Abstract
Background
There are no clear recommendations to guide post-treatment surveillance in patients with pancreatic cancer. Our goal was to describe the post-treatment surveillance patterns in patients undergoing curative-intent resection for pancreatic cancer.
Methods
We used SEER-Medicare linked data (1992-2005) to identify CT scans and physician visits in patients with pancreatic cancer who underwent curative resection (n= 2,393). Surveillance began 90 days after surgery and patients were followed for 2 years in 6-month periods. Patients were censored if they died, recurred, or entered hospice. Chi-square tests were two-sided.
Results
2,045 patients survived uncensored to the beginning of the surveillance period. CT scan use decreased from 20.9% of patients in month 4 to 6.4% in month 27. There was no temporal pattern in CT use to suggest regular surveillance. 23% of patients did not receive a CT scan in the year after surgery, increasing to 42% the second year. Patients who underwent adjuvant therapy and patients diagnosed in later years had higher CT scan use over the surveillance periods. Most patients visited both a primary care physician and a cancer specialist in each 6-month surveillance period. Patients who visited cancer specialists were more likely to have any CT scan and to be scanned more frequently.
Conclusions
Current surveillance patterns after resection for pancreatic cancer reflect the lack of established guidelines, implying a need for evaluation and standardization of surveillance protocols. The lack of a temporal pattern in CT testing suggests that most were obtained to evaluate symptoms rather than for routine surveillance.
Keywords: pancreatic resection, cancer surveillance, pancreatic neoplasms, computed, tomography, SEER program, Medicare
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer deaths in the U.S., with 43,140 new cases and 36,800 deaths in 2010.1 Surgical resection is the only potentially curative treatment.2 However, over 80% of patients experience recurrence within 2 years of surgery.3, 4 Distant recurrences occur in about 75% of cases, with the liver as the most common site of metastatic recurrence.5, 6 Local recurrences occur in nearly one-third of cases.5, 7, 8 Median survival following recurrence is 7 months for local and 3 months for metastatic recurrence.9
The primary goal of surveillance following curative treatment for any cancer is to detect local or distant recurrence when available interventions can prolong survival.10 Secondary goals include patient and physician reassurance, introduction of noncurative chemotherapy and/or radiation to slow disease progression, and early hospice referral when further therapy is not indicated. For pancreatic cancer, there is little evidence that early identification of metastatic disease in asymptomatic patients improves long-term survival. Current gemcitabine-based chemotherapy provides only modest survival benefit11 and local recurrences are usually not amenable to surgery.3, 5, 12
Surveillance methods used to monitor for recurrence include routine physical exam, imaging studies, and tumor marker CA 19-9 levels.9, 13 The American Society of Clinical Oncology (ASCO) does not make formal recommendations regarding post-treatment surveillance in pancreatic cancer. The National Comprehensive Cancer Network (NCCN) recommends history and physical examination for symptom assessment every 3-6 months for 2 years after curative treatment based on lower-level evidence with uniform NCCN consensus. The NCCN guidelines suggest the use of CA 19-9 determinations and follow-up CT scans every 3-6 months for 2 years after surgical resection based on lower-level evidence with non-uniform NCCN consensus.14
Little is known about current surveillance patterns after curative resection for pancreatic cancer. The purpose of this study is to describe the current population-based patterns of surveillance in patients diagnosed with locoregional pancreatic cancer and treated with curative intent. Using Surveillance, Epidemiology, and End Results (SEER) and linked Medicare claims data, we describe the use of abdominal CT scans and physician visits over a 2-year follow-up period.
METHODS
This study was approved by the Institutional Review Board at the University of Texas Medical Branch.
Data Source
SEER-Medicare data come from the linkage of two large population-based data sources, the SEER tumor registry and Medicare claims data collected by the Center for Medicare and Medicaid Services for covered health care services for Medicare beneficiaries. The SEER tumor registry, sponsored by the National Cancer Institute, is derived from specific geographic areas currently representing 28% of the U.S. population. Approximately 93% of all SEER patients older than 65 are matched with Medicare enrollment files. SEER data include information on patient demographics, clinical characteristics, and cause of death. Medicare data include information on inpatient hospital stays, physician services, hospital outpatient services, and hospice use.
