Abstract
Background
The reported high rate of local recurrence (LR) in myxofibrosarcoma raises the question of whether this sarcoma histology should be considered radioresistant. We compared rates and patterns of LR of high-grade (HG) myxofibrosarcoma to HG leiomyosarcoma, chosen due to similarity in incidence and general treatment approach.
Methods
202 patients with primary non-metastatic extremity myxofibrosarcoma (n = 114) and leiomyosarcoma (n = 88) underwent limb-sparing surgery and were prospectively followed. All 202 patients had HG tumors; 138 (68%) received adjuvant radiotherapy.
Results
The groups were comparable in age, sex, and chemotherapy use. Compared with leiomyosarcoma, myxofibrosarcoma presented more frequently with tumors >5 cm (p < 0.001), deep location (p = 0.036), and upper extremity site (p = 0.015). In addition, rates of positive/close margins (p < 0.001) and use of RT (<0.001) were significantly higher in myxofibrosarcoma. The 5-year overall LR rate was not significantly different according to histology: 14.6% for myxofibrosarcoma, 13.2% for leiomyosarcoma (p = 0.594). The only predictor of LR for the whole cohort of patients was positive/close margins (p = 0.01). Of 17 myxofibrosarcoma LRs, 8 (47%) occurred out of field, vs 1 of 12 (8%) leiomyosarcoma LRs (p=0.04). Leiomyosarcoma more commonly recurred distantly (54.1 vs 24.3% at 5 years, p=0.014)
Conclusion
Despite more adverse clinical features, myxofibrosarcoma recurred less often distantly than leiomyosarcoma whereas the LR rate was comparable between the two groups, suggesting that adjuvant RT is effective in myxofibrosarcoma. Myxofibrosarcoma LRs more commonly occurred out of field. Reduction in radiation-field margins may not be advisable in myxofibrosarcoma.
Keywords: soft tissue sarcoma, local recurrence, radiation, myxofibrosarcoma
Over the past several years there have been improvements and greater consensus in the histopathological classification of soft tissue sarcoma (STS).1 These efforts, combined with advances in molecular genetics, have led to an improved understanding of the pathogenesis of STS and, in some cases, better prediction of biological behavior and response to therapy.2–4 One example for which improved molecular analysis has impacted multidisciplinary management is myxoid liposarcoma histology. Myxoid liposarcoma is a common variant of liposarcoma with a characteristic reciprocal translocation, unique predilection for nonpulmonary skeletal metastases,5, 6 and an exquisite radiosensitivity.7–9 Notwithstanding these advances, in most cases it remains largely unclear how histopathological classification of sarcomas should impact local management.
Myxofibrosarcoma, one of the common histologic types of STS that frequently involves the extremities, has previously been reported to recur significantly more frequently than other STS histologies.10 Various series have reported local recurrence (LR) rates ranging from 32% to 60%.10–12 The perceived high propensity for LR in myxofibrosarcoma raises the important question of whether myxofibrosarcoma histology should be considered radioresistant. We therefore examined the prevalence and pattern of recurrence in high-grade (HG) extremity myxofibrosarcoma and compared these findings with patients with HG leiomyosarcoma, another common extremity STS. Leiomyosarcoma was chosen due to similarities in incidence and general treatment approach.13–15 Other histologies were considered as comparison groups, including malignant peripheral nerve sheath tumor (MPSNT) and high grade liposarcoma. These were not selected because myxoid liposarcoma is exquisitely radiosensitive.7–9 In addition, other high grade liposarcomas and MPNST are rare in comparison to myxofibrosarcoma.
MATERIALS AND METHODS
Patient and Tumor Characteristics
From April 1991 to December 2006, a total of 887 consecutive patients 16 years of age or older underwent definitive management of STS at MSKCC and met the following criteria: primary non-metastatic presentation, extremity site, HG histologic features, and limb-sparing surgery. Of these 887 patients, a total of 114 consecutive patients with HG myxofibrosarcoma and 88 consecutive patients with HG leiomyosarcoma were prospectively followed and were the subjects of this study. All patients were reviewed by a STS pathologist prior to entry into our prospective database. The 114 myxofibrosarcoma patients were derived from our prospective database by querying the different malignant fibrous histiocytoma (MFH) histologic variants. Patients with pleomorphic, storiform and MFH not otherwise specified were excluded. Of the 114 patients, 80 were entered as myxofibrosarcoma. The remaining 34 patients were re-classified as myxofibrosarcoma based on pathologic diagnosis of MFH, myxoid variant.16 Exclusion criteria included those who underwent amputation (including ray amputation for hand sarcomas), recurrent tumors, low-grade histologic features, distant metastases at presentation, or surgical resection outside of MSKCC.
The median age at presentation of the 202 patients in the study was 63 years (range, 22–95 years). There were 95 (47%) male and 107 (53%) female subjects. Tumors were considered to be in the upper extremity if they were at or beyond the shoulder (n = 52, 26%) and in the lower extremity if they were at or beyond the groin (n = 150, 74.3%). The anatomic depth of each tumor was evaluated relative to the investing superficial fascia of the extremity. A deep location of a tumor (versus superficial location) was defined as any invasion of or through the superficial fascia. Tumor size was defined as the maximal diameter of the tumor at pathologic analysis. At the time of microscopic examination of the specimen, the surgical margins were defined as positive if the tumor cells extended to the margin and close when a tumor was ≤1 mm of the surgical margin.
