Abstract
Neurogenesis, the production of new neurons, occurs in two specialized niches in the adult brain, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. In the SGZ, neural stem cells (NSCs) give rise to glutamatergic granule neurons that integrate into the granule cell layer. In the SVZ, NSCs generate a more diverse cohort of new neurons, including GABAergic, dopaminergic, and glutamatergic neurons, all of which migrate to the olfactory bulb through the rostral migratory stream. In both adult neurogenic niches, specific transcription factors have been shown to direct fate specification and lineage commitment. This review summarizes current progress on the transcriptional control of glutamatergic neurogenesis in the SGZ and SVZ, highlighting commonalities as well as differences in their transcriptional programs. In particular, we focus on work from our laboratory and others indicating that precise, sequential expression of transcription factors regulates the progression from NSC to lineage-committed progenitor, and ultimately regulates the production and differentiation of adult-born glutamatergic neurons.
Keywords: Adult neurogenesis, Neural stem cells, Glutamatergic neurons, Subventricular zone, Subgranular zone, Transcription factor
Introduction
In the adult hippocampus and subventricular zone (SVZ), neurogenesis (the generation, differentiation and integration of new neurons) is an ongoing process that occurs throughout the mammalian lifespan. Within these brain regions, adult neurogenesis occurs in specialized stem cell niches. In the hippocampus, adult-born neurons are generated exclusively in the dentate gyrus (DG) from neural stem cells (NSCs) that reside in the subgranular zone (SGZ). These NSCs produce granule neurons, a glutamatergic population of cells that integrate into the existing granule cell layer (GCL). Adult hippocampal neurogenesis is thought to contribute to learning and memory, and may also be involved in the regulation of emotional behaviors [1–4]. NSCs in the SVZ, a region that lies adjacent to the lateral ventricles, produce multiple lineages of new neurons that include dopaminergic, GABAergic, and a recently described subset of glutamatergic neurons, all of which migrate through the rostral migratory stream (RMS) to populate several areas of the olfactory bulb [5–8]. Adult-born olfactory neurons are thought to contribute to olfactory memory, odor discrimination, and, interestingly, to some maternal and sexual behaviors [9–12].
The molecular mechanisms controlling adult neurogenesis in the SVZ and DG have been the subject of much recent research. Transcription factors that regulate gene expression by activating and/or repressing downstream target genes have been implicated in controlling neurogenesis in many brain regions. Indeed, in both the SVZ and SGZ, coordinated, sequential expression of transcription factors has been demonstrated during the generation and differentiation of newborn neurons [5, 13–16]. In this review we will summarize the current research on transcription factor expression and function during adult neurogenesis in the SVZ and SGZ, focusing specifically on regulation of glutamatergic neurogenesis in these regions.
The subgranular zone neurogenic niche
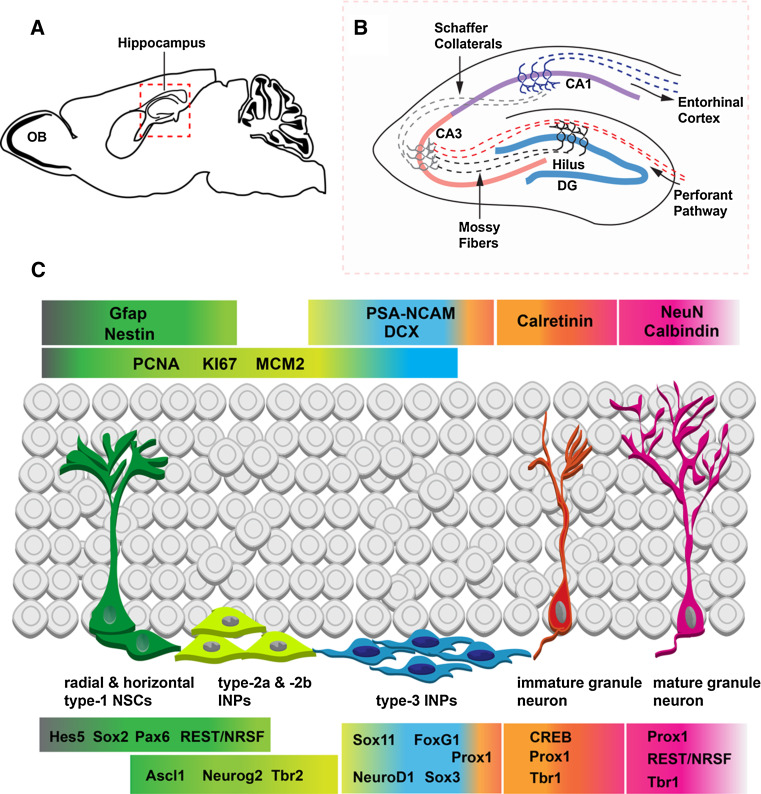
NSCs in the SGZ reside adjacent to the dentate hilus at the border of the GCL (Fig. 1). Multipotent SGZ NSCs can be divided into several subtypes according to their morphology and proliferative characteristics. Radial (type-1) NSCs are typically quiescent, dividing slowly and infrequently (Fig. 1c); [17, 18]. These radial NSCs are characterized morphologically by a single radial process that passes through the GCL, ending in multiple branches in the molecular layer, and immunohistochemically by expression of GFAP and nestin [17]. Horizontal (type-1) NSCs also express GFAP and nestin, but divide at a faster rate than radial NSCs and are morphologically characterized by short, horizontal processes (Fig. 1c); [17]. The lineage relationship between radial and horizontal NSCs is not currently clear. However, the existing model of adult hippocampal neurogenesis suggests that one or both of these NSC subtypes divides to give rise to transit-amplifying intermediate neuronal progenitors (INPs) [19]. INPs are neuronal lineage committed progenitors that divide rapidly in the SGZ [13, 20]. Morphologically, they are small cells that have very short tangential processes, and they tend to be present in clusters in the SGZ (Fig. 1c). Several types of INPs have been distinguished on the basis of morphology and molecular expression. Late-stage (type-2b/type-3) INPs are characterized by their expression of neuronal lineage markers such as Doublecortin (DCX) and PSA-NCAM [20]. INPs divide to give rise to new glutamatergic granule neurons, which downregulate DCX and upregulate calretinin, a calcium-binding protein, and NeuN, a marker of neuronal differentiation. If these adult-born neurons survive, they will be integrated into the existing hippocampal circuitry in approximately 4–7 weeks [20].