Cohort Selection
We selected patients aged ≥ 66 with a first diagnosis of pancreatic adenocarcinoma (neuroendocrine and acinar cell cancers excluded) from 1992-2005 based on International Classification of Diseases for Oncology histology codes, who were enrolled in Medicare Part A and B fee-for-service coverage for 12 months before and 27 months after diagnosis. We restricted the cohort to patients with localized or regional pancreatic cancer (based on SEER historic stage) who underwent curative resection. Curative resection was identified by ICD-9-CM codes for total pancreatectomy, pancreaticoduodenectomy, distal pancreatectomy, or other pancreatic resection (Appendix).
Surveillance Testing
The surveillance period began 90 days post-surgery and patients were followed for 2 years or until last follow-up in the claims data. The 90-day lag was used to exclude tests and visits related to postoperative complications. Of the 2,393 patients who met inclusion criteria, 2,045 survived 90 days to the beginning of the surveillance period.
We defined four six-month surveillance periods: months 4-9, 10-15, 16-21, and 22-27 after the date of surgery. Patients who died, showed evidence of possible recurrence (defined below), or were referred to hospice were censored at the event date, in an attempt to identify disease-free cohorts for examination of surveillance testing. Nevertheless, we examined the overall use of CT rather than CT specifically for surveillance purposes, which cannot be determined from claims data.
For inclusion in analyses of a surveillance period, patients had to remain alive and uncensored to the end of the period. Table 1 shows the cohort size for each surveillance period and reasons for censoring. The most common reason for censoring was receipt of a new course of chemotherapy or radiation therapy. The median time to any censoring mechanism (death, hospice, or treatment for recurrence) was 9.2 months. By the end of surveillance period 4, 26.4% of patients were cumulatively censored for death, 30.4% for recurrent disease, and 24.4% for hospice enrollment; in total, 81.2% of patients were censored by the end of surveillance. Although only 26% of patients were censored for death, by the end of month 27 about 70% of patients had died. Median survival for the cohort was 14.6 months.
Table 1. Reasons for censoring from cohort of patients by surveillance period.
| Surveillance period from date of surgery |
1-3 mo. N |
% | 4-9 mo. N |
% | 10-15 mo. N |
% | 16-21 mo. N |
% | 22-27 mo. N |
% |
|---|---|---|---|---|---|---|---|---|---|---|
| Followed to end of period | 2045 | 85.5 | 1214 | 59.4 | 741 | 61.0 | 558 | 75.3 | 452 | 81.0 |
| Death | 280 | 11.7 | 193 | 9.4 | 87 | 7.2 | 39 | 5.3 | 32 | 5.7 |
| Recurrent disease | 0 | 0 | 344 | 16.8 | 254 | 20.9 | 87 | 11.7 | 38 | 6.8 |
| Enrolled in hospice | 68 | 2.8 | 294 | 14.4 | 132 | 10.9 | 57 | 7.7 | 36 | 6.5 |
| Total at start of period | 2393 | 2045 | 1214 | 741 | 558 | |||||
Abdominal/Pelvis CT Scans
Medicare claims in inpatient, outpatient, and carrier files were searched for ICD-9 and Current Procedural Terminology codes for abdominal/pelvis CT scans (Appendix). We identified CT scans done for any reason in each month of follow-up during the surveillance periods.
Physician Visits
We identified all outpatient physician visits over the surveillance period. We obtained physicians’ specialties from Medicare Health Care Financing Administration specialty claims codes. We categorized specialty as primary care physician (PCP; general practitioner, family practice, internal medicine, geriatrician), medical oncologist (medical oncology, hematology/oncology), radiation oncologist, gastroenterologist, and surgeon (general surgeon, surgical oncologist). Many patients visited multiple types of providers during a surveillance period. Therefore, we categorized patients into four exclusive categories according to the types of providers seen during each surveillance period, consistent with previous cancer surveillance research:15 PCP but no cancer specialist(medical oncologist, radiation oncologist, surgeon), cancer specialist but no PCP, cancer specialist and PCP, and neither cancer specialist nor PCP.