Treatment
The surgical technique used in this study has been previously described.17, 18 In brief, all visible or palpable disease was resected in an en-bloc fashion. Previous biopsy scars and drain sites, when present, were included in the resection. When the tumor was intermuscular or intramuscular, resection included one or more of the involved muscle bundles. For tumors situated near major neurovascular structures, resection was performed with margins limited by the lack of expendable soft tissues.
Of the 202 patients in the study, 138 (68%) received adjuvant radiation therapy. Postoperative brachytherapy (BRT) alone was given to 33 patients (24%). External beam radiation therapy (EBRT) alone was given to 101 patients (73%) and was delivered preoperatively in 7 (7%) patients and postoperatively in 94 (94%) patients. Four (3%) patients received a combination radiation therapy approach with BRT followed by EBRT. At our institution, patients with small (<5cm) superficial tumors are not routinely irradiated when negative pathological margins are obtained.
The median dose of BRT alone was 45 Gy (range, 34–45 Gy) with a median dose rate of 0.41 Gy/h. All four patients treated with BRT as a boost received 20 Gy, and their median EBRT dose was 50.4 Gy (range, 45–50.4 Gy). For the purpose of this analysis, the four patients who received BRT as a boost were included in the BRT group for a total 37 patients.
For the 94 patients undergoing postoperative EBRT alone, treatment was given 4–6 weeks after surgery to a median dose of 63 Gy (range, 50.0–70.4 Gy) at 1.8–2.5 Gy/fraction. The initial target volume included the tumor bed plus 5–10 cm margins to a dose of 45–50 Gy. This was usually followed by one or two cone downs to bring the median total dose to 63 Gy. The seven patients treated with preoperative EBRT alone received a median dose of 50 Gy (range, 46.8–50.4 Gy) in 25 fractions, followed by surgery in 4–6 weeks.
At MSKCC, chemotherapy is not generally recommended for all patients with high-grade STS of the extremity. Adriamycin-based chemotherapy was given to 23 (11%) patients. Those who received chemotherapy were generally part of early in-house protocols that were trying to address the role of adjuvant chemotherapy or they had tumors that were >10 cm.
Follow-Up
The time of follow-up was calculated from the date of the first operation at MSKCC. The median follow-up time was 48 months. In patients who were still alive at the last follow-up visit, the median follow-up time was 56 months. For patients treated with surgery alone, we defined an out-of-field LR as a LR outside the tumor bed, and for those treated with adjuvant RT as a LR outside the radiation-therapy field.
Statistical Analysis
The Fisher Exact test was used to examine differences in the clinicopathologic categorical variables between myxofibrosarcoma patients and leiomyosarcoma patients. The Wilcoxon Rank Sum test was used for the continuous variables. The cumulative incidence function was used for the competing-risks analysis to describe local and distant recurrence (death without recurrence was regarded as a competing risk).19 The recurrence time was defined as time (in months) elapsed from the date of surgery to the recurrence date, the death date, or the last follow-up date. The Kaplan-Meier method20 was used to describe overall survival and the log-rank test was used for comparing overall survival differences non-parametrically. Survival time was defined as the time (in months) elapsed from the date of surgery to the death date, or the last follow-up date. Regression analysis was performed using the Cox proportional hazards model21 for overall survival and Fine and Gray model22 for the competing-risks analysis.
RESULTS
Patients
The two groups of patients were comparable according to age, sex, and the use of chemotherapy (Table 1). Compared with HG leiomyosarcoma, however, HG myxofibrosarcoma patients presented more frequently with tumors >5 cm (78% vs 51%; p < 0.001), deep location (80% vs 66%; p = 0.036), and upper extremity site (32% vs 17%; p = 0.015). In addition, the rate of positive/close margins was significantly higher for myxofibrosarcoma (42% vs 18%; p < 0.001). Forty-one percent of patients in this study underwent excisions at outside hospitals prior to undergoing en-bloc definitive wide local excision at MSKCC. The rate of prior excision was not significantly different according to histology. It was 37% for myxofibrosarcoma compared to 47% for leiomyosarcoma, p=0.195. Fewer previously excised myxofibrosarcoma patients had negative pathological specimens following definitive limb-sparing surgery at our institution (40.5% vs 80.5%, p < 0.001). The decision on whether to give adjuvant radiation to such patients was based on the presence of adverse prognostic features. Thus, the use of adjuvant radiation was significantly higher in myxofibrosarcoma than in leiomyosarcoma (80% vs 53%, p < 0.001). The type of adjuvant radiotherapy also differed, where significantly less myxofibrosarcoma patients received BRT (20% vs 40%; p = 0.014).
Table 1.
