comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389–397. doi: 10.1007/s11136-006-9133-9. [DOI] [PubMed] [Google Scholar]
- 36.Lai HL, Lin SY, Yeh SH. [Exploring uncertainty, quality of life and related factors in patients with liver cancer] Hu Li Za Zhi. 2007;54:41–52. In Chinese. [PubMed] [Google Scholar]
- 37.Shun SC, Chiou JF, Lai YH, et al. Changes in quality of life and its related factors in liver cancer patients receiving stereotactic radiation therapy. Support Care Cancer. 2008;16:1059–1065. doi: 10.1007/s00520-007-0384-y. [DOI] [PubMed] [Google Scholar]
- 38.Wang YB, Chen MH, Yan K, et al. [Quality of life of primary hepatocellular carcinoma patients after radiofrequency ablation] Ai Zheng. 2005;24:827–833. In Chinese. [PubMed] [Google Scholar]
- 39.Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39:1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun V, Ferrell B, Juarez G, et al. Symptom concerns and quality of life in hepatobiliary cancers. Oncol Nurs Forum. 2008;35:E45–E52. doi: 10.1188/08.ONF.E45-E52. [DOI] [PubMed] [Google Scholar]
- 41.Shun SC, Hsiao FH, Lai YH. Relationship between hope and fatigue characteristics in newly diagnosed outpatients with cancer. Oncol Nurs Forum. 2011;38:E81–E86. doi: 10.1188/11.ONF.E81-E86. [DOI] [PubMed] [Google Scholar]
- 42.Yeo W, Mo FK, Koh J, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2006;17:1083–1089. doi: 10.1093/annonc/mdl065. [DOI] [PubMed] [Google Scholar]
- 43.Passik SD, Kirsh KL, Donaghy K, et al. Patient-related barriers to fatigue communication: Initial validation of the fatigue management barriers questionnaire. J Pain Symptom Manage. 2002;24:481–493. doi: 10.1016/s0885-3924(02)00518-3. [DOI] [PubMed] [Google Scholar]
- 44.Shun SC, Lai YH, Hsiao FH. Patient-related barriers to fatigue communication in cancer patients receiving active treatment. The Oncologist. 2009;14:936–943. doi: 10.1634/theoncologist.2009-0048. [DOI] [PubMed] [Google Scholar]
- 45.McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: Evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8:945–953. doi: 10.6004/jnccn.2010.0069. [DOI] [PubMed] [Google Scholar]
- 46.Benson AB, 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: Hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]