Abstract
Most glandular tissues comprise polarized epithelial cells organized around a single central lumen. Although there is active research investigating the molecular networks involved in the regulation of lumenogenesis, little is known about the extracellular factors that influence lumen formation and maintenance. Using a three-dimensional culture system of epithelial endometrial cells, we have revealed a new role for pro-inflammatory cytokines such as TNFα and IL1α in the formation and, more importantly, maintenance of a single central lumen. We also studied the mechanism by which glucocorticoids repress TNFα and IL1α expression. Interestingly, regulation of pro-inflammatory cytokine expression and subsequent lumen formation is mediated by estrogen receptor α (ERα) but not by the glucocorticoid receptor. Finally, we investigated the signaling pathways involved in the regulation of lumen formation by pro-inflammatory cytokines. Our results demonstrate that activation of the ERK/MAPK signaling pathway, but not the PI3K/Akt signaling pathway, is important for the formation and maintenance of a single central lumen. In summary, our results suggest a novel role for ERα-regulated pro-inflammatory cytokine expression in lumen formation and maintenance.
Key words: Pro-inflammatory cytokines, Lumen, Epithelial cell polarity, ERα, ERK/MAPK
Introduction
In glandular epithelial tissues such as the mammary gland or the endometrium, epithelial cells interact with neighboring cells and the underlying extracellular matrix to develop polarized and well-organized glands. Increasing evidence indicates that appropriate three-dimensional (3D) organization is crucial for tissue homeostasis (Bryant and Mostov, 2008). Maintenance of cell polarity, cell-to-cell contacts and cell-to-matrix adhesion plays a pivotal role in the regulation of glandular homeostasis and epithelial cell proliferation, differentiation and survival.
Over the past few years, development of 3D cultures derived from epithelial cells has provided important advances in the knowledge of the cellular and molecular mechanisms underlying epithelial cell morphogenesis and oncogenesis. 3D cultures of epithelial cells were first established from different epithelial tissues or cell lines using collagen-based matrices (Elsdale and Bard, 1972; Emerman et al., 1979; Emerman and Pitelka, 1977; Hall et al., 1982). More recently, growth of Madin-Darby canine kidney (MDCK) epithelial cells or MCF-10 mammary epithelial cells over reconstituted basement membranes are the most widely used in vitro 3D models for investigation of cell polarity during epithelial morphogenesis and of the mechanisms involved in epithelial oncogenesis (Debnath et al., 2003a; Hebner et al., 2008; Kim, 2005; Kim et al., 2004; Mailleux et al., 2008; Martin-Belmonte and Mostov, 2008; Shaw et al., 2004; Yamada and Cukierman, 2007).
During formation of glandular epithelial tissues, epithelial cells surround a single central lumen with their apical surfaces facing towards it. Two mechanisms of lumen formation have been reported: hollowing and cavitation (Bryant and Mostov, 2008; Mailleux et al., 2008; Martin-Belmonte and Mostov, 2008; Martin-Belmonte et al., 2008). In the first model, lumen is formed by membrane separation of glandular cells; in the second model, death of central cells by apoptosis (Debnath et al., 2002; Mailleux et al., 2007) is the main mechanism for lumen formation.
Different intracellular signaling pathways have been shown to participate in the establishment of epithelial cell polarity and lumen formation. Among them, activation of phosphatidylinositol 3-kinase (PI3K)/Akt has been extensively involved in the control of lumenogenesis. Overexpression of Akt in MCF10 cells (Debnath et al., 2003b) and either exogenous expression of phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] (Gassama-Diagne et al., 2006) or downregulation of phosphatase and tensin homolog (PTEN) in MDCK cells (Martin-Belmonte et al., 2007; Martin-Belmonte and Mostov, 2007) disrupts acinar architecture, leading to formation of multiple-lumened glands. On the other hand, the extracellular signal-regulated kinases (ERKs)/mitogen-activated protein kinases (MAPKs) transduce different extracellular signals that are important for normal morphogenesis as well as for tumorigenesis of epithelial tissues such as the mammary gland (Fata et al., 2007; Whyte et al., 2009).
Pro-inflammatory cytokines such as interleukin-1α (IL1α) and tumor necrosis factor α (TNFα) play a pivotal role in inflammation and immunity. However, in addition to their function as chemical mediators of inflammation, pro-inflammatory cytokines regulate a wide range of cellular processes. This multiple functionality has been particularly studied in the case of TNFα. TNFα was initially identified as an inductor of apoptosis in tumor cells (Carswell et al., 1975) but, over the past few years, it has been shown that TNFα plays additional roles. At the cellular level, the best-characterized function of TNFα is related to its ability to induce apoptosis but, depending on cell type and cellular environment, it can also promote cellular proliferation, survival or differentiation (Aggarwal, 2003; Gaur and Aggarwal, 2003). Furthermore, increasing evidence demonstrates that TNFα plays an important role in morphogenesis by regulating cytoskeleton organization and remodelling, which result in changes in cell shape, adhesion or migration (Mathew et al., 2009). These multiple functions of TNFα are translated to complex intracellular signaling pathways. Apart from the apoptotic arm of TNFα signaling, multiple signaling pathways such as nuclear factor kappa B (NF-κB), PI3K/Akt, p38 MAPK, ERK/MAPK or Jun N-terminal kinases (JNK) (Aggarwal, 2003; Baud and Karin, 2001; Mathew et al., 2009; Wajant et al., 2003) have been shown to transduce TNFα signals.
Formation and maintenance of polarized glands with a central lumen are key processes in morphogenesis and homeostasis of epithelial tissues. Although many works have investigated the intracellular signaling networks involved in establishment of epithelial cell polarity and lumenogenesis, little is known about the extracellular molecules such as growth factors, hormones or cytokines involved in the control of those processes. Here, we have demonstrated that the anti-inflammatory properties of glucocorticoids disrupt endometrial glandular architecture, revealing an important role for pro-inflammatory cytokines such as IL1α and TNFα in the formation of well-polarized glands and, more importantly, in the maintenance of a single central lumen surrounded by epithelial cells displaying appropriate cell polarity. Furthermore, we demonstrate that repression of TNFα and IL1α expression is mediated by the estrogen receptor α (ERα) but not by the glucocorticoid receptor (GR). Finally, we show that ERK/MAPK is required downstream of TNFα or IL1α to transduce their effects on correct formation of a central lumen and establishment of epithelial cell polarity.
Results
Glucocorticoids induce multiple lumen formation in endometrial glandular cultures
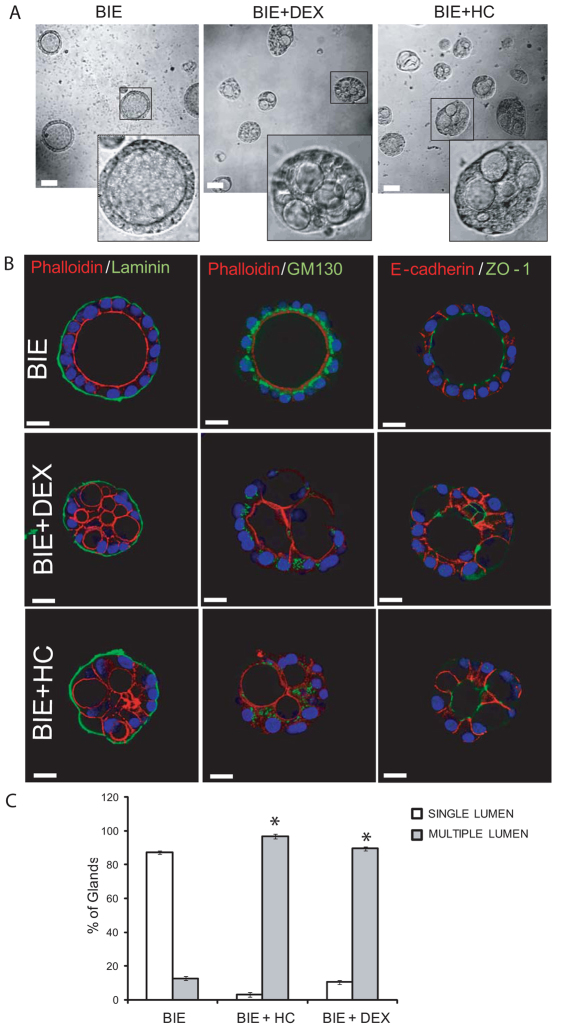
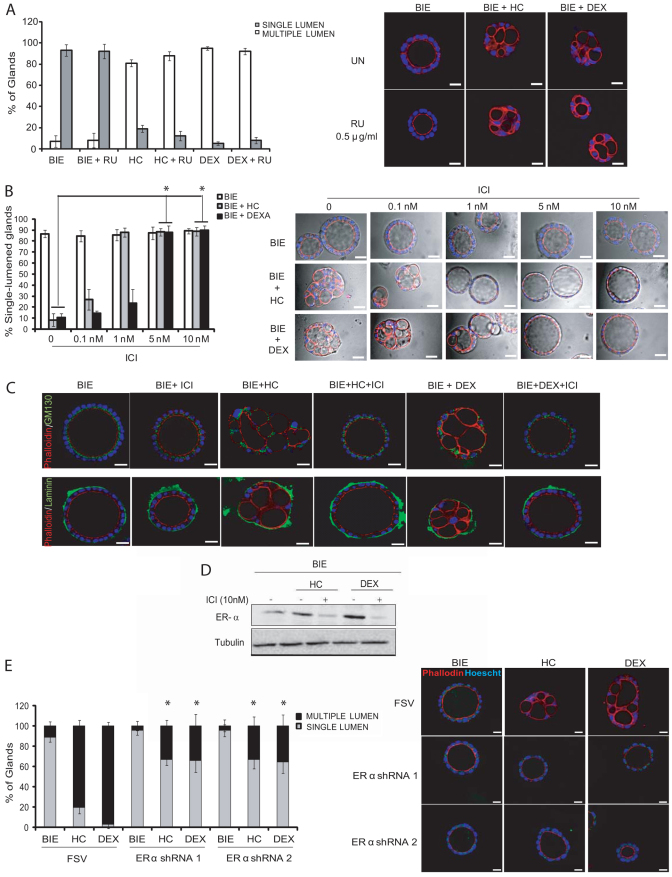
We have recently established a novel 3D culture of primary endometrial epithelial cells that leads to the development of polarized 3D glandular structures (Eritja et al., 2010). In this 3D culture system, most of glands display a single central lumen and a correct apico–basal cell polarization. Surprisingly, addition of glucocorticoids, such as hydrocortisone or dexamethasone, to the culture medium caused the development of morphologically aberrant glands (Fig. 1A). Double immunostaining of glands with laminin and phalloidin (to evidence the apical actin cytoskeleton) revealed that glands contained multiple lumens (Fig. 1B). Moreover, glands cultured in the presence of glucocorticoids displayed aberrant morphological features. An immunofluorescence study using markers of apico–basal polarity revealed an aberrant positioning of Golgi apparatus (evidenced by GM130 immunostaining) as well as an aberrant location of adherens and tight junctions (evidenced by E-cadherin and ZO-1 immunostaining) (Fig. 1B). Quantification of glands displaying one lumen versus glands displaying multiple lumens demonstrated that glucocorticoid treatment dramatically increased the number of glands with multiple lumens, yielding a total of 75–90% of multiple-lumened glands in the culture (Fig. 1C).