Adjuvant Therapy and Disease Recurrence
Adjuvant therapy was defined as a cycle of chemotherapy or radiation beginning within 6 months of surgery. ICD-9 and CPT codes were used to identify chemotherapy16 and radiation (see Appendix).
For the purposes of censoring patients at recurrence, “noncurative” therapy was defined as chemotherapy and/or radiation initiated > 6 months after the date of surgery, or initiated after the last date of adjuvant therapy for patients whose initial treatment cycle lasted > 6 months. Patients were censored at the first date of noncurative therapy.
Hospice enrollment was recorded from the Medicare Hospice file as the date of first hospice claim. We censored patients at the date of hospice enrollment, as they were no longer candidates for post-treatment surveillance.
Covariates
Sociodemographic characteristics included age, sex, race/ethnicity, marital status, income, and population of the Metropolitan Statistical Area. Zip-code level median income was obtained from the 2000 U.S. Census. Patient lymph node involvement was obtained from SEER data, and tumor location was categorized as head, body/tail, or not stated. Charlson comorbidity index was used as a measure of patient comorbidity.
Analysis
We calculated the proportion of patients receiving CT scans for each month of the surveillance period and generated bar graphs. Chi-square tests were used to examine bivariate associations between surveillance testing and demographic, tumor, and treatment characteristics. We calculated the proportion of patients with physician visits and the number of visits during each follow-up period. We examined bivariate associations between physician visits and surveillance testing during that period using chi-square tests. All P values were from two-sided tests. All statistical analysis was performed using SAS software, version 9.2 (Cary, N.C.).
RESULTS
A total of 2,045 patients survived 90 days uncensored to the beginning of the surveillance period. The mean age was 73.6 ± 5.2 years, 54.9% were women, and 84.1% were white. Cancer was located in the head of the pancreas for 74.6% of patients, in the body/tail for 14.4%, and was unspecified for 11.1%. Approximately 51.2% had node positive disease, and 58.5% underwent adjuvant therapy. More than 63% of patients had no comorbid conditions.
CT scanning
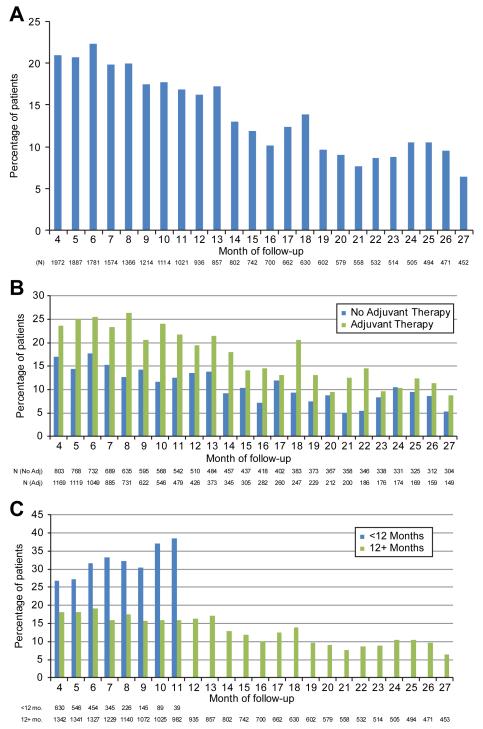
Figure 1A shows the proportion of patients who underwent a CT scan during each month of follow-up. The denominator is the number of uncensored patients at the end of each month. There is no evidence of periodicity in CT scans (i.e. spikes every 6 months) suggesting regular surveillance. The percentage of patients undergoing CT scans decreased over the surveillance period, from 20.9% in month 4 to 6.4% in month 27. Figure 1B shows CT scan use by receipt of adjuvant therapy. Patients who received adjuvant therapy were more likely to undergo CT scans over the follow-up period. Again, there was no pattern suggesting regular surveillance for either group. Figure 1C shows the pattern of CT scans by length of survival. For patients who died <12 months after surgery, CT scanning increased from 27% in month 4 to 38% in month 11. For patients who died 12 or more months after surgery, CT scanning rates were lower and gradually declined over follow-up, from 18% in month 4 to 6% in month 27.