Fig. 1.
The subgranular zone (SGZ) neurogenic niche of the dentate gyrus (DG). a–b The DG is part of the hippocampal formation (dashed red box). b Schematic diagram of the hippocampal formation outlined in the red box in a. Granule neurons in the DG receive inputs from the perforant pathway, and in turn, send axonal projections via the mossy fiber pathway to the CA3 field. The tri-synaptic hippocampal circuitry is completed by Schaffer collateral projections from CA3 to CA1, which sends reciprocal axonal projections to entorhinal cortex. c The SGZ neurogenic niche is made up of radial and horizontal type-1 NSCs (green), early stage type-2a and -2b INPs (yellow), and late-stage type-3 INPs. These progenitor cells are located along the base of the granule cell layer (GCL), adjacent to the dentate hilus. This progenitor pool gives rise to immature granule neuroblasts (orange), which, if they survive, integrate into the existing GCL circuitry (pink, mature neurons). The progression from NSC to mature granule neuron is signaled by expression of a number of stage-specific cellular markers (upper colored panels corresponding to individual cell types) and transcription factors (lower colored panels). Color gradients indicate overlap of transcription factor expression into multiple cell types
Transcriptional control of glutamatergic neurogenesis in the SGZ
The transcriptional program regulating progression from multipotent NSCs to differentiated glutamatergic granule neurons in the SGZ is beginning to emerge. As NSCs divide to generate INPs they transition through expression of a series of transcription factors that signal commitment to a glutamatergic neuronal lineage. However, radial and horizontal NSCs initially express a number of transcription factors that maintain their undifferentiated state. One of the best-studied transcription factors expressed in DG NSCs is the SRY-related HMG-box (Sox) family member Sox2, a transcription factor important for stem cell maintenance in many systems. Sox2 is expressed in both the radial and horizontal subtypes of NSCs, where it colocalizes with GFAP, Blbp, and nestin [17, 21]. Sox2 expression persists at decreasing levels in early-stage (type-2a/type-2b) INPs, as it colocalizes in a subset of cells with the INP specific transcription factor Tbr2 [13]. Accordingly, Sox2 is expressed in numerous dividing cells in the SGZ and has been shown to colocalize with as many as 90% of PCNA + (dividing) cells in the neurogenic niche [22, 23]. Functionally, Sox2 appears to be involved in the maintenance of NSCs in the SGZ, as conditional deletion of the Sox2 gene results in depletion of type-1 NSCs [24]. Interestingly, Sox2 represses expression of NeuroD1, a transcription factor expressed in late-stage (type-3) INPs and newborn granule neurons, and this repression must be removed in order for granule neurogenesis to proceed [25]. Indeed, Sox2 is downregulated in late-stage INPs and is not present in newborn granule neurons [13, 21]. Maintenance of Sox2 expression itself appears to be dependent upon active Notch signaling, which increases expression of Sox2, whereas loss of activated Notch in RBPJκ-deficient NSCs decreases expression of Sox2 [26]. In line with a role for Notch in regulating Sox2 expression, Hes5, a bHLH transcription factor that is a known read-out of canonical Notch signaling, is expressed in both radial and horizontal NSCs where it frequently colocalizes with Sox2 [17]. However, the function of Hes5 during adult hippocampal neurogenesis is not currently known.
In addition to Sox2, NSCs in the SGZ characteristically express the paired domain and homeodomain-containing transcription factor Pax6 (Fig. 1c); [13, 27, 28]. Expression of Pax6 in DG NSCs parallels expression of this transcription factor in the embryonic cerebral cortex, where it is present in radial glia that give rise to glutamatergic cortical projection neurons [29, 30]. In the SGZ, Pax6 is expressed by radial and horizontal type-1 NSCs, as well as at least a subset of Tbr2 + INPs [13, 15]. However, Pax6 expression is absent from late stage INPs and granule neurons [13, 15]. Little information is available on the function of Pax6 in the SGZ; however, in heterozygous Pax6-deficient mice, GFAP + NSCs are reduced in the SGZ, as is overall proliferation, suggesting that Pax6 may be involved in regulating the NSC pool in the adult DG [28].