Fig. 1.
Glucocorticoids induce multiple lumen formation in endometrial glandular cultures. Endometrial epithelial cells were grown in 3D conditions for 7 days in BIE medium alone, BIE plus 500 ng/ml hydrocortisone (BIE+HC) or BIE plus 200 ng/ml dexamethasone (BIE+DEX). (A) Representative images of 3D cultures show glands with single lumen or multiple lumens. (B) Double immunostaining of glands with laminin (green) and phalloidin (red), GM-130 (green) and phalloidin (red) or ZO-1 (green) and E-cadherin (red). Nuclei were counterstained with Hoechst 33342. (C) Quantification of the number of single-lumened glands versus glands displaying two or more lumens. Results are expressed as a percentage of the total number of glands. Scale bars: 20 μm. *P≤0.05.
We have previously demonstrated that, in our endometrial model, lumen formation takes place via a membrane separation event rather than by luminal apoptosis. To rule out the possibility that glucocorticoid treatment switched the mechanism of lumen formation, we performed an immunofluorescence analysis of caspase-3 activation at different time-points of gland formation. Glucocorticoid treatment did not cause any substantial increase in luminal apoptotic cells compared with control cultures (supplementary material Fig. S1).
Multiple lumen formation is caused by a decrease in TNFα and IL1α expression
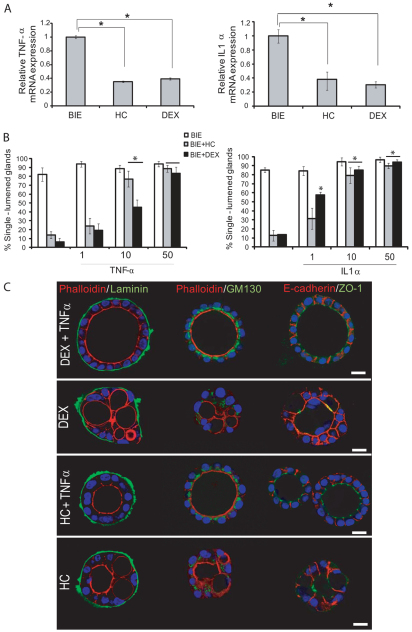
To ascertain the mechanisms by which glucocorticoids induce multiple lumen formation, we first investigated whether it could be a process related to the anti-inflammatory properties of glucocorticoids. For this reason, we performed a quantitative PCR array analysis of genes related to inflammation (supplementary material Table S1). In this study, we found that TNFα and IL1α were the main pro-inflammatory cytokines downregulated by glucocorticoids. Results were further confirmed by single-gene quantitative real-time PCR. As we show in Fig. 2A, addition of glucocorticoids for 16 hours caused a dramatic decrease in TNFα and IL1α mRNA expression. This result enabled us to check whether the decrease in pro-inflammatory cytokines was responsible for formation of multiple-lumened glands. For this purpose, we performed a complementation experiment in which we grew endometrial glands in the presence of glucocorticoids alone or glucocorticoids plus increasing doses of either TNFα or IL1α. Addition of increasing doses of TNFα or IL1α caused a dose-dependent increase in the number of glands displaying a single central lumen (Fig. 2B). Finally, to demonstrate establishment of a correct cell polarity, we performed double immunofluorescence staining with phalloidin (to evidence the apical actin cytoskeleton) and different antibodies against laminin, ZO-1 or GM130. Addition of TNFα completely restored normal cell polarity in glands grown in the presence of glucocorticoids (Fig. 2C). The same results were obtained in the case of addition of IL1α (supplementary material Fig. S2).
Fig. 2.
Multiple lumen formation is caused by a decrease in TNFα and IL1α expression. (A) Glucocorticoids reduce pro-inflammatory cytokine expression. Endometrial epithelial cells were grown in 3D conditions for 7 days in BIE, BIE plus hydrocortisone (HC) or BIE plus dexamethasone (DEX). Real-time PCR analysis of TNFα (left) and IL1α (right) expression. Results are expressed as relative mRNA levels. (B) Addition of either TNFα (50 ng/ml) or IL1α (50 ng/ml) causes a dose-dependent blockage of multiple lumen formation induced by glucocorticoids. Quantification of single-lumened glands grown for 7 days in BIE medium, BIE+HC or BIE+DEX containing increasing doses of either TNFα (left) or IL1α (right). (C) Addition of 50 ng/ml TNFα restores correct glandular architecture. Glands were cultured in the presence of HC, DEX, HC+TNFα or DEX+TNFα. Double immunostaining of glands with phalloidin (red) plus laminin (green), phalloidin (red) plus GM130 (green) or E-cadherin (red) plus ZO-1 (green). Nuclei were counterstained with Hoechst 33342. Scale bars: 20 μm. *P≤0.05.
Expression of TNFα and IL1α is required for maintenance of a single lumen
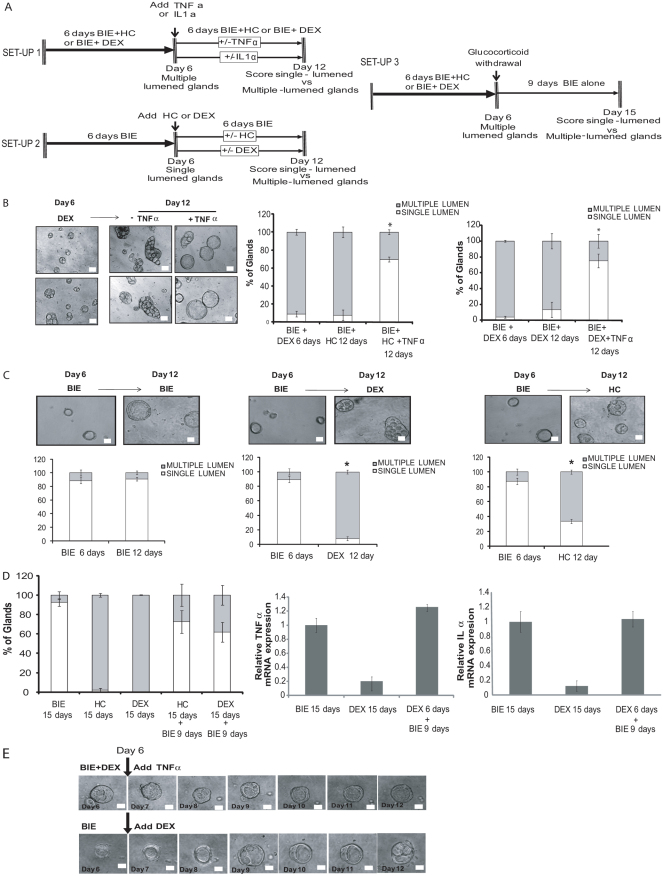
Next, we investigated whether expression of TNFα or IL1α was only required for lumen formation or, alternatively, whether cytokines were further required to upkeep one single lumen once the gland was formed. To assess this point, we designed three different experimental set-ups (Fig. 3A). In the first, glandular cultures were grown in the presence of hydrocortisone or dexamethasone for 6 days. At day 6, medium was replaced by medium containing glucocorticoids supplemented or not with pro-inflammatory cytokines and the cultures were grown for 6 additional days (until day 12) (Fig. 3A, see experimental set-up 1). After the first 6 days in culture with glucocorticoids, most of glands displayed multiple lumens, but addition of TNFα caused a restructuration of multiple lumens into one single central lumen (Fig. 3B). These results demonstrate that TNFα was able to reverse multiple-lumened glands to single-lumened glands. In the second experimental set-up (Fig. 3A, experimental set-up 2), we cultured glands in BIE medium (see Materials and Methods) without glucocorticoids for 6 days, allowing the development of single-lumened glands. At day 6, medium was replaced by BIE medium containing or not glucocorticoids for 6 additional days (day 12). At the end of the incubation period, glucocorticoid treatment had transformed most of the single-lumened glands into multiple-lumened glands (Fig. 3C). In the third experimental approach (Fig. 3A, experimental set-up 3), glandular cultures were grown in the presence of hydrocortisone or dexamethasone for 6 days. At day 6, medium was replaced by medium without glucocorticoids. However, in this set-up we did not add an exogenous source of pro-inflammatory cytokines. The rationale of this experimental set-up was to ascertain whether re-expression of endogenous cytokine expression was enough to change multiple-lumened glands to single-lumened ones. In this third set-up, to allow re-expression of endogenous cytokines, we extended the period of incubation without glucocorticoids to 9 days. As shown in Fig. 3D, glucocorticoid withdrawal was sufficient to restore mRNA expression levels of endogenous pro-inflammatory cytokines and, subsequently, reversion of multiple-lumened glands to single-lumened ones.