Figure 1A-C.
Percentage of patients who received CT scan per month of follow-up among pancreatic cancer patients aged 66 years or older, Surveillance, Epidemiology, and End Results-Medicare 1992-2005: A) for the overall cohort; B) by receipt of adjuvant therapy; and C) by length of survival. Patients who died, entered hospice, or began noncurative therapy were censored at the date of event. N represents the number of patients who survived uncensored to the end of the month.
Table 2 shows CT utilization for each 6-month surveillance period and for the first 2 years of follow-up. In each period, a substantial proportion of patients (32% in months 4-9 to 62% in months 22-27) did not have a CT. Other patients appeared to receive CT scans every 3-6 months. In the 4-9 months following surgery, 60% of patients received 1-2 CT scans, while 8% received 3 or more. The percent of patients who received 1-2 CT scans in a 6-month follow-up period declined from 60% in months 4-9 to 37% in months 22-27. In the first year following surgery, 52% of patients received CT scans every 3-6 months (≥ 2 scans in 12 months), while 27% received CT scans every 3-6 months in the second year of follow-up. The average number of scans decreased somewhat over the surveillance periods, from 1.63 in months 4-9 to 1.22 in months 22-27.
Table 2. CT utilization by surveillance period in patients surviving uncensored to end of surveillance period.
| 4-9 months N = 1214 |
10-15 months N = 741 |
16-21 months N = 558 |
22-27 months N = 452 |
4-15 months N = 741 |
16-27 months N = 452 |
|
|---|---|---|---|---|---|---|
| Any CT scan | 68.5% | 57.5% | 43.2% | 38.3% | 76.9% | 58.0% |
| No CT scans | 31.6% | 42.5% | 56.8% | 61.7% | 23.1% | 42.0% |
| 1 CT | 38.4% | 39.7% | 32.3% | 31.6% | 25.4% | 30.8% |
| 2 CT scans | 21.7% | 13.1% | 8.2% | 5.3% | 26.1% | 16.8% |
| 3 CT scans | 5.3% | 3.5% | 1.6% | 1.1% | 14.0% | 7.3% |
| ≥4 CT scans | 3.1% | 1.1% | 1.1% | 0.2% | 11.5% | 3.1% |
| Mean (SD)* | 1.63 (0.89) |
1.42 (0.74) |
1.34 (0.69) |
1.22 (0.55) |
2.26 (1.32) |
1.72 (0.94) |
SD = standard deviation; CT = computed tomography
Table 3 shows the percent of patients who underwent at least one CT scan by patient characteristics for each surveillance period. Younger and white patients were more likely to receive CT scans, while those living in low income areas were less likely. Patients with node positive disease and those who underwent adjuvant therapy were more likely to receive CT scans in each surveillance period. Patients diagnosed in 2000-2005 were more likely to receive CT scans compared to those diagnosed in 1992-1999.