Ascl1, a basic helix loop helix (bHLH) transcription factor, is also expressed in both radial and horizontal type-1 NSCs in the adult SGZ [13, 15, 31, 32]. Like Sox2 and Pax6, Ascl1 expression persists in early stage INPs, where it overlaps with Tbr2, and is downregulated prior to terminal INP division [13]. Expression of Ascl1 in DG NSCs is confirmed by lineage-tracing studies, which show that Ascl1 + NSCs predominantly give rise to granule neurons in the normal context of the SGZ neurogenic niche [31, 32]. However, when Ascl1 is overexpressed in SGZ NSCs, the progeny produced are predominantly oligodendrocytes, indicating that the level of Ascl1 expression is an important determinant of its in vivo function [33]. Regardless, it is not currently known if Ascl1 is explicitly required for glutamatergic neurogenesis in the context of the adult SGZ.
The transcriptional repressor REST/NRSF (repressor element-1 silencing transcription/neuron-restrictive silencer factor) exhibits an interesting pattern of biphasic expression in the adult DG [34]. REST/NRSF is expressed in quiescent and proliferating type-1 cells, and its expression is maintained into type-2a INPs. REST/NRSF is downregulated in late-stage type-3 INPs, but then increases again in postmitotic neurons. In fact, REST/NRSF expression is maintained at high levels in all postmitotic granule neurons in the DG [34]. Within the adult SGZ, REST/NRSF functions to maintain the NSC pool. Knockdown of REST/NRSF in hippocampal stem cell cultures results in accelerated neuronal differentiation. Similarly, deletion of REST/NRSF in vivo in adult NSCs causes NSCs to exit from quiescence, leading to a transient increase in neurogenesis. However, the stem cell pool in the SGZ is ultimately depleted in the absence of REST/NRSF, eventually leading to reduced production of new granule neurons, suggesting that REST/NRSF acts to regulate the balance between stem cell maintenance and neuronal differentiation [34].
The transition from uncommitted NSC to neuronal lineage committed INP is signaled by the upregulation of several transcription factors. First among these is the bHLH transcription factor Neurog2, which colocalizes with a small subset of Sox2 +/Pax6 +/nestin + NSCs, presumably those committed to neurogenesis [15]. More broadly, Neurog2 expression overlaps substantially with the INP marker Tbr2, and many Neurog2 + cells also express proliferation markers such as Ki67, typical of rapidly dividing INPs [13, 15]. Neurog2 expression is generally transient and discrete in the SGZ, as it is not usually coexpressed with DCX or PSA-NCAM [15]. Little is known about the role of Neurog2 in regulating neurogenesis specifically within the adult SGZ, but Neurog2 null mutants do exhibit impaired DG development [15, 35]. Retroviral overexpression studies show that increased expression of Neurog2 promotes neuronal fate, indicating that this transcription factor is likely involved in specifying glutamatergic granule neurons in the adult DG [15].
The T-box transcription factor Tbr2 is perhaps the best-documented and most specific marker of INPs in the adult SGZ [13]. Tbr2 expression begins in type-2a INPs, where it is coexpressed with Sox2, Pax6, and Ascl1. As expression of Tbr2 increases with INP maturation, markers of neuronal commitment such as NeuroD1, DCX, and PSA-NCAM increase in parallel (Fig. 1c); [13, 15]. Consistent with expression in INPs, Tbr2 + cells are typically small with short horizontal processes, rapidly dividing, and often found in clusters in the SGZ [13]. While the function of Tbr2 in adult hippocampal neurogenesis is not currently known, Tbr2 is essential for development of the DG, suggesting that it likely has a role in regulating granule neurogenesis [36]. Indeed, ongoing studies from our laboratory suggest that Tbr2 is required in the adult SGZ for production and maturation of granule neurons. This requirement can be traced at least in part to Tbr2 control over the transition from NSC to INP (RDH and RFH, manuscript submitted).
As INPs mature to later stages and become committed to neuronal differentiation, expression of the bHLH transcription factor NeuroD1 is highly upregulated [13, 15, 37]. NeuroD1 is rarely coexpressed with Sox2 or Pax6 in DG NSCs, but is frequently present in Tbr2 + INPs [13, 15]. However, unlike Tbr2, NeuroD1 expression persists into postmitotic granule neuroblasts. Accordingly, only a subset of NeuroD1 + cells actively proliferates in the adult SGZ [15]. Consistent with expression in late stage INPs and new glutamatergic neurons, the majority of NeuroD1 + cells in the GCL coexpress DCX and PSA-NCAM, and many are positive for calretinin (Fig. 1c). NeuroD1 expression is transient in adult-born granule neurons, and as new neuroblasts mature in the GCL, NeuroD1 is downregulated [15, 37]. Expression of NeuroD1 is essential for development of the DG [38], and recent studies suggest that NeuroD1 is also a critical regulator of adult hippocampal neurogenesis [38]. In conditional NeuroD1 knockouts, adult-born granule neurons are greatly decreased because of a failure of these cells to survive and properly integrate into the adult hippocampus [38]. This loss of adult granule neurogenesis in conditional NeuroD1 knockouts does not appear to result from decreased progenitor proliferation or numbers, indicating that NeuroD1 acts mainly to promote differentiation and survival of adult-born glutamatergic neurons [38]. Intriguingly, a recent study suggests that increased expression of NeuroD1 in late-stage INPs results from Wnt/β-catenin mediated transcriptional activation and removal of Sox2-mediated repression from the NeuroD1 promoter [25].