Fig. 3.
TNFα and IL1α are required for single lumen maintenance. (A) Diagram showing the three different experimental set-ups for studying the role of pro-inflammatory cytokines in maintenance of single central lumen. In set-up 1, epithelial 3D cultures were grown in BIE supplemented with dexamethasone or hydrocortisone (BIE+DEX or BIE+HC) for 6 days. After 6 days, TNFα or IL1α were added to the culture and the percentages of multiple-lumened and single-lumened glands were scored after 6 additional days. In set-up 2, epithelial 3D cultures were grown in BIE for 6 days. After 6 initial days, medium was supplemented with HC or DEX and glands were cultured for 6 additional days. In set-up 3, epithelial 3D cultures were grown in BIE+DEX or BIE+HC for 6 days. At this point, medium was replaced by BIE medium with or without glucocorticoids and cultures grown for 9 additional days. (B) Reversible switch of phenotypes from multiple lumen to one single lumen. Representative images (left) and quantification (right) of single-versus multiple-lumened glands at day 6 and at the end of the first experimental set-up (day 12). (C) Representative images (top) and quantifications (bottom) of single-versus multiple-lumened glands at day 6 and at the end of the second experimental set-up (day 12). (D) Glucocorticoid removal is sufficient to restore single-lumened glands and expression of pro-inflammatory cytokines. Left: Quantification of single-versus multiple-lumened glands at the end of the third experimental set up (day 15). Real-time PCR analysis of TNFα (middle) and IL1α (right) expression at the indicated days of treatment. Results are expressed as relative mRNA levels. (E) Representative images of time-lapse experiments corresponding to experimental set-up 1 (top) and experimental set-up 2 (bottom). Scale bars: 50 μm. *P≤0.05.
Finally, in order to evidence the role of pro-inflammatory cytokines in lumen dynamics, we performed a set of time-lapse experiments (Fig. 3E). For this purpose, we grew 3D cultures as shown in set-ups 1 and 2. The first 6 days of culture were maintained in a normal incubator and, at day 6 (just after the medium switch), 3D cultures were transferred to the incubator of a time-lapse recording system. Under the first experimental set-up conditions, addition of TNFα progressively restructured multiple lumens into one single lumen (Fig. 3E; supplementary material Movies 1 and 2). By contrast, addition of glucocorticoids to single-lumened glands resulted in the disruption of one single central lumen into multiple lumens (Fig. 3E). This evidence suggests that pro-inflammatory cytokine expression is important for both formation and maintenance of luminal space.
Glucocorticoids repress pro-inflammatory cytokine expression through ERα
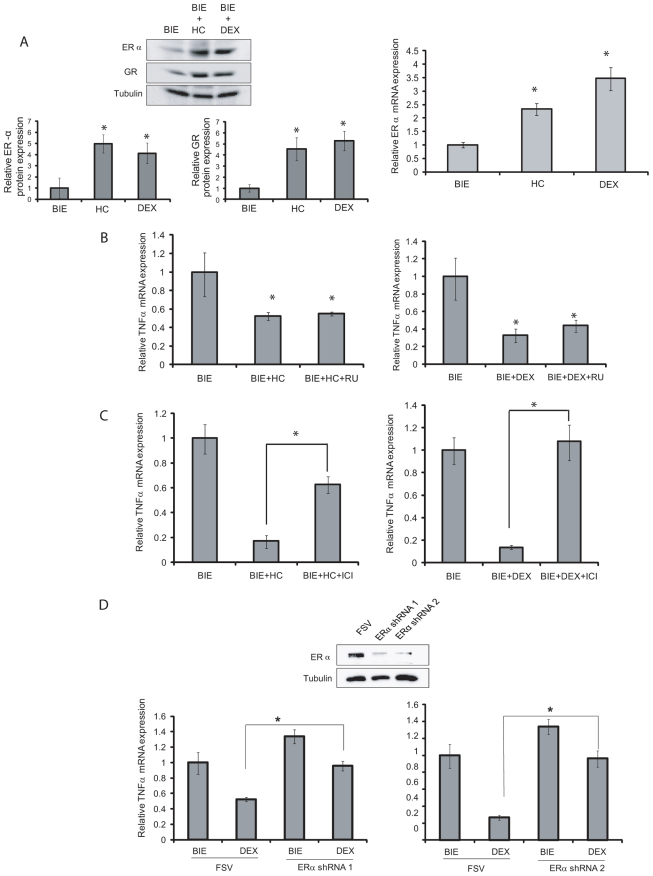
Having ascertained that glucocorticoids regulate lumen formation and cell polarity, we decided to investigate the molecular mechanism by which glucocorticoids regulate expression of pro-inflammatory cytokines. Recent evidence demonstrates that glucocorticoids can use ERα to drive their anti-inflammatory actions (Cvoro et al., 2011). Therefore, we analyzed the expression of both GR and ERα after glucocorticoid treatment. Addition of glucocorticoids to glandular cultures led to increased expression of both GR and ERα receptors (Fig. 4A). The increase in ERα expression was further analyzed by RT-PCR. As shown in Fig. 4A, glucocorticoids caused a marked increase in mRNA expression of ERα, suggesting that glucocorticoids cause transcriptional activation of ERα.
Fig. 4.
Glucocorticoids repress pro-inflammatory cytokine expression through ERα. (A) Glucocorticoids increase expression of both GR and ERα. Left: Western blot from cultures treated for 7 days with BIE alone or BIE supplemented with either hydrocortisone (HC) or dexamethasone (DEX) shows increased expression of both ERα and GR. Membranes were reproved with tubulin to ensure equal protein loading. Quantification of band intensity in three different western blots is shown below. Right: Real-time PCR analysis of ERα expression in cultures treated with BIE alone or BIE supplemented with HC or DEX. Results are expressed as relative mRNA levels. (B) GR does not to repress TNFα expression. Left: Endometrial epithelial cells were grown in 3D conditions for 7 days in BIE, BIE+HC or BIE+HC plus 0.5 μg/ml RU-486 (BIE+HC+RU). Right: Endometrial epithelial cells were grown in 3D conditions for 7 days in BIE, BIE+DEX or BIE+DEX plus 0.5 μg/ml RU-486 (BIE+DEX+RU). mRNA was extracted and TNFα expression was analyzed by real-time PCR. Results are expressed as relative mRNA levels. (C) ERα is required to repress TNFα expression. Left: Endometrial epithelial cells were grown in 3D conditions for 7 days in BIE, BIE+HC or BIE+HC plus 10 nM ICI182170 (BIE+HC+ICI). Right: Endometrial epithelial cells were grown in 3D conditions for 7 days in BIEBIE+DEX or BIE+DEX plus 10 nM ICI1821700 (BIE+DEX+ICI). mRNA was extracted and TNFα expression was analyzed by real-time PCR. Results are expressed as relative mRNA levels. (D) shRNAs targeting ERα restore pro-inflammatory cytokine expression. Endometrial epithelial cells were infected with lentiviruses carrying two different shRNAs targeting ERα (ERα shRNA1 or ERα shRNA2) and grown in 3D conditions in the presence or absence of DEX. Western blot shows downregulation of ERα expression by both shRNAs (top). mRNA was extracted and TNFα expression was analyzed by real-time PCR. Results are expressed as relative mRNA levels (bottom). *P≤0.05.
To investigate the role of GR and ERα upregulation in the control of TNFα transcription, we treated glandular cultures with the ERα antagonist ICI182170 or the GR antagonist RU486. Treatment of 3D cultures with RU486 did not restore TNFα expression (Fig. 4B). By contrast, ICI182170 restored TNFα mRNA to the levels observed in control cultures (Fig. 4C). To further assess the role of ERα in lumen formation, we performed experiments using two different short hairpin RNAs (shRNAs) targeting ERα. Analysis of mRNA expression by RT-PCR revealed that ERα knockdown restored the levels of TNFα mRNA to levels similar to those shown in control cultures (Fig. 4D).
Repression of pro-inflammatory expression by ERα causes multiple lumen formation and loss of cell polarity in 3D endometrial cultures
The above results suggest that ERα, but not GR, mediates repression of pro-inflammatory cytokine expression in response to glucocorticoid treatment. In the light of these results, we would expect that ICI182170 but not RU486 treatment leads to the formation of single-lumened glands in the presence of glucocorticoids. Indeed, treatment of glandular cultures with RU485 did not block formation of multiple lumens induced by glucocorticoids (Fig. 5A). By contrast, treatment of 3D cultures with dexamethasone or hydrocortisone plus ICI182170 caused a dose-dependent increase in single-lumened glands (Fig. 5B). Double immunostaining with phalloidin plus laminin or phalloidin plus GM130 revealed that ICI182170 addition restored correct cell polarity even in the presence of glucocorticoids (Fig. 5C). As expected, ICI182170 caused downregulation of ERα expression (Fig. 5D). To further demonstrate that inhibition of ERα expression was important in restoring cell polarity, we infected 3D cultures with two shRNAs targeting ERα. In agreement with the results obtained with ICI, downregulation of ERα expression by shRNAs 1 and 2, prevented formation of multiple-lumened glands induced by glucocorticoids (Fig. 5E).
Fig. 5.