The Distribution of Clinical Variables
| Variable | Whole Cohort | MFS | LMS | P Value |
|---|---|---|---|---|
| All | 202 | 114 | 88 | |
| Age at primary surgery | ||||
| Median (Mean) | 63 (61.3) | 63 (62.2) | 61.5 (60.2) | 0.49* |
| Range | 22–95 | 25–95 | 22–88 | |
| <50 | 47 (23.3%) | 27 (23.7%) | 20 (22.7%) | 1 |
| ≥50 | 155 (76.7%) | 87 (76.3%) | 68 (77.3%) | |
| Sex | ||||
| Male | 95 (47%) | 56 (49.1%) | 39 (44.3%) | 0.57 |
| Female | 107 (53%) | 58 (50.9%) | 49 (55.7%) | |
| Depth | ||||
| Superficial | 53 (26.2%) | 23 (20.2%) | 30 (34.1%) | 0.036 |
| Deep | 149 (73.8%) | 91 (79.8%) | 58 (65.9%) | |
| Site | ||||
| Lower | 150 (74.3%) | 77 (67.5%) | 73 (83%) | 0.015 |
| Upper | 52 (25.7%) | 37 (32.5%) | 15 (17%) | |
| Size | ||||
| ≤5 cm | 68 (33.7%) | 25 (21.9%) | 43 (48.9%) | <0.001 |
| >5 cm | 134 (66.3%) | 89 (78.1%) | 45 (51.1%) | |
| Positive or Close Margin | ||||
| No | 138 (68.3%) | 66 (57.9%) | 72 (81.8%) | <0.001 |
| Yes | 64 (31.7%) | 48 (42.1%) | 16 (18.2%) | |
| MSKCC WLE >1 | ||||
| No | 195 (96.5%) | 109 (95.6%) | 86 (97.7%) | 0.701 |
| Yes | 7 (3.5%) | 5 (4.4%) | 2 (2.3%) | |
| Prior Excision at OSH | ||||
| No | 119 (58.9%) | 72 (63.2%) | 47 (53.4%) | 0.195 |
| Yes | 83 (43.6%) | 42 (36.8%) | 41 (46.6%) | |
| Negative Re-excision | ||||
| No | 33/83 (39.8%) | 25/42 (59.5%) | 8/41 (19.5%) | <0.001 |
| Yes | 50/83 (60.2%) | 17/42 (40.5%) | 33/41 (80.5%) | |
| Adjuvant Radiation | ||||
| No | 64 (31.7%) | 23 (20.2%) | 41 (46.6%) | <0.001 |
| Yes | 138 (68.3%) | 91 (79.8%) | 47 (53.4%) | |
| RT type | ||||
| EBRT | 101/138 (73.2%) | 73/91 (80.2%) | 28/47 (59.6%) | 0.014 |
| BRT | 37/138 (26.8%) | 18/91 (19.8%) | 19/47 (40.4%) | |
| Chemotherapy | ||||
| No | 179 (88.6%) | 99 (86.8%) | 80 (90.9%) | 0.504 |
| Yes | 23 (11.4%) | 15 (13.2%) | 8 (9.1%) |
p value with * is obtained by using the Wilcoxon Rank Sum test; all other p values are obtained by using Fisher’s exact tests. MFS indicates myxofibrosarcoma; LMS, leiomyosarcoma; RT, radiotherapy; WLE, wide local excision; EBRT, external beam RT; BRT, brachytherapy; MSKCC, Memorial-Sloan-Kettering Cancer Center; OSH, outside hospital.
Local Recurrence
Of the 202 patients in the study, 29 (14.3 %) had LRs (Table 2). The median time to LR for myxofibrosarcoma and leiomyosarcoma patients was 8.2 and 12.9 months, respectively. The initial management of the 17 myxofibrosarcoma patients that recurred locally was biopsy only in three (18%) patients, wide local excision alone in six (35%) patients, wide local excision and radiation in four (22%) patients, and amputation in four (22%) patients. For the 12 leiomyosarcoma local recurrences, the initial management was biopsy only in 6 (50%), wide local excision alone in 2 (17%), wide local excision and radiation in 3 (25%), and amputation in 1 (8%). Thirty-five percent (6/17) of patients with myxofibrosarcoma who developed LRs went on to develop subsequent and sometimes multiple LRs at the same site, compared with none in the leiomyosarcoma group (p = 0.056). In addition, 41% (7 of 17) of myxofibrosarcoma patients that recurred locally eventually required amputation, compared with 8% (1 of 12) for leiomyosarcoma (p = 0.09).
Table 2.
Univariate Competing-Risks Analysis for Local Recurrence
| Variable | N | 5-Year CIF for LR (95% CI) | Hazard Ratio | p Value |
|---|---|---|---|---|
| All | 29/202 | 14% (8.9%–19%) | ||
| Sex | ||||
| Male | 13/95 | 13.8% (6.4%–21.2%) | Reference level | 0.781 |
| Female | 16/107 | 14.1% (7.1%–21.1%) | 1.1 (0.53–2.28) | |
| Age | ||||
| <50 | 5/47 | 11.1% (1.8%–20.4%) | Reference level | 0.452