Several Sox-family transcription factors are also expressed in INPs in the adult SGZ, although their roles in regulating adult neurogenesis have not been extensively studied (Fig. 1). Sox3, a member of the SoxB1 subgroup, is expressed in late stage DCX + INPs, some of which are actively proliferating (acute BrdU+), and in new postmitotic neuroblasts [39]. Sox3 is rapidly downregulated and, as such, is not present in NeuN + granule neurons [39]. Similarly, Sox11, a member of the SoxC subgroup, is expressed in DCX + INPs and new neuroblasts [40]. Overexpression of Sox11 in NSCs promotes neuron generation in vitro, suggesting that Sox11 may act to promote differentiation [40].
The forkhead transcription factor FoxG1 is also expressed predominantly in INPs in the SGZ [41]. Evidence of a role for FoxG1 in adult hippocampal neurogenesis comes from analyses of heterozygous FoxG1 mutant mice. BrdU labeling studies indicate a reduction in progenitor proliferation in the adult SGZ, as well as decreased numbers of new neuroblasts due to impaired neuronal differentiation, suggesting that FoxG1 may regulate both the progenitor pool in the SGZ and the maturation of adult-born granule neurons [41].
Several transcription factors have been identified as important regulators of the maturation and survival of adult-born granule neurons. Perhaps the most well studied of these transcription factors is the prospero-related homeobox gene Prox1, which is upregulated in late stage (type-3) INPs as they terminally divide and then constitutively expressed in newborn granule cells, as well as in all neurons in the GCL [42]. Several recent studies have demonstrated the requirement for Prox1 expression during adult hippocampal neurogenesis. Conditional knockout of Prox1 in the adult SGZ results in reduced numbers of DCX + and calretinin + newborn granule neurons, indicating that Prox1 is necessary for the survival and maturation of adult-born neurons [43]. This finding is confirmed by shRNA knockdown studies that show reduced glutamatergic neuron production in vitro and in the adult hippocampus in the absence of Prox1 expression [44]. Conditional Prox1 knockouts also show reduced numbers of INPs in the adult SGZ and transiently increased numbers of NSCs, suggesting that Prox1 functions indirectly to regulate the SGZ progenitor pool through feedback from postmitotic cells [43]. Interestingly, the Prox1 gene is a target of Wnt/β-catenin regulation, similar to NeuroD1 [44]. Additionally, the role of Prox1 in regulating granule neuron survival is specific to newborn adult-generated neurons, as knockdown of Prox1 in mature granule neurons does not impact their survival [44].
The T-box transcription factor Tbr1 has a very similar expression pattern to that of Prox1, although its functions during adult hippocampal neurogenesis are unknown [13]. Tbr1 is best known for its role in specifying glutamatergic pyramidal neurons in the cerebral cortex [45, 46]. In the adult SGZ, Tbr1 is upregulated in late-stage (type-3) INPs and maintained in maturing adult-born granule neurons. Like Prox1, Tbr1 is constitutively expressed by mature granule neurons, but its function in this context is also unknown [13].
Lastly, the transcription factor cAMP response-element binding protein (Creb1, CREB), a member of the CREB transcription factor family, also appears to regulate the survival and maturation of adult-born granule cells [47]. In particular, increased expression of CREB results in enhanced dendrite length and branching, whereas loss of CREB expression has the converse effect of decreasing dendritic branching [47]. Moreover, DCX and NeuroD1 are both downregulated in the SGZ following loss of CREB function, suggestive of decreased glutamatergic differentiation and survival [47].
The subventricular zone neurogenic niche
The SVZ is the larger of the two neurogenic niches in the adult brain, spanning approximately 6 mm2 in mice. During the first postnatal weeks, the germinal niche of the adult subventricular zone develops from the embryonic progenitor compartment. Radial glia (NSCs in the embryonic brain) that generated multiple cell types during embryonic neurogenesis differentiate to become a heterogeneous group of astrocyte-like NSCs that line the lateral ventricles and produce diverse types of interneurons for the olfactory bulb [6, 7]. The adult SVZ consists of primary neural stem cells (NSCs, type B cells) that give rise to rapidly dividing intermediate neuronal progenitors (INPs, type C cells) and ultimately immature neuroblasts (type A cells) (Fig. 2). Adult SVZ neurogenesis is instructed by a microenvironment of astrocytes, ependymal cells, blood vessels, extracellular matrix, cerebrospinal fluid, and microglia [48, 49]. Genetic and retroviral approaches to fate mapping have identified regional (rostral/caudal and dorsal/ventral) variations in neuronal output from the SVZ. The ventral SVZ produces deep granule cells and calbindin + periglomerular cells, while the dorsal SVZ gives rise to superficial granule cells and tyrosine hydroxylase-positive periglomerular cells, and the medial face produces calretinin-positive superficial granule and periglomerular cells [7, 8, 50]. While the majority of newborn neurons are inhibitory (GABAergic), a new subtype of glutamatergic juxtaglomerular short axon interneuron, produced in the dorsal compartment of the adult SVZ, has recently been identified [5], broadening the discussion surrounding progenitor identity and the mechanisms of differentiation during adult SVZ neurogenesis.