ERα but not GR expression prevents multiple lumen formation. (A) Inhibition of GR does not block formation of multiple-lumened glands. Endometrial epithelial cells were grown for 7 days in BIE, BIE plus hydrocortisone (HC) or BIE plus dexamethasone (DEX) with or without 0.5 μg/ml of RU-486. Quantification of the percentage of single-lumened and multiple-lumened glands (left) and representative images showing phalloidin immunostaining to evidence the number of multiple-lumened glands (right). (B) Dose-dependent inhibition of ERα leads to increasing number of single-lumened glands. Endometrial epithelial cells were grown for 7 days in BIE, BIE+HC, BIE+DEX alone or with increasing doses of ICI1821700. Quantification of the percentage of single-lumened glands (left). Representative images showing phase contrast plus phalloidin immunostaining (red) to evidence the switch from multiple-lumened to single-lumened glands (right). (C) ICI1821700 restores epithelial cell polarity and the formation of single-lumened glands. Endometrial epithelial cells were grown for 7 days in BIE, BIE+HC, BIE+DEX alone or with ICI1821700 (BIE+ICI; BIE+HC+ICI; BIE+DEX+ICI). Double immunostaining of glands with phalloidin (red) and either laminin (green) or GM130 (green) was carried out to evidence cell polarity and lumen morphology. (D) Glucocorticoids increase ERα expression. Western blot shows increase in ERα protein levels in glands cultured for 7 days in BIE, BIE+HC or BIE+DEX. Membranes were reproved with tubulin to demonstrate equal protein loading. (E) Endometrial epithelial cells were infected with lentiviruses carrying two different shRNAs targeting ERα and grown in 3D conditions in the presence or absence of DEX or HC. Left: Quantification of the percentages of single-lumened and multiple-lumened glands. Right: Representative images showing phalloidin immunostaining to evidence the number of multiple-lumened glands. Nuclei were counterstained with Hoechst 33342 (blue) in all samples. Scale bars: 50 μm. *P≤0.05.
It is important to point out that, although the effects of glucocorticoids on pro-inflammatory cytokine expression and formation of lumen were mediated by ERα, treatment of glandular cultures with estradiol did not cause reduction of pro-inflammatory cytokine expression or disruption of cell polarity (supplementary material Fig. S3).
PTEN deficiency does not disrupt epithelial cell polarity and lumen formation
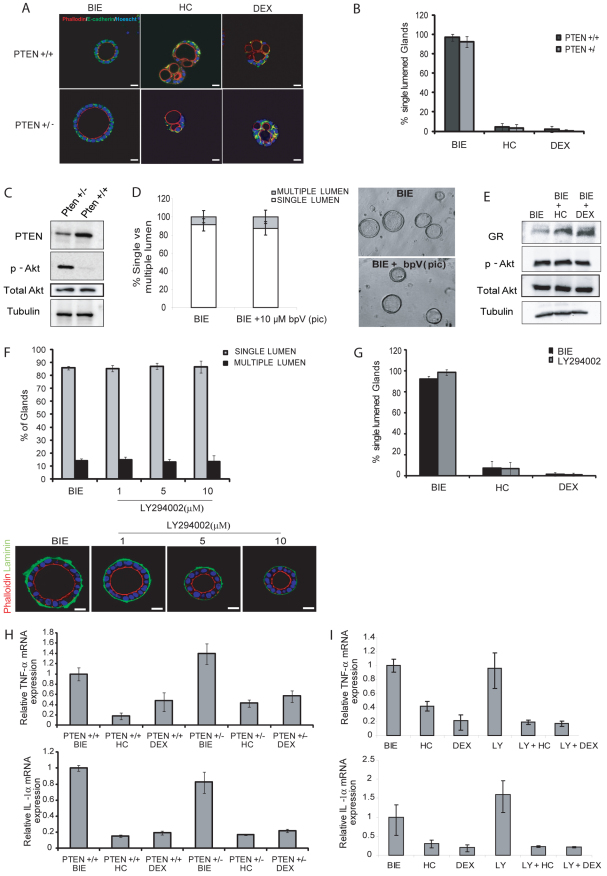
Recent evidence has identified PTEN as crucial phosphatase in the establishment of cell polarity and lumen formation in 3D cultures (Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007; Martin-Belmonte and Mostov, 2008). Moreover, deregulation of PI3K/Akt signaling is a common event in endometrial carcinogenesis (Bussaglia et al., 2000; Llobet et al., 2009; Velasco et al., 2006). Such evidence enabled us to analyze the role of PTEN in establishment of cell polarity and lumen formation in primary 3D cultures of endometrial epithelial cells. To assess the effect of PTEN deletion on cell polarity cytoarchitecture, we isolated primary endometrial epithelial cells from PTEN+/+ or PTEN+/− mice. Isolated epithelial cells were grown in BIE to develop polarized 3D glandular structures. Surprisingly, after 10 days in culture, PTEN+/− endometrial glands displayed normal morphology, with a single lumen and no apparent changes compared with PTEN+/+ glands (Fig. 6A). Moreover, we performed immunofluorescence analysis of polarity and epithelial architecture markers on PTEN+/+ and PTEN+/− glands treated with or without glucocorticoids. PTEN+/− glands cultured in BIE without glucocorticoids displayed normal apical actin cytoskeleton (phalloidin staining) and correct E-cadherin positioning, as observed in PTEN+/− glands, indicating no alterations in gland formation. Addition of either hydrocortisone or dexamethasone disrupted normal acinar morphology regardless of PTEN status (Fig. 6A). Quantification of single-lumened versus multiple-lumened glands revealed no differences between PTEN+/+ and PTEN+/− glands regardless of glucocorticoid treatment (Fig. 6B). As a control for correct deregulation of PI3K/Akt signaling in PTEN-deficient glands, we checked that that PTEN+/− glands displayed increased levels of phosphorylated Akt (Fig. 6C). Similar results were obtained in epithelial cells treated with 10 μM of the PTEN inhibitor bpV(pic) (Fig. 6D) or infected with lentiviruses carrying shRNA targeting PTEN (supplementary material Fig. S4). Accordingly, glucocorticoids did not change the phosphorylation status of Akt even though they completely disrupted lumen formation (Fig. 6E). Finally, we wanted to investigate whether inhibition of PI3/Akt signaling would result in disruption of lumen formation. Increasing doses of the PI3K/Akt inhibitor LY294002 did not disrupt the formation of normal single central lumen glands (Fig. 6F). Addition of glucocorticoids plus LY294002 did not modify the effect of glucocorticoids alone on lumen formation (Fig. 6G). In concordance with these results, neither PTEN deficiency nor LY294002 treatment modified the decrease in TNFα and IL1α expression caused by glucocorticoids (Fig. 6H,I).
Fig. 6.
PTEN downregulation or PI3K inhibition does not affect lumen formation or maintenance. Endometrial epithelial cells isolated from PTEN+/+ or PTEN+/− mice were grown in 3D conditions for 10 days in defined BIE medium, in BIE plus hydrocortisone (HC) or in BIE plus dexamethasone (DEX). (A) Double immunostaining of PTEN+/+ and PTEN+/− glands with E-cadherin (green) and phalloidin (red). Nuclei were counterstained with Hoechst 33342 in all samples. (B) PTEN deficiency does not modify the number of lumens. The percentages of single-lumened glands in PTEN+/+ and PTEN−/− cultures are shown. (C) Western blots from PTEN+/+ and PTEN−/− glands show decreased PTEN levels and increased Akt phosphorylation in PTEN+/− mice. (D) Quantification (left) and representative images (right) of single- versus multiple-lumened glands grown for 7 days with BIE or BIE plus 10 mM of PTEN inhibitor bpV(pic). (E) Glucocorticoids do not affect Akt phosphorylation. Western blots from cultures treated with BIE alone or BIE supplemented with HC or DEX show no differences in Akt phosphorylation after 7 days in culture. (F) Inhibition of PI3K does not affect lumen formation. Quantification of single- versus multiple-lumened glands grown for 7 days with the indicated doses of the PI3K inhibitor LY294002 (top). Double laminin (green) plus phalloidin (red) immunostaining of representative images of single-lumened glands (bottom). (G) Quantification of single- versus multiple-lumened glands grown for 7 days with BIE or BIE plus LY294002 in BIE medium alone or BIE containing HC or DEX. (H) PTEN status does not affect the reduction of cytokine expression caused by glucocorticoids. Endometrial epithelial cells from PTEN+/+ or PTEN+/− mice were grown in 3D conditions for 7 days in defined BIE medium alone or BIE supplemented with HC or DEX. Real-time PCR analysis of TNFα (top) and IL1α (bottom) expression is shown. Results are expressed as relative mRNA levels. (I) PI3K/Akt inhibition does not affect the reduction of cytokine expression caused by glucocorticoids. Endometrial epithelial cells were grown in 3D conditions for 7 days in defined BIE medium alone or BIE supplemented with HC or DEX in the presence or absence of the PI3K inhibitor LY294002 (LY). Real-time PCR analysis of TNFα (top) and IL1α (bottom) expression is shown. Results are expressed as relative mRNA levels. Scale bars: 50 μm.