Table 3. CT scan utilization by patient characteristicsa in patients surviving uncensored to end of surveillance period.
| 4-9 months N= 1214 |
10-15 months N= 741 |
16-21 months N= 558 |
22-27 months N= 452 |
|
|---|---|---|---|---|
| n (%)b | n (%)b | n (%)b | n (%)b | |
| Total | 831 (68.5) | 426 (57.5) | 241 (43.2) | 173 (38.3) |
| Age | ||||
| < 75 | 519 (74.1)§ | 252 (63.0)† | 146 (48.3)† | 98 (40.5) |
| ≥ 75 | 312 (60.7) | 174 (51.0) | 95 (37.1) | 75 (35.7) |
| Race | ||||
| White | 727 (69.4) | 374 (58.4) | 218 (45.0)* | 152 (38.8) |
| Nonwhite Year of diagnosis |
104 (62.6) | 52 (51.5) | 23 (31.1) | 21 (35.0) |
| 1992-1999 | 232 (57.3) § | 123 (46.8) § | 71 (36.6)* | 51 (32.5) |
| 2000-2005 | 599 (74.0) | 303 (63.4) | 170 (46.7) | 122 (41.4) |
| Income | ||||
| 1st quartile | 169 (63.8) | 92 (55.4) | 43 (36.1) | 33 (34.9) |
| 2nd quartile | 180 (68.7) | 103 (60.2) | 54 (42.5) | 34 (32.7) |
| 3rd quartile | 196 (70.5) | 95 (58.6) | 66 (52.8) | 44 (44.9) |
| 4th quartile | 248 (69.9) | 116 (57.1) | 63 (40.4) | 55 (43.3) |
| Tumor site | ||||
| Head | 610 (68.8)* | 302 (57.9) | 157 (42.0) | 117 (39.7) |
| Body/tail | 134 (73.6) | 68 (54.4) | 50 (46.3) | 31 (33.3) |
| Not specified | 87 (60.0) | 56 (59.6) | 34 (44.7) | 25 (39.1) |
| Node | ||||
| Positive | 397 (72.7)* | 165 (59.1) | 92 (47.4) | 55 (37.4) |
| Negative | 357 (65.5) | 212 (56.7) | 118 (40.4) | 96 (39.5) |
| Adjuvant therapy | ||||
| Any adjuvant | 503 (81.3) § | 216 (71.1) § | 112 (56.0) § | 65 (43.6) |
| Surgery only | 328 (55.1) | 210 (48.1) | 129 (36.0) | 108 (35.6) |
P<0.05
P<0.01
P<0.0001
Note: P values represent the statistical significance of differences between subcategories of patient characteristics within a particular surveillance period (e.g., < 75 vs. ≥ 75 in the 4-9 month surveillance period)
Other covariates showed no statistically significant differences, and are excluded from this table.
n(%) indicates the number and percent of patients within each subcategory of patient characteristics who had a CT scan during the surveillance period indicated in the column heading. The number and percent of patients within each subcategory who did not have a CT scan are not presented in the table, but may be estimated within each subcategory and surveillance period by subtracting the percentage from 100.
Physician Visits
In the first 6-month surveillance period, 52% of patients visited both PCPs and cancer specialists, 17% visited PCPs only, 23% visited cancer specialists only, and 6% did not visit a physician (Table 4). Over subsequent periods, the percentage of patients who visited both PCPs and cancer specialists decreased to 29% in months 22-27, and the percentage of patients who visited cancer specialists only decreased to 15%. Correspondingly, the percentage of patients who visited a PCP only increased from 17% in months 4-9 to 31% in months 22-27, and the percentage of patients who had no physician visits increased from 6% to 23%. The average number of visits to cancer specialists decreased between the first and second surveillance periods. In each surveillance period, patients who visited cancer specialists were more likely to receive a CT scan compared to patients who visited PCPs only or who had no physician visits. There were also differences in CT use across cancer specialists, irrespective of PCP involvement (not shown). In each surveillance period, patients who visited multiple oncologists were most likely to have undergone a CT scan (e.g., 82.3% in months 4-9), followed by patients who visited a medical oncologist only (78.3% in months 4-9), patients who visited a surgical oncologist only (61.5% in months 4-9) and patients who had no oncologist visits (47.8% in months 4-9).