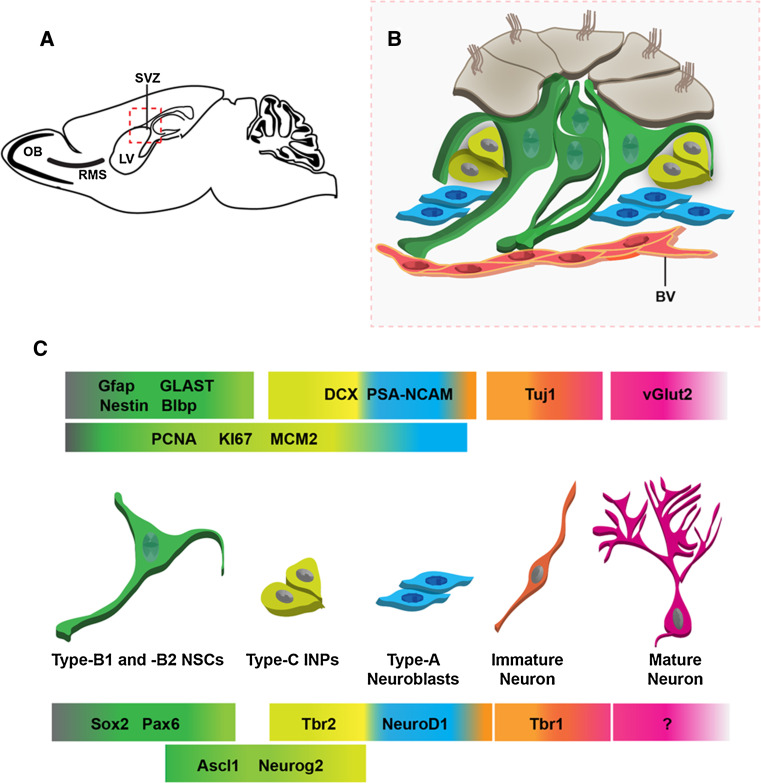
Fig. 2.
The subventricular zone (SVZ) neurogenic niche. a The SVZ neurogenic niche (dashed red box) is located adjacent to the lateral ventricle (LV). b Schematic diagram of the SVZ neurogenic niche corresponding to the red box outlined in a. Type B NSCs (green) maintain contact with the ventricle and are surrounded in a pinwheel formation by ependymal cells (brown). Type B NSCs often contact blood vessels (BV, red). NSCs give rise to type C INPs (yellow), which in turn produce type A new neuroblasts (blue). These cells migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB). c The progression from adult NSC to new adult-born OB neuron is signaled by a number of cellular markers (upper colored panels correspond to individual cell types). In the glutamatergic lineage, expression of vGlut2 is critical in mature juxtaglomerular OB interneurons (pink). Neurons in the glutamatergic lineage are specified by sequential expression of a number of transcription factors (lower colored panels) that characterize the transition from uncommitted NSC to fate specified neuron. However, there is no known transcription factor that characteristically labels adult-born glutamatergic OB neurons. Color gradients indicate overlap of transcription factor expression into multiple cell types
An array of molecular and morphological markers have been utilized to characterize different cell types and their differentiation during neurogenesis in the adult SVZ. Type-B1 NSCs extend an apical process with a nonmotile primary cilium that contacts the ventricle and a basal process that contacts blood vessels and migrating neuroblasts (type-A cells) (Fig. 2b). Type-B2 NSCs do not contact the ventricle, but maintain astrocytic morphology and ultrastructure. Although there is no marker with absolute sensitivity and specificity for each cellular subtype, NSCs in the adult SVZ typically express astrocyte-specific glutamate transporter (GLAST), Blbp, connexin-30, GFAP, vimentin, and nestin. Activated type-B NSCs divide to produce type-C INPs, which can, in the GABAergic lineages, be distinguished by their upregulation of the transcription factors Ascl1 and Dlx2, and by loss of contact with the ventricular surface (Fig. 2b). INPs (type-C cells) are highly proliferative and tend to cluster around blood vessels. Type-C INPs generate type-A migratory neuroblasts, identified by their elongated cell bodies with leading and lagging processes, and by their production of the cytoskeletal proteins DCX, PSA-NCAM, and Tuj1 (β-tubulin class III) (Fig. 2). In the adult SVZ, the type-A neuroblasts coalesce into chains within glial tubes and migrate tangentially through the rostral migratory stream (RMS) into the core of the olfactory bulb (OB). There, neuroblasts detach from chains and migrate radially to integrate at their final positions in the granule cell layer or superficial layers of the OB. The time course from birth to synaptic integration of a newborn neuron takes approximately 3 weeks in the adult mouse [51].