Activation of ERK/MAPK downstream of pro-inflammatory cytokines is required for correct lumen formation
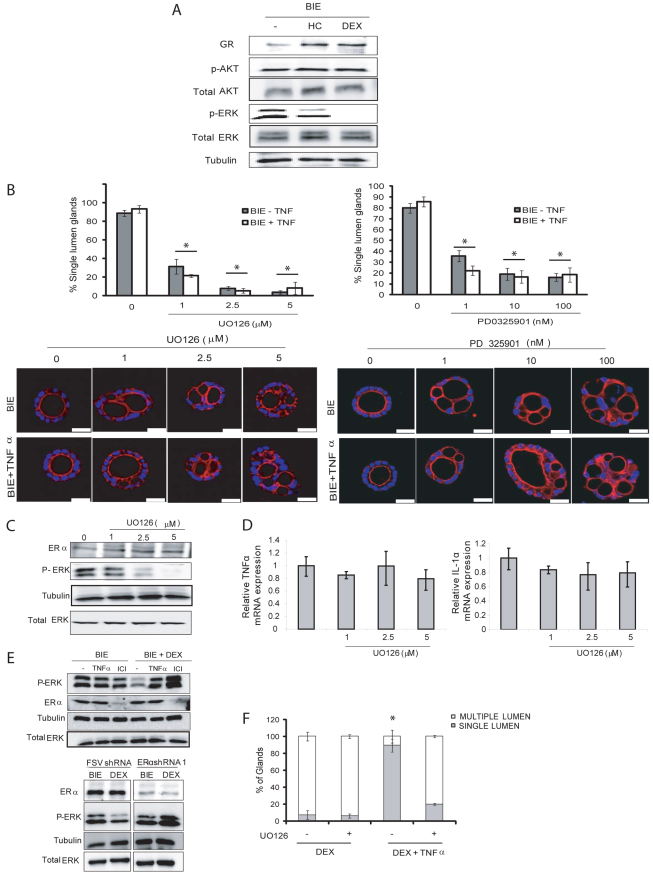
As shown in Fig. 6, neither activation nor inhibition of the PI3K/Akt signaling caused abnormalities in lumen formation. Consistently, glucocorticoids did not change the levels of Akt phosphorylation. By contrast, glucocorticoids caused a marked decrease in ERK phosphorylation (Fig. 7A). This result enabled us to investigate the role of ERK activity in lumen formation. For this purpose, we grew 3D cultures in BIE and in BIE plus TNFα supplemented with increasing doses of the MAPK kinase (MEK) inhibitors UO126 or PD0325901. Both ERK inhibitors caused a dose-dependent increase in the number of glands bearing multiple lumens in either glands grown in BIE alone or glands grown in BIE supplemented with TNFα (Fig. 7B). To rule out the possibility that ERK inhibition lead to formation of multiple-lumened glands by changing ERα repression of pro-inflammatory cytokines, we analyzed the levels of ERα by western blot and the levels of TNFα and IL1α mRNA expression by real-time PCR. Noteworthy, although UO126 caused an efficient inhibition of ERK phosphorylation it did not change the expression of ERα (Fig. 7C). Consistently, ERK inhibitors did not cause repression of pro-inflammatory cytokine mRNA levels (Fig. 7D), supporting the idea that ERK activation is required downstream of pro-inflammatory cytokine expression to promote correct lumen formation. Accordingly, addition of TNFα or inhibition of ERα expression by shRNAs targeting ICI182170 or ERα prevented the decrease in ERK phosphorylation caused by glucocorticoids (Fig. 7E). Moreover, ERK inhibition prevented correct lumen formation in cultures treated with glucocorticoids plus TNFα (Fig. 7F), suggesting that ERK regulates lumen formation downstream of pro-inflammatory cytokines.
Fig. 7.
Inhibition of ERK/MAPK signaling results in multiple lumen formation downstream of cytokine expression. (A) Glucocorticoids reduce ERK phosphorylation. Western blot from 3D culture grown for 7 days in BIE, BIE plus dexamethasone (DEX) or BIE plus hydrocortisone (HC) shows no modification of Akt phosphorylation, increased GR expression and reduction of ERK phosphorylation. Membranes were reproved with tubulin to show equal protein loading. (B) Inhibition of ERK/MAPK causes a dose-dependent increase in multiple-lumened glands. Endometrial epithelial glands were grown for 7 days in BIE medium alone or BIE plus TNFα and increasing doses of the ERK inhibitors UO126 or PD0325901. Quantification of single-lumened glands (top) and representative images (bottom) showing phalloidin immunostaining (red). Nuclei were evidenced by Hoechst 33342 staining (bue). (C) Inhibition of ERK/MAPK does not change ERα expression. Western blot from 3D cultures grown for 7 days in BIE plus increasing doses of UO126 shows an effective inhibition of ERK phosphorylation and no modification of ERα protein levels. Membranes were reproved with tubulin to show equal protein loading. (D) Inhibition of ERK/MAPK does not repress TNFα or IL1α expression. Endometrial epithelial glands were grown for 7 days in BIE plus increasing doses of UO126. mRNA was extracted and TNFα (left) and IL1α (right) expression was analyzed by real-time PCR. Results are expressed as relative mRNA levels. (E) TNFα, ICI182170 and shRNA targeting ERα restore ERK phosphorylation in glucocorticoid-treated cells. Top: Western blot shows ERK phosphorylation of cultures treated with BIE or BIE+DEX and 50 ng/ml TNFα, 10 nM ICI182170 (ICI) or no additives (−) for 7 days. Bottom: Western blot shows ERK phosphorylation in cultures infected with FSV vector or ERα shRNA1 and treated with BIE alone or BIE+DEX. Membranes were reproved with ERα antibodies to ensure correct inhibition of ERα. Membranes were also reproved with tubulin and total ERK to ensure equal loading. (F) Percentage of single- versus multiple-lumened glands grown for 7 days in BIE+DEX or BIE+DEX plus TNFα with (+) or without (−) 5 μM UO126. Scale bars: 50 μm. *P≤0.05.
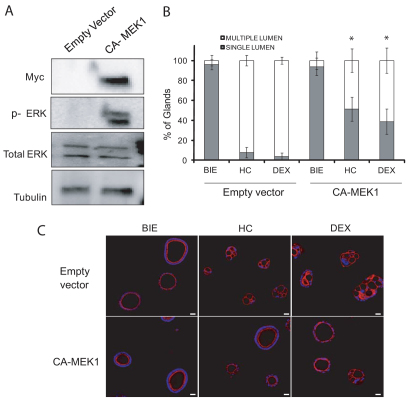
Finally, to further investigate the role of ERK signaling in the formation of single-lumened glands, we infected 3D cultures with lentiviruses carrying a Myc-tagged constitutively active form of MEK1 (CA-MEK1). MEK1 is the kinase immediately upstream of ERK and is commonly used to activate ERKs. CA-MEK1 expression caused a marked increase in ERK phosphorylation, indicating an activation of the pathway (Fig. 8A). More importantly, CA-MEK1 expression reduced the formation of multiple-lumened glands in the presence of glucocorticoids (Fig. 8B,C). Expression of CA-MEK1 did not cause a complete restoration of the number of single-lumened glands. This is because 3D cultures are hard to infect with lentiviruses carrying transgenes. In our hands, around 70% of glands in the culture were efficiently infected by lentiviruses carrying transgenes. These results suggest that activation of ERK is sufficient to restore cell polarity and formation of single lumen.
Fig. 8.
Activation of ERK is sufficient to prevent multiple lumen formation induced by glucocorticoids. Glandular cultures were infected with vector expression of constitutively active form of MEK1 (CA-MEK1) or the empty vector and grown for 7 days in BIE. (A) Western blot shows increased phosphorylation of ERK in cells infected with CA-MEK1. Membranes were reproved with Myc to ensure expression of the construct and with total ERK and tubulin to ensure equal protein loading. (B) Quantification of single-lumened versus multiple-lumened glands. (C) Representative images of 3D cultures infected with empty vector or vector carrying CA-MEK1 stained with phalloidin (red). Nuclei were evidenced by Hoechst 33342 staining (blue). Scale bars: 50 μm. *P≤0.05.
Discussion
Establishment and maintenance of cell polarity is important for development and homeostasis of epithelial tissues. During the last few years, the development of 3D cultures of epithelial cells has provided an important tool for advancing knowledge of the molecular mechanisms that participate in correct epithelial cell polarization and the formation of lumens in epithelial glandular tissues such as the mammary gland. We have recently developed a novel serum-free 3D culture of primary endometrial epithelial cells that can be used for both morphogenesis and oncogenesis studies. Surprisingly, we have found that addition of glucocorticoids to the culture medium (a common component of 3D cultures of epithelial cells) caused the formation of multiple-lumened glands displaying aberrant polarization of epithelial endometrial cells. The dramatic effects of glucocorticoids in lumenogenesis and polarization of epithelial cells enabled us to investigate the molecular mechanisms by which they drive these effects. It is important to point out that glucocorticoids are widely used as anti-inflammatory drugs, making the study of glucocorticoid effects on cell polarity even more interesting. In a PCR-array study we have identified TNFα and IL1α as the main cytokines regulated by glucocorticoid treatment. Thus, we have focused our investigations on these pro-inflammatory cytokines. However, it is reasonable to think that other pro-inflammatory cytokines contribute together with TNFα and IL1α to the regulation of cell polarity. It is worth mentioning that cytokines represent a diverse group of molecules that collectively exert a wide range of actions. Many cytokines are pleiotropic and display multiple and overlapping actions. This overlap results in a functional redundancy and compensatory mechanisms of action (Kelso, 1994; Ozaki and Leonard, 2002; Paul, 1989). Glucocorticoid treatment does not exclusively decrease expression of TNFα and IL1α, but also decreases the expression of other pro-inflammatory cytokines (Barnes, 2006; Hayashi et al., 2004). Therefore, it is unlikely that inhibition of TNFα and IL1α results in dramatic changes in cell polarity. Nonetheless, to analyze the specific effects of TNFα and IL1α inhibition, we performed experiments using blocking antibodies to TNFα and IL1α (data not shown). Neither inhibition of TNFα nor inhibition of IL1α was sufficient to induce the formation of multiple-lumened glands. This result suggests that other cytokines apart from TNFα and IL1α can participate in the establishment of cell polarity. These compensatory effects are further supported by the fact that addition of either TNFα or IL1α separately causes a complete restoration of the number of glands displaying a single central lumen.