Table 4. Distribution of physician visits and CT utilization by specialty and surveillance period in patients surviving uncensored to end of period.
| 4-9 months | 10-15 months | 16-21 months | 22-27 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physician Visits | CT | Physician Visits | CT | Physician Visits | CT | Physician Visits | CT | |||||
| % | Mean (SD) | % | % | Mean (SD) | % | % | Mean (SD) | % | % | Mean (SD) | % | |
| PCP only | 17.2 | 4.56 (4.01) | 51.7 | 22.3 | 3.65 (2.73) | 34.6 | 28.0 | 3.22 (2.21) | 30.1 | 31.0 | 3.29 (2.29) | 30.0 |
| Oncology only | 23.4 | 7.10 (7.46) | 72.2 | 18.5 | 3.36 (3.47) | 68.6 | 16.1 | 2.45 (2.66) | 56.7 | 15.0 | 2.40 (2.34) | 64.7 |
| Medical | 8.7 | 6.8 | 6.5 | 6.0 | ||||||||
| Radiation | 0.6 | 0.9 | 0.4 | 0.2 | ||||||||
| Surgical | 5.6 | 5.8 | 6.5 | 6.0 | ||||||||
| Multiple | 8.6 | 5.0 | 2.9 | 2.9 | ||||||||
| PCP and oncology | 52.0 | 10.43 (7.55) | 76.6 | 47.8 | 6.19 (4.53) | 69.8 | 36.4 | 5.50 (3.45) | 61.6 | 29.4 | 5.44 (2.62) | 58.7 |
| PCP and medical | 14.2 | 17.0 | 14.3 | 11.7 | ||||||||
| PCP and radiation | 1.2 | 1.5 | 0.7 | 0.4 | ||||||||
| PCP and surgical | 16.2 | 17.1 | 11.5 | 11.5 | ||||||||
| PCP and multiple | 20.3 | 12.1 | 9.9 | 5.7 | ||||||||
| Neither PCP nor oncology |
7.4 | 38.9 | 11.5 | 32.9 | 19.5 | 16.5 | 24.6 | 8.1 | ||||
| GI physician only | 1.1 | 1.92 (0.95) | 1.1 | 2.37 (1.19) | 1.4 | 2.12 (1.55) | 1.3 | 3.67 (2.25) | ||||
| No physician | 6.3 | 10.4 | 18.1 | 23.2 | ||||||||
SD = standard deviation; PCP = primary care physician; GI = gastrointestinal
DISCUSSION
This study is the first to describe post-treatment surveillance in a large cohort of Medicare beneficiaries with locoregional pancreatic cancer. The surveillance patterns observed in this study reflect the lack of established guidelines for surveillance of pancreatic cancer patients. There was no discernible periodicity in CT scans suggestive of surveillance testing at regular intervals. In contrast, surveillance studies in breast or colon cancer patients have shown temporal patterns consistent with consensus recommendations.17, 18 CT scans and physician visits decreased over the surveillance period for all patients. Our data demonstrate a wide range of CT utilization, with 42% of long-term survivors receiving no CT scans in the second year of surveillance and 27% receiving multiple scans.
In each monthly interval during the first year of follow-up, CT scans were performed in 20% of patients. CT use was lower in patients who survived more than 12 months after surgery. In these “long-term survivors,” CT scans more likely represent surveillance, rather than diagnostic imaging. Patients who survived less than 12 months likely exhibited symptoms that would prompt CT scans from care providers. We did not examine the indication for CT scans in this study. We were more interested in utilization and temporal patterns. The accuracy of primary indication for CT scans ordered for surveillance is unclear, as physicians must specify a billable diagnosis for reimbursement. For any given patient, the indication for a CT scan may have been development of symptoms, indications unrelated to pancreatic cancer, or routine protocol/surveillance. Cooper19 examined procedures performed after curative-intent treatment in patients with cancer and found that office visits and guideline-recommended tests for local recurrence were most frequently performed for routine surveillance, while tests not recommended in guidelines were ordered to detect metastatic recurrence.