Transcription factors regulating adult glutamatergic SVZ neurogenesis
Recently, we and others determined that not only GABAergic, but also glutamatergic neurons are produced from the adult SVZ. In addition, there appears to be significant temporal heterogeneity with regard to the glutamatergic neuron subtypes produced during postnatal versus adult ages. Glutamatergic neurons generated from the SVZ during early postnatal development are juxtaglomerular interneurons that virtually all co-express the transcription factors Tbr2 and Tbr1 as well as either of the vesicular glutamate transporter genes, vGlut1 or vGlut2 [52]. Adult-born neurons are also juxtaglomerular interneurons, but these cells do not constitutively express Tbr2 or Tbr1, and express vGlut2 exclusively [5]. Interestingly, many of the same transcription factors are expressed during the generation of these distinct glutamatergic subtypes, suggesting that there is a conserved (albeit temporally modulated) genetic program regulating glutamatergic fate specification in the SVZ.
With some exceptions, the program of transcription factors expressed in glutamatergic lineages of the adult DG and embryonic cerebral cortex is largely conserved in the adult SVZ. Sox2 is expressed in virtually all dividing progenitors along the entire rostrocaudal axis of the adult SVZ [22]. Although not specifically studied in the glutamatergic lineage, Sox2 is important for the maintenance of the proliferative NSC pool in the SVZ, with Sox2 mutants showing a 55% decrease in proliferating cells as well as an 80% reduction in the generation of DCX+ neuroblasts [22]. Pax6 is also transiently expressed in the vast majority of proliferating, multipotent type B NSCs (GLAST+/Nestin+/GFAP+), including those that give rise to glutamatergic neurons, along the entire axis of the SVZ [5, 16] (Fig. 2c). However, expression of Pax6 is also observed in a subset of migrating neuroblasts and tyrosine hydroxylase-positive dopaminergic periglomerular neurons, demonstrating its role as a determinant of periglomerular subtype specification and survival in the adult SVZ and olfactory bulb [53–55].
Ascl1 expression is upregulated as type B NSCs transition to INPs in the adult SVZ. Although they lose the NSC marker GLAST [16], Ascl1+ cells are multipotent progenitors capable of generating neurons as well as oligodendrocytes [31, 56]. Accordingly, Ascl1 is expressed in both the GABAergic lineage, where coexpression with Pax6 is critical, and in the glutamatergic lineage. In order to specify glutamatergic fate, Ascl1 must be coexpressed with the transcription factors Neurog2 and Tbr2 [5]. Conversely, oligodendrocytes are generated from the co-expression of Olig2 with Ascl1 [56].
Neurog2 is expressed in a limited subset of progenitors along the dorsal SVZ, and expression of this transcription factor is a key determinant of glutamatergic fate specification. Neurog2 expression labels about one-third of actively dividing cells in the SVZ (presumably, mainly type-C INPs) and is coexpressed with Tbr2, and to a lesser extent with Tbr1 [5, 16]. Neuronal lineage commitment after onset of Neurog2 expression is signaled by upregulation of neuroblast marker DCX. While the function of Neurog2 has not been explicitly determined in vivo, overexpression of Neurog2 in vitro results in upregulation of Tbr1 and subsequent acquisition of glutamatergic identity [57]. The mechanisms directing GABAergic versus glutamatergic fate at this critical differentiation step in the SVZ are currently unknown, although β-catenin and Lef1 have been shown by in vitro ChIP experiments to directly bind to the Neurog1 promoter and to upregulate Neurog2, suggesting that they may be important in directing fate specification [58]. However, it may be that the adult SVZ contains distinct lineages of NSCs that are already committed to GABAergic or glutamatergic fates, as in embryonic neurogenesis. Alternatively, a single type of uncommitted NSC may give rise to multiple neuronal lineages.
Tbr2, a transcription factor implicated in glutamatergic fate specification in the cerebral cortex and DG, is also expressed in Neurog2+ INPs in the dorsal compartment of the adult SVZ [5]. Fate mapping with Ascl1 BAC-GFP transgenic mice demonstrates that Tbr2 + INPs are derived from multipotent Ascl1-expressing progenitors, although very few Tbr2+ INPs actually co-express Ascl1 protein [5]. Tbr2+ INPs are proliferative, although only about 10% are labeled acutely with BrdU, and many co-express the neuroblast marker DCX. Tbr2+ progenitors are committed to the glutamatergic lineage. It is important to note that Tbr2 is downregulated as neuroblasts exit the cell cycle, distinguishing adult born juxtaglomerular glutamatergic neurons from those that are born during embryonic and early postnatal development that constitutively express Tbr2 [5, 52].
The bHLH transcription factor NeuroD1 is also expressed in the program of glutamatergic differentiation in the SVZ. NeuroD1 is not co-expressed with Ascl1 in multipotent NSCs, but NeuroD1 expression increases with the onset of Tbr2 expression in INPs, and Tbr1 in postmitotic glutamatergic neuroblasts. NeuroD1 + cells upregulate neuroblast markers PSA-NCAM and DCX [16]. NeuroD1 also participates in the generation of GABAergic neurons, as it is co-expressed in nearly all Dlx1/2–positive cells [16]. Although there have been no functional studies of NeuroD1 in the specific context of glutamatergic neurogenesis in the SVZ, recent work in the postnatal SVZ demonstrates that NeuroD1 plays a key role in neuroblast exit from the cell cycle and terminal differentiation in the OB [59].
Tbr1 is expressed in later stages of glutamatergic neuronal differentiation in the SVZ, predominantly in post-mitotic neuroblasts derived from lineages expressing Neurog2, Tbr2, and NeuroD1 (Fig. 2c); [5]. Only approximately 1% of Tbr1+ neuroblasts are actively dividing, and virtually all Tbr1 + cells in the SVZ and RMS express DCX. Short- and long-term lineage tracing methods have demonstrated that Tbr1+ neuroblasts originate from Tbr2 + progenitors and are exclusively glutamatergic [5]. Tbr1 is downregulated during terminal differentiation of adult-born glutamatergic neuroblasts in the OB, and like Tbr2, is not constitutively expressed in adult born glutamatergic neurons. Tbr1+ neuroblasts ultimately differentiate into short axon, juxtaglomerular neurons that mediate glutamatergic transmission between glomeruli in the OB [5]. They have dendritic arbors that project over two to three neighboring glomeruli and express vGlut2 on their presynaptic terminals. These adult-born glutamatergic interneurons represent approximately 2% of the total output of adult SVZ neurogenesis [5]. Although expression of Tbr1 appears to be restricted to glutamatergic OB neurogenesis in vivo, in vitro overexpression of Tbr1 in adult OB-derived stem cells results in an increase in neurons as well as oligodendrocytes, while inhibiting astrocyte production [60]. Interestingly, astrocyte production was similarly inhibited after retroviral-mediated overexpression of Tbr1 in OB progenitors in vivo, but oligodendrocyte production was unaffected [60].
Given that the discovery of glutamatergic neurogenesis in the adult SVZ is quite recent and constitutes a minority of adult SVZ neuronal output, very little is known about the functions of the above transcription factors in this specific context. Clearly, more research is needed to define the mechanisms regulating not only glutamatergic fate determinants during adult SVZ neurogenesis, but also the lineage-specific and context-dependent effects of transcriptional programs and individual transcription factors.
Summary
Sequential expression of transcription factors defines a genetic program regulating glutamatergic neurogenesis in the adult neurogenic niches. In general, a similar sequence of transcription factors is expressed during glutamatergic neurogenesis in both the SGZ and SVZ, suggesting that the genetic program specifying glutamatergic fate is largely conserved across diverse subtypes of these neurons. Intriguingly, the pattern of transcription factor expression during adult glutamatergic neurogenesis closely resembles the sequence of transcription factors expressed during embryonic generation of cortical pyramidal neurons, suggesting that a general genetic program may regulate glutamatergic neurogenesis at many different stages of development and in many brain regions. However, it remains to be determined if the functions of individual transcription factors are strictly the same in the embryonic and adult brain, or if distinct, context-dependent functions and downstream targets may exist within individual niches. What is clear from studies of these adult and embryonic niches is that expression of Neurog2 and Tbr2 signals commitment to glutamatergic fate, and expression of NeuroD1 and Tbr1 occur at the onset of glutamatergic neuronal differentiation. Although the sequence of transcription factors expressed during glutamatergic neurogenesis in the adult niches has been well described, relatively little is known about the functions and downstream targets of individual transcription factors in this context. Specifically, it is not clear if individual transcription factors have distinct functional roles or if there is redundancy in the genetic program specifying adult-born glutamatergic neurons. Many transcription factors exhibit overlapping expression patterns during neurogenesis (NeuroD1, Tbr1, and Prox1 in the SGZ, for example), and it is currently unclear if all of these transcription factors have distinct and critical roles in regulating glutamatergic differentiation or if there might be a hierarchy in the genetic program with some transcription factors being functionally more important than others. As well, it is uncertain what mechanisms are at play in defining the transcriptional code expressed during glutamatergic neurogenesis in the adult brain, but it is likely that extrinsic, intrinsic, and epigenetic factors all have contributory roles. Thus, there is clearly much room for future work to define the roles of key transcription factors and broaden our understanding of the molecular networks that regulate adult glutamatergic neurogenesis. Experiments performed in the Hevner laboratory conformed to all current, applicable laws in the United States of America.
Acknowledgments
Research in the Hevner laboratory was supported by the National Institute of Mental Health R01 080766. R.D.H. received research fellowships from the Heart & Stroke Foundation of Canada and the American Heart Association (10POST2610067). R.J.K. is a NICHD fellow of the Pediatric Scientist Development Program (NIH K12 HD000850).
Conflict of Interest
The authors declare that no conflicts of interest exist.