We have found that glucocorticoids cause an increase in ERα expression. One conserved function of steroid hormone receptors is that they autoregulate the expression of their own genes (Schmidt and Meyer, 1994). Estrogens upregulate ERα gene expression in uteri of all mammalian species examined, including rodents (Bergman et al., 1992) primates (Hild-Petito et al., 1992; Koji and Brenner, 1993) and humans (Lessey et al., 1988; Snijders et al., 1992). Thus, expression of ERα is increased after its binding to estradiol, resulting in a positive feedback mechanism that controls transcription of ERα. Similarly, increased expression of ERα upon glucocorticoid addition is probably the result of ERα stimulation by glucocorticoids.
One of the most important results from our investigations is the ERα-dependent regulation of pro-inflammatory cytokine expression and its link to cell polarity. Previous evidence clearly demonstrates that unligated ERα potentiates transcriptional activation of TNFα (Cvoro et al., 2006). Recently, the same group has demonstrated that ERα can drive pro-inflammatory cytokine repression caused by glucocorticoids (Cvoro et al., 2011). Accordingly, in vivo addition of estrogen receptor antagonist ICI182780 inhibits the anti-inflammatory effect of glucocorticoids in mice (Cuzzocrea et al., 2007). In line with the above evidence, we have found that addition of glucocorticoids caused an expected decrease in pro-inflammatory cytokine levels through ERα. Furthermore, we found a novel function for ERα modulation of pro-inflammatory cytokine expression in the control of lumen formation, maintenance and acquisition of epithelial cell polarity. Interestingly, the effects on lumen observed after glucocorticoid treatment were not reproduced by addition of estradiol. These results can be explained by the differential gene regulation of ERα engaged by estradiol or glucocorticoids (Cvoro et al., 2011). Because ICI182170 targets both ERα and ERβ, another reasonable explanation for the effects of ICI182170 on lumen could be the inhibition of ERβ rather than inhibition of ERα. However, this possibility was ruled out by the fact that ERβ is not expressed in mouse epithelial endometrial cells (Couse et al., 1997). Furthermore, shRNA-mediated knockdown of ERα caused similar effects on lumen formation to those observed after ICI treatment, suggesting that ERα is involved in the regulation of lumen formation. Another key finding derived from our studies is the role of TNFα and IL1α in the maintenance of one single central lumen. Most works have assessed the mechanisms of lumen formation but, once the lumen is formed, little is known about the extracellular factors and the molecular mechanisms that participate in maintenance of a single central lumen. We have demonstrated that, once formed, a single central lumen is not passively maintained. Instead, maintenance of single central lumen seems to be an active process that requires active signaling.
Moreover, we have shown that endogenous pro-inflammatory cytokine expression is sufficient to maintain lumen and epithelial organization. Finally, we have assessed the signaling pathways involved in the regulation of lumen formation by pro-inflammatory cytokines. A basic feature of cell polarity is the asymmetrical organization of the plasma membrane. This organization is basically achieved through membrane trafficking along the cytoskeleton tracks under the control of signaling molecules (Bonifacino and Glick, 2004; Cai et al., 2007). Rho GTPases are molecules that control a wide range of signaling pathways decisive for the acquisition of the polarized phenotype. The function of the Rho family of small GTPases, especially Cdc42, in polarized membrane traffic, has been shown from yeast to mammalian cells (Nelson, 2009; Rodriguez-Fraticelli et al., 2010; Yu et al., 2005; Yu et al., 2003). Cdc42 also controls lumen formation in MCDK cells (Martin-Belmonte et al., 2007) and more recently Cdc42 activity has been involved in regulation of the mitotic spindle orientation (Jaffe et al., 2008; Rodriguez-Fraticelli et al., 2010). In addition to small GTPases, another set of membrane traffic regulators are the lipids phosphatidylinositol and its phosphorylated species. In polarized MCDK cells PtdIns(3,4,5)P3 is localized to the basolateral membrane (Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007). Studies using 3D cultures of MCDK cells, showed that PTEN localizes to the apical membrane during epithelial morphogenesis, where it excludes PtdIns(3,4,5)P3 from this domain (Martin-Belmonte et al., 2007). It has been also demonstrated that PtdIns(4,5)P2 recruits the adaptor protein anexin2 to the apical domain which, in turn, recruits Cdc42. This causes the organization of the subapical actin cytoskeleton and formation of the apical surface. Cdc42 binds to the Par6–aPKC complex and they promote the establishment of polarity (Martin-Belmonte et al., 2007; Martin-Belmonte and Mostov, 2007). Alterations in Cdc42, PTEN or anexin2 expression in polarized MCDK cells, result in aberrant cysts, which present multiple lumens as well as an abnormal polarization (Jaffe et al., 2008; Martin-Belmonte et al., 2007). It has also been reported that E-cadherin regulates both the PTEN expression and its recruitment to cell–cell junctions to regulate lumen formation in breast cancer cells (Fournier et al., 2009).
In the present study, our results demonstrate that a deficiency in PTEN expression in polarized epithelial endometrial cells does not cause formation of aberrant or multiple-lumened glands. This result initially appears to contradict the findings in MCDK cells, but these differences can be explained in several ways. First, because our cells are grown in a defined medium different to that used in the MDCK model, the microenvironment signals might be different, and cues from the extracellular environment act directly on the positioning of the apical lumen (Lipschutz et al., 2001; Martin-Belmonte et al., 2008; Myllymaki et al., 2011; O'Brien et al., 2001). It has been shown that, depending on the microenvironment, two distinct integrin-dependent pathways can regulate epithelial cytogenesis (Myllymaki et al., 2011). Second, PtdIns(4,5)P2 can be distributed in equivalent densities in the apical and basolateral membranes in endometrial epithelial glands; as happens in pancreatic acinars (Ozato-Sakurai et al., 2011). Finally, these differences can be caused by intrinsic cell-specific differences in the mechanisms involved in apical lumen formation. In this regard, it is important to point out that PTEN hemizygous mice or mice with deletion of both copies of PTEN, which show dramatic susceptibility to develop endometrial carcinoma and other malignancies, do not display aberrant development of endometrial epithelium or other glandular epitheliums even though they have increased levels of phosphorylated Akt (Di Cristofano et al., 1998; Podsypanina et al., 1999). This evidence suggests that other signaling pathways can operate in the regulation of cell polarity in those epithelial tissues. Our results suggest that activation of ERK/MAPK is required for correct formation of one single lumen. The ERK/MAPK pathway is involved in the regulation of many cellular processes, including proliferation, migration, differentiation and apoptosis (Shaul and Seger, 2007). Among these functions, ERK activation has been involved in the regulation of the cytoskeleton, migration and formation of cell-to-matrix and cell-to-cell adhesion (Howe et al., 2002; Pullikuth and Catling, 2007; Viala and Pouyssegur, 2004; Yee et al., 2008). Therefore, it is reasonable to hypothesize that ERK can regulate lumen formation and cell polarity by regulating the cytoskeleton and cellular adhesion. We demonstrate that inhibition of ERK causes a disruption of cell polarity and the formation of multiple lumens without affecting TNFα or IL1α expression, suggesting that ERK/MAPK act downstream of these cytokines to induce correct epithelial polarization. More importantly, the activation of ERK signaling by a constitutively active form of MEK is sufficient to prevent multiple lumen formation induced by glucocorticoids. The importance of ERK/MAPK as mediator of inflammatory signaling induced by TNFα has suggested the ERK/MAPKs as a putative therapeutic target for inflammation (Hommes et al., 2003; Kaminska, 2005; Karin, 2004). In summary, our investigations provide new insights into the regulation of cellular polarity and lumen formation in a 3D model of endometrial glands. We have identified a novel function for pro-inflammatory cytokines such as TNFα and IL1α in lumen formation and, more importantly, in maintenance of a single central lumen with appropriate epithelial cell polarity. We have further demonstrated that ERα plays a crucial role in the regulation of cytokine expression and, finally, we have shown that the ERK/MAPK signaling pathway drives the effects of TNFα and IL1α on lumen formation and cell polarity.
Materials and Methods
Reagents and antibodies
The recombinant basement membrane Matrigel was purchased from BD Biosciences (San Jose, CA). Epidermal growth factor, hydrocortisone, dexamethasone, RU-486, ICI182170 and LY294002 were obtained from Sigma (St Louis, MO); insulin-transferrin-sodium selenite (ITS) supplement was obtained from Invitrogen (Carlsbad, CA); bpV(pic), PD0325901 and UO126 were purchased from Calbiochem (Calbiochem-Novabiochem, UK). Antibodies to E-cadherin and GM130 were from BD Biosciences; ZO-1 was from Zymed (San Francisco, CA); and bisBenzimide H33342 trihydrochloride from Hoechst. Rhodamine-conjugated phalloidin and antibodies to laminin and tubulin were obtained from Sigma. Alexa-Fluor-594- and Alexa-Fluor-488-conjugated anti-rabbit, and Alexa-Fluor-546- and Alexa-Fluor-488-conjugated anti-mouse antibodies were from Invitrogen. Antibodies against phosphorylated Akt and phosphorylated ERK were from Cell Signaling Technology (Beverly, MA). Antibodies against GR and ERα were obtained from Santa Cruz Biotechnology (Santa Cruz, CA.). Peroxidase-conjugated anti-mouse and anti-rabbit antibodies were from Jackson ImmunoResearch Europe (Suffolk, UK). All other regents were obtained from Sigma unless specified.