Our results showed little evidence of testing at regular surveillance intervals. Heterogeneity of patient events may have contributed to these results. Pancreatic cancer is an aggressive disease with high rates of recurrence. In many cases, physicians may have begun postoperative surveillance with the intent of testing at regular intervals, only to have this schedule disrupted by the onset of unplanned events (e.g., clinical manifestations of disease recurrence). In a supplementary analysis we identified the most common time intervals between imaging studies, using Kaplan-Meier curves (not shown) to estimate median time from surgery to first CT scan (5.67 months), from first CT scan to second CT scan (3.80 months), and from second CT scan to third CT scan (3.97 months). These results suggest that 3-4 month intervals may have been used for some patients.
The majority of patients visited a PCP and a cancer specialist in each surveillance period, though the percentage decreased over time. The percentage of patients who saw only a PCP increased to 31% by months 22-27. These results may reflect transition of care from the cancer specialist to the PCP following treatment. Our data suggest that this transition is not ideal and many patients are lost to follow-up. While the percentage of patients seeing a PCP only increased, the number of patients without physician visits quadrupled. According to an Institute of Medicine report, cancer patients are often lost to follow-up within the health care system following completion of curative-intent treatment.20 Patients who visited cancer specialists were more likely to receive CT scans. Studies have shown that surveillance procedures occur more frequently for breast and colorectal cancer patients followed by oncology specialists.21, 22
Currently, there is no clear survival benefit in the early detection of local or metastatic recurrences in pancreatic cancer. Most recurrences involve metastatic disease, and local recurrences are usually not amenable to curative resection.3, 5, 12 Treatment of local recurrences with additional resection or chemoradiotherapy does not significantly improve survival.11, 23 This study does not attempt to evaluate the effectiveness of regular surveillance since we cannot determine the reasons for CT use and would be unable to control for the obvious selection bias. Prospective clinical data are required to evaluate the comparative effectiveness of post-treatment surveillance strategies. Ideally, several methodologic approaches, including prospective clinical trials, patient registries, and medical record data collection, would be combined.
CT scanning is the standard modality for detecting pancreatic cancer recurrences following resection. The use and costs of imaging are increasing among Medicare beneficiaries. From 1999-2006, the cost of diagnostic imaging in Medicare beneficiaries with cancer grew 5.1-10.3% per year, outpacing the increase in total costs in these patients.24 It may be difficult to avoid performing CT scans in pancreatic cancer patients because they frequently experience symptoms that would prompt diagnostic imaging. Use of imaging in these patients may accomplish secondary goals of post-treatment surveillance. In the absence of evidence-based guidelines, we recommend that physicians follow the NCCN suggestion of CT scans every 3-6 months for 2 years after surgical resection. However, physicians may need to approach surveillance on a case-by-case basis according to estimated recurrence risk and likelihood for therapy benefit should recurrent disease be identified.
This study has several limitations. First, we did not attempt to distinguish surveillance imaging from diagnostic imaging because the accuracy of indications for CT scans is uncertain in administrative data. Second, the accuracy of procedure coding and physician visits in the Medicare population has not been formally examined. However, Cooper and colleagues25 demonstrated good-to-excellent concordance between medical records and administrative claims data for most procedures and examinations in cancer patients following treatment. Third, we did not examine CA 19-9 utilization because Medicare did not cover this test before 2003. Fourth, the SEER database does not contain data on cancer recurrence. We used claims for chemotherapy/radiation and hospice enrollment as indirect markers of recurrence. The high proportion of patients censored for these events suggests that our algorithm is sensitive. Fifth, the cohort was limited to Medicare beneficiaries older than 65. Finally, due to potential selection biases in administrative data, this study was unable to evaluate surveillance strategies and determine their impact on survival. However, study results showed population-based utilization patterns that were previously unknown. These data may be useful for the purposes of hypothesis generation. Potential research questions include: what is the impact of surveillance on care decisions, and what is the impact of early detection of recurrence on hospice utilization?
This was the first study to describe surveillance practice patterns in a population-based cohort of patients with pancreatic cancer. Our data show a wide range of CT utilization and demonstrate a need to evaluate current practice and standardize post-treatment surveillance. More research is necessary to determine the potential benefits and harms of post-treatment surveillance in these patients in order to improve care and limit unnecessary imaging.