Footnotes
R. D. Hodge and R. J. Kahoud contributed equally.
References
- 1.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning Mem. 2009;16(2):147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29(43):13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 4.Dranovsky A, Leonardo ED. Is there a role for young hippocampal neurons in adaptation to stress? Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascón S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Götz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12(12):1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13(6):580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 7.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101(50):17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31(8):392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouret A, Lepousez G, Gras J, Gabellec M-M, Lledo P-M. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29(39):12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kageyama R, Imayoshi I, Sakamoto M. The role of neurogenesis in olfaction-dependent behaviors. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Oboti L, Schellino R, Giachino C, Chamero P, Pyrski M, Leinders-Zufall T, Zufall F, Fasolo A, Peretto P. Newborn interneurons in the accessory olfactory bulb promote mate recognition in female mice. Frontiers Neurosci. 2011;5:113. doi: 10.3389/fnins.2011.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci USA. 2011;108(20):8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28(14):3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hevner RF, Hodge RD, Daza RAM, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55(3):223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Roybon L, Hjalt T, Stott S, Guillemot F, Li J-Y, Brundin P, Reh TA. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4(3):e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roybon L, Deierborg T, Brundin P, Li J-Y. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci. 2009;29(2):232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 17.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14(2):186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331(1):243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 20.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferri ALM, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131(15):3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 23.Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369(1):24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Favaro R, Valotta M, Ferri ALM, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12(10):1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. RBPJ-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30(41):13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nacher J, Varea E, Blasco-Ibañez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res. 2005;81(6):753–761. doi: 10.1002/jnr.20596. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa M, Takashima N, Arai Y, Nomura T, Inokuchi K, Yuasa S, Osumi N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells. 2005;10(10):1001–1014. doi: 10.1111/j.1365-2443.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- 29.Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21(5):1031–1044. doi: 10.1016/S0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 30.Englund C, Fink A, Lau C, Pham D, Daza RAM, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25(1):247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27(47):12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE. 2011;6(3):e18472. doi: 10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessberger S, Toni N, Clemenson Jr GD, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11(8):883–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, Ure K, Ding P, Nashaat M, Yuan L, Ma J, Hammer RE, Hsieh J. The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci. 2011;31(26):9772–9786. doi: 10.1523/JNEUROSCI.1604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galichet C, Guillemot F, Parras C. Neurogenin 2 has an essential role in development of the dentate gyrus. Development. 2008;135(11):2031. doi: 10.1242/dev.015115. [DOI] [PubMed] [Google Scholar]
- 36.Arnold SJ, Huang G-J, Cheung AFP, Era T, Nishikawa S-I, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22(18):2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstein DH. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci USA. 2000;97(2):865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T-W, Stromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J Comp Neurol. 2006;497(1):88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- 40.Haslinger A, Schwarz TJ, Covic M, Chichung Lie D. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci. 2009;29(11):2103–2114. doi: 10.1111/j.1460-9568.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Nam H-S, Song P, Moore H, Anderson SA. FoxG1 haploinsufficiency results in impaired neurogenesis in the postnatal hippocampus and contextual memory deficits. Hippocampus. 2006;16(10):875–890. doi: 10.1002/hipo.20218. [DOI] [PubMed] [Google Scholar]
- 42.Lavado A, Oliver G. Prox1 expression patterns in the developing and adult murine brain. Dev Dyn. 2007;236(2):518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- 43.Lavado A, Lagutin OV, Chow LML, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8(8):e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, Lie DC, Jessberger S. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2011;108(14):5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29(2):353–366. doi: 10.1016/S0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 46.Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RAM, Beyer RP, Bammler TK, Rubenstein JLR, Hevner RF. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107(29):13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29(25):7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331(1):179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 50.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 51.G-l Ming, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 52.Winpenny E, Lebel-Potter M, Fernandez ME, Brill MS, Gotz M, Guillemot F, Raineteau O. Sequential generation of olfactory bulb glutamatergic neurons by Neurog2-expressing precursor cells. Neural Dev. 2011;6:12. doi: 10.1186/1749-8104-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brill M, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick G, Ashery-Padan R, Saghatelyan A, Berninger B, Gotz M. A Dlx2- and Pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci. 2008;28(25):6439. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JLR, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27(26):6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohwi M, Osumi N, Rubenstein JLR, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25(30):6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23(22):4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berninger B, Guillemot F, Gotz M. Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur J Neurosci. 2007;25(9):2581–2590. doi: 10.1111/j.1460-9568.2007.05509.x. [DOI] [PubMed] [Google Scholar]
- 58.Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Bio. 2004;268(1):220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 59.Boutin C, Hardt O, de Chevigny A, Core N, Goebbels S, Seidenfaden R, Bosio A, Cremer H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci USA. 2010;107(3):1201–1206. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Méndez-Gómez HR, Vergaño-Vera E, Abad JL, Bulfone A, Moratalla R, de Pablo F, Vicario-Abejón C. The T-box brain 1 (Tbr1) transcription factor inhibits astrocyte formation in the olfactory bulb and regulates neural stem cell fate. Mol Cell Neurosci. 2010;46(1):108–121. doi: 10.1016/j.mcn.2010.08.011. [DOI] [PubMed] [Google Scholar]