Animals and Isolation of endometrial epithelial cells
PTEN knockout mice (strain nomenclature B6.129-Ptentm1Rps) were obtained from the National Cancer Institute (NCI, Frederick, MD) mouse repository. The C57BL6 and PTEN knockout mice used to isolate endometrial cells were maintained in temperature- and light-controlled conditions and fed ab libitum. The Institutional Animal Care Committee of the IRBLleida approved all experimental procedures. The isolation of endometrial epithelial cells was processed as described previously (Eritja et al, 2010). In brief, uterine horns were dissected from C57BL6 mice of 3–4 weeks old. Uteri were washed with Hank's balanced salt solution (HBSS) and digested with trypsin (Invitrogen). After trypsin digestion, epithelial sheets were squeezed-out of the uterine pieces. Epithelial sheets were washed twice with PBS and resuspended in 1 ml of DMEM/F12 medium (Invitrogen) supplemented with 1 mM HEPES (Sigma), 1% of penicillin/streptomycin (Sigma) and fungizone (Invitrogen) (basal medium). Epithelial sheets were mechanically disrupted in basal medium. Cells were diluted in basal medium containing 2% dextran-coated charcoal-stripped (DCC-S) serum (Hyclone, Logan, UT) and plated in culture dishes (BD Falcon, Bedford, MA). Cells were cultured for 24 hours in a incubator at 37°C with saturating humidity and 5% CO2.
Three-dimensional glandular cultures
Growth of endometrial epithelial cells in 3D cultures was performed as described previously (Eritja et al, 2010). At 24 hours after platting in plastic, cells were washed with HBSS and incubated with trypsin/EDTA solution (Sigma) for 5 minutes at 37°C. Trypsin activity was stopped by adding DMEM containing 10% FBS. Clumps of 2–8 cells were obtained. Cells were centrifuged at 1000 r.p.m. for 3 minutes and diluted in basal medium containing 3% Matrigel to obtain 4×104 cell clumps/ml. For immunofluorescence, cells were seeded in a volume of 40 μl/well in 96-well plates that were black with micro-clear bottom (Greiner Bio-one). For western blotting, cells were placed in a volume of 200 μl in 24-well plates (BD Biosciences). In all cases, 24 hours after plating, medium was replaced by basal medium supplemented with 5 ng/ml EGF and 1:100 dilution of ITS supplement and 3% of fresh Matrigel (this medium is referred to as BIE). Medium was replaced every 2–3 days.
Immunofluorescence
3D cultures were fixed with formalin for 5 minutes at room temperature and washed twice with PBS. Depending on the primary antibody, cells were permeabilized with 0.2% Triton X-100 in PBS for 10 minutes (indicated by T) or permeabilized with 100% methanol for 2 minutes (indicated by M). Next, cultures were incubated overnight at 4°C with the indicated dilutions of antibodies: anti-laminin 1:500 (T), rhodamine conjugated-phalloidin 1:500 (T), anti-E-cadherin 1:250, ZO-1 1:250 and anti-GM130 1:100 (M). After 1 day, cells were washed twice with PBS and incubated with PBS containing a 5 μg/ml of Hoechst 33342 and 1:500 dilution of Alexa Fluor secondary anti-mouse or anti-rabbit antibodies for 2 hours at room temperature. For double immunofluorescence staining, cells were incubated with the second round of primary and secondary antibodies. We would like to point out that in all double immunofluorescence stains, first and second primary antibodies were from different isotopes. Immunofluorescence staining was visualized and analyzed using a confocal microscopy (FV1000, Olympus) using the oil-immersion 60× magnification objective. Analysis of images was made with Fluoview FV100 software.
Confocal imaging and evaluation of lumen and cell polarity
Endometrial epithelial glands were analyzed on a confocal microscope Fluoview FV1000. The presence of one or multiple lumens (more than two lumens) was revealed by immunostaining with GM130 and phalloidin. For each experiment, we quantified the number of single lumens and multiple lumens in at least 100 glands. Cell polarity of epithelial cells forming glandular structures was evidenced by double immunostaining as indicated in each figure.
Time-lapse experiments
Endometrial glands were grown for 6 days in BIE plus glucocorticoids. After 6 days, medium was replaced by medium containing BIE supplemented with TNFα or IL1α. At this point, cultures were incubated in a Zeiss Axiovert inverted microscope equipped with a motorized slide and CO2 and temperature incubation modules. AxioVision Rel. 4.6 software was programmed to take a picture of the selected glands once a day or every 3 hours for 6 consecutive days.
Lentiviral production and infection
Oligonucleotides to produce plasmid-based shRNA were cloned into the FSV vector using AgeI-BamHI restriction sites. shRNA target sequences were: PTEN, 5′-ATATAGGTCAAGTCTAAGTCG-3′; ERα-1, 5′-GGTGCCCTACTACCTGGAA-3′; ERα-2, 5′-GTGCAAGGAGACTCTCGCTATC-3′. Lentiviral particles were produced as previously described with some modifications (Llobet et al., 2008). 293T human embryonic kidney (HEK) cells were co-transfected using the polyethylenimine (PEI) method with virion packaging elements (psPAX2 and pMD2G) and the shRNA-producing vector (FSV). 293T cells were allowed to produce lentiviral particles over 3–4 days in the same culture medium used for endometrial cell lines and explants. Culture medium was collected, centrifuged for 5 minutes at 1000 r.p.m. and filtered through a 0.45 μM filter (Millipore). The medium was concentrated by centrifugation through Vivaspin20 100000 MWCO columns (Sartorious Stedium Biotech, Aubagne, France). Freshly isolated or thawed mouse endometrial cells were diluted in DMEM/F12 plus 2% DCC-S as described above and plated into 24-well plastic dishes. At 2–3 hours after plating, 10–20 μl of concentrated lentiviral particles plus 8 μg/ml of hexadimethrine bromide (Polybrene; Sigma) were added to the cultures and incubated for 24 hours. After this infection period, cells were processed to establish 3D cultures as described above.
Western blot analysis
Glandular endometrial 3D cultures stimulated for the indicated periods of time were washed with HBSS and incubated with trypsin/EDTA solution for 5 minutes at 37°C. Incubation with trypsin was done to allow separation of the glandular structures from Matrigel. Trypsin activity was stopped by adding DMEM containing 10% FBS and the cells lysed with lysis buffer (2% SDS, 125 mM Tris-HCL pH 6.8). Relative protein concentrations were determined by loading an 8% acrylamide gel, transferring to PVDF membranes and blotting with anti-tubulin antibody. Band density was determined using Quantity One software (Bio-Rad, Richmond, CA). Equal amounts of proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA). Nonspecific binding was blocked by incubation with TBST (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% Tween-20) containing 5% non-fat milk. Membranes were incubated with the primary antibodies overnight at 4°C followed by 1 hour incubation with secondary antibody diluted 1:10,000 in TBST. Signal was detected with ECL Advance (Amersham-Pharmacia, Buckinghamshire, UK).
Real-time PCR
Total RNA was prepared using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. Reverse transcription reactions were performed using a 1 μg total RNA with TaqMan Reverse Transcription Kit from Applied Biosystems. Quantitative real-time PCR detection of gene expression was performed with the ABI Prism 7000 Sequence Detection System using the TaqMan Universal PCR Master Mix (Applied Biosystems). The sequences of primers used for PCR were obtained commercially from Applied Biosystems Assay-on-Demand Gene Expression Products: (TNFα) Mm00443258_m1, (IL1α) Mm00439620_m1 and (GADPH) Mm99999915_g1. Relative expression was determinates from cycle threshold (Ct) values, which were normalized to GADPH as the endogenous control.
Statistical analysis
Experiments were performed at least three times and statistical significance was determined by Student's t-test with P≤0.05. Unless otherwise indicated, asterisks indicate P≤0.05. Results are shown as means ± s.e.m.
Supplementary Material
Acknowledgements
We thank David D. Schlaepfer (Department of Reproductive Medicine, University of California San Diego, La Jolla, CA) for the gift of lentiviral vector expressing a constitutively active form of MEK1.