Acknowledgments
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should it be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicate database.
Funding
This work was supported by the Cancer Prevention Research Institute of Texas (RP101207) and a Cancer Prevention, Control, and Population Sciences Career Development Award (1K07CA13098-01A1) from the National Cancer Institute at the National Institutes of Health.
Appendix
Surgical resection: ICD-9 procedure codes 52.6, 52.7, 52.51, 52.52, 52.53, 52.59
Abdominal/pelvis CT scan: CPT codes 74150, 74160, 74170, 74175; ICD-9 procedure codes 88.01, 88.02
Chemotherapy: ICD-9 diagnosis codes V58.1, V66.2, V67.2; ICD-9 procedure code 99.25; and Healthcare Common Procedure Coding System (HCPCS)/CPT codes Q0083-Q0085, 51720, J9000-9999, 964xx,, 965xx, J8510, J8520, J8530-J8999, G0355-G0363
Radiation: ICD-9 diagnosis codes V58.0, V66.1. V67.1; ICD-9 procedure codes: 92.21-92.29;revenue center codes: 0330, 0333; and HCPCS: 77401-77499, 77520, 77523, 77750-77799, G0256, G0261
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004 May;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 3.Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009 Aug 15;115(16):3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez JM, Morton CA, Al-Saadi S, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg. 2010 May;76(5):480–485. [PubMed] [Google Scholar]
- 5.Asiyanbola B, Gleisner A, Herman JM, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. 2009 Apr;13(4):752–759. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]
- 6.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006 Apr;10(4):511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Annals of Surgery. 2007 Jul;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008 Oct;31(5):446–453. doi: 10.1097/COC.0b013e318168f6c4. [DOI] [PubMed] [Google Scholar]
- 9.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997 Feb;21(2):195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 10.Wertheimer MD. Against minimalism in breast cancer follow-up. JAMA. 1991 Jan 16;265(3):396–397. [PubMed] [Google Scholar]
- 11.Wilkowski R, Thoma M, Bruns C, Duhmke E, Heinemann V. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP. 2006;7(1):34–40. [PubMed] [Google Scholar]
- 12.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009 Jun;35(6):600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Kang CM, Kim JY, Choi GH, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007 Jun 1;140(1):31–35. doi: 10.1016/j.jss.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.NCCN (National Comprehensive Cancer Network) NCCN Clinical Practice Guidelines in Oncology; Pancreatic Adenocarcinoma, V.2.2010. 2010 http://www.nccn.org.
- 15.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007 Mar 20;25(9):1074–1081. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001 Feb 15;19(4):1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 17.Lafata JE, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005 Jun;43(6):592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 18.Knopf KB, Warren JL, Feuer EJ, Brown ML. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc. 2001 Nov;54(5):563–571. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 19.Cooper GS, Johnson CC, Lamerato L, et al. Use of guideline recommended follow-up care in cancer survivors: routine or diagnostic indications? Med Care. 2006 Jun;44(6):590–594. doi: 10.1097/01.mlr.0000215902.50543.77. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt ME, Greenfield S, Stovall E, National Cancer Policy Board (U.S.) From cancer patient to cancer survivor: lost in transition. The National Academies Press; Washington, DC: 2006. Committee on Cancer Survivorship: Improving Care and Quality of Life. [Google Scholar]
- 21.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008 Mar 1;26(7):1073–1079. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 22.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006 Jan 1;24(1):85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 23.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Annals of Surgery. 2007 Apr;245(4):566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA. 2010 Apr 28;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 25.Cooper GS, Schultz L, Simpkins J, Lafata JE. The utility of administrative data for measuring adherence to cancer surveillance care guidelines. Med Care. 2007 Jan;45(1):66–72. doi: 10.1097/01.mlr.0000241107.15133.54. [DOI] [PubMed] [Google Scholar]