Footnotes
Funding
Supported by grants from Fondo de Investigaciones Sanitarias (FIS) [grant numbers FISPI10/00604, FISPI070304, FISPI070276]; Ministerio de Ciencia e Innovacion [grant numbers SAF2002-10529-E, SAF2004-05250]; National Cancer Institute [grant number CA90400]; Marató de TV3 2005-47 (Fundació la Marató de TV3), RD06/0020/1034 and RD06/0020/0013(Red Temática de Investigación Cooperativa en Cáncer), 2009SGR794 (Grups Consolidats de Recerca) and 2004XT00090 (Xarxa Temàtica); AECC, Catalunya contra el cancer and programa de intensificación de la investigación; and Instituto Carlos III. N.E. holds a fellowship from Fundación Alicia Cuello de Merigó and a predoctoral fellowship from FIS [grant number FI08/0012]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.095067/-/DC1
References
- Aggarwal B. B. (2003). Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745-756 [DOI] [PubMed] [Google Scholar]
- Barnes P. J. (2006). How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 148, 245-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V., Karin M. (2001). Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11, 372-377 [DOI] [PubMed] [Google Scholar]
- Bergman M. D., Schachter B. S., Karelus K., Combatsiaris E. P., Garcia T., Nelson J. F. (1992). Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle: the role of estradiol. Endocrinology 130, 1923-1930 [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153-166 [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. (2008). From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussaglia E., del Rio E., Matias-Guiu X., Prat J. (2000). PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum. Pathol. 31, 312-317 [DOI] [PubMed] [Google Scholar]
- Cai H., Reinisch K., Ferro-Novick S. (2007). Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 12, 671-682 [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. (1975). An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 72, 3666-3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse J. F., Lindzey J., Grandien K., Gustafsson J. A., Korach K. S. (1997). Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138, 4613-4621 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Bruscoli S., Crisafulli C., Mazzon E., Agostini M., Muia C., Esposito E., Di Virgilio R., Meli R., Vegeto E., et al. (2007). Estrogen receptor antagonist fulvestrant (ICI 182,780) inhibits the anti-inflammatory effect of glucocorticoids. Mol. Pharmacol. 71, 132-144 [DOI] [PubMed] [Google Scholar]
- Cvoro A., Tzagarakis-Foster C., Tatomer D., Paruthiyil S., Fox M. S., Leitman D. C. (2006). Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol. Cell 21, 555-564 [DOI] [PubMed] [Google Scholar]
- Cvoro A., Yuan C., Paruthiyil S., Miller O. H., Yamamoto K. R., Leitman D. C. (2011). Cross talk between glucocorticoid and estrogen receptors occurs at a subset of proinflammatory genes. J. Immunol. 186, 4354-4360 [DOI] [PubMed] [Google Scholar]
- Debnath J., Mills K. R., Collins N. L., Reginato M. J., Muthuswamy S. K., Brugge J. S. (2002). The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29-40 [DOI] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S. K., Brugge J. S. (2003a). Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256-268 [DOI] [PubMed] [Google Scholar]
- Debnath J., Walker S. J., Brugge J. S. (2003b). Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J. Cell Biol. 163, 315-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. (1998). Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348-355 [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. (1972). Collagen substrata for studies on cell behavior. J. Cell Biol. 54, 626-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. (1977). Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13, 316-328 [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. (1979). Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 11, 109-119 [DOI] [PubMed] [Google Scholar]
- Eritja N., Llobet D., Domingo M., Santacana M., Yeramian A., Matias-Guiu X., Dolcet X. (2010). A novel three-dimensional culture system of polarized epithelial cells to study endometrial carcinogenesis. Am. J. Pathol. 176, 2722-2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J. E., Mori H., Ewald A. J., Zhang H., Yao E., Werb Z., Bissell M. J. (2007). The MAPERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 306, 193-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. V., Fata J. E., Martin K. J., Yaswen P., Bissell M. J. (2009). Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res. 69, 4545-4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J., Mostov K. (2006). Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 8, 963-970 [DOI] [PubMed] [Google Scholar]
- Gaur U., Aggarwal B. B. (2003). Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 66, 1403-1418 [DOI] [PubMed] [Google Scholar]
- Hall H. G., Farson D. A., Bissell M. J. (1982). Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc. Natl. Acad. Sci. USA 79, 4672-4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Wada H., Ito K., Adcock I. M. (2004). Effects of glucocorticoids on gene transcription. Eur. J. Pharmacol. 500, 51-62 [DOI] [PubMed] [Google Scholar]
- Hebner C., Weaver V. M., Debnath J. (2008). Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu. Rev. Pathol. 3, 313-339 [DOI] [PubMed] [Google Scholar]
- Hild-Petito S., Verhage H. G., Fazleabas A. T. (1992). Immunocytochemical localization of estrogen and progestin receptors in the baboon (Papio anubis) uterus during implantation and pregnancy. Endocrinology 130, 2343-2353 [DOI] [PubMed] [Google Scholar]
- Hommes D. W., Peppelenbosch M. P., van Deventer S. J. (2003). Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 52, 144-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A. K., Aplin A. E., Juliano R. L. (2002). Anchorage-dependent ERK signaling – mechanisms and consequences. Curr. Opin. Genet Dev. 12, 30-35 [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Kaji N., Durgan J., Hall A. (2008). Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B. (2005). MAPK signalling pathways as molecular targets for anti-inflammatory therapy – from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 1754, 2532-2562 [DOI] [PubMed] [Google Scholar]
- Karin M. (2004). Mitogen activated protein kinases as targets for development of novel anti-inflammatory drugs. Ann. Rheum. Dis. 63 Suppl. 2, ii62-ii64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A. (1994). The enigma of cytokine redundancy. Immunol. Cell Biol. 72, 97-101 [DOI] [PubMed] [Google Scholar]
- Kim J. B. (2005). Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 15, 365-377 [DOI] [PubMed] [Google Scholar]
- Kim J. B., Stein R., O'Hare M. J. (2004). Three-dimensional in vitro tissue culture models of breast cancer – a review. Breast. Cancer Res. Treat 85, 281-291 [DOI] [PubMed] [Google Scholar]
- Koji T., Brenner R. M. (1993). Localization of estrogen receptor messenger ribonucleic acid in rhesus monkey uterus by nonradioactive in situ hybridization with digoxigenin-labeled oligodeoxynucleotides. Endocrinology 132, 382-392 [DOI] [PubMed] [Google Scholar]
- Lessey B. A., Killam A. P., Metzger D. A., Haney A. F., Greene G. L., McCarty K. S., Jr (1988). Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J. Clin. Endocrinol. Metab. 67, 334-340 [DOI] [PubMed] [Google Scholar]
- Lipschutz J. H., O'Brien L. E., Altschuler Y., Avrahami D., Nguyen Y., Tang K., Mostov K. E. (2001). Analysis of membrane traffic in polarized epithelial cells. Curr. Protoc. Cell Biol. Chapter 15, Unit 15 5 [DOI] [PubMed] [Google Scholar]
- Llobet D., Eritja N., Encinas M., Llecha N., Yeramian A., Pallares J., Sorolla A., Gonzalez-Tallada F. J., Matias-Guiu X., Dolcet X. (2008). CK2 controls TRAIL and Fas sensitivity by regulating FLIP levels in endometrial carcinoma cells. Oncogene 27, 2513-2524 [DOI] [PubMed] [Google Scholar]
- Llobet D., Pallares J., Yeramian A., Santacana M., Eritja N., Velasco A., Dolcet X., Matias-Guiu X. (2009). Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J. Clin. Pathol. 62, 777-785 [DOI] [PubMed] [Google Scholar]
- Mailleux A. A., Overholtzer M., Schmelzle T., Bouillet P., Strasser A., Brugge J. S. (2007). BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell 12, 221-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux A. A., Overholtzer M., Brugge J. S. (2008). Lumen formation during mammary epithelial morphogenesis: insights from in vitro and in vivo models. Cell Cycle 7, 57-62 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Mostov K. (2007). Phosphoinositides control epithelial development. Cell Cycle 6, 1957-1961 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Mostov K. (2008). Regulation of cell polarity during epithelial morphogenesis. Curr. Opin. Cell Biol. 20, 227-234 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007). PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F., Yu W., Rodriguez-Fraticelli A. E., Ewald A. J., Werb Z., Alonso M. A., Mostov K. (2008). Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr. Biol. 18, 5075-5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S. J., Haubert D., Kronke M., Leptin M. (2009). Looking beyond death: a morphogenetic role for the TNF signalling pathway. J. Cell Sci. 122, 1939-1946 [DOI] [PubMed] [Google Scholar]
- Myllymaki S. M., Teravainen T. P., Manninen A. (2011). Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS ONE 6, e19453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. (2009). Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 1, a000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. (2001). Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3, 831-838 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Leonard W. J. (2002). Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 277, 29355-29358 [DOI] [PubMed] [Google Scholar]
- Ozato-Sakurai N., Fujita A., Fujimoto T. (2011). The distribution of phosphatidylinositol 4,5-bisphosphate in acinar cells of rat pancreas revealed with the freeze-fracture replica labeling method. PLoS ONE 6, e23567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. (1989). Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell 57, 521-524 [DOI] [PubMed] [Google Scholar]
- Podsypanina K., Ellenson L. H., Nemes A., Gu J., Tamura M., Yamada K. M., Cordon-Cardo C., Catoretti G., Fisher P. E., Parsons R. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 96, 1563-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth A. K., Catling A. D. (2007). Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell. Signal. 19, 1621-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli A. E., Vergarajauregui S., Eastburn D. J., Datta A., Alonso M. A., Mostov K., Martin-Belmonte F. (2010). The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J. Cell Biol. 189, 725-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. J., Meyer A. S. (1994). Autoregulation of corticosteroid receptors. How, when, where, and why? Receptor 4, 229-257 [PubMed] [Google Scholar]
- Shaul Y. D., Seger R. (2007). The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim. Biophys. Acta 1773, 1213-1226 [DOI] [PubMed] [Google Scholar]
- Shaw K. R., Wrobel C. N., Brugge J. S. (2004). Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J. Mammary Gland Biol. Neoplasia 9, 297-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders M. P., de Goeij A. F., Debets-Te, Baerts M. J, Rousch M. J, Koudstaal J., Bosman F. T. (1992). Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J. Reprod. Fertil. 94, 363-371 [DOI] [PubMed] [Google Scholar]
- Velasco A., Bussaglia E., Pallares J., Dolcet X., Llobet D., Encinas M., Llecha N., Palacios J., Prat J., Matias-Guiu X. (2006). PIK3CA gene mutations in endometrial carcinoma: correlation with PTEN and K-RAS alterations. Hum. Pathol. 37, 1465-1472 [DOI] [PubMed] [Google Scholar]
- Viala E., Pouyssegur J. (2004). Regulation of tumor cell motility by ERK mitogen-activated protein kinases. Ann. New York Acad. Sci. 1030, 208-218 [DOI] [PubMed] [Google Scholar]
- Wajant H., Pfizenmaier K., Scheurich P. (2003). Tumor necrosis factor signaling. Cell Death Differ. 10, 45-65 [DOI] [PubMed] [Google Scholar]
- Whyte J., Bergin O., Bianchi A., McNally S., Martin F. (2009). Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 11, 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Cukierman E. (2007). Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601-610 [DOI] [PubMed] [Google Scholar]
- Yee K. L., Weaver V. M., Hammer D. A. (2008). Integrin-mediated signalling through the MAP-kinase pathway. IET Syst. Biol. 2, 8-15 [DOI] [PubMed] [Google Scholar]
- Yu W., O'Brien L. E., Wang F., Bourne H., Mostov K. E., Zegers M. M. (2003). Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol. Biol. Cell 14, 748-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Datta A., Leroy P., O'Brien L. E., Mak G., Jou T. S., Matlin K. S., Mostov K. E., Zegers M. M. (2005). Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 16, 433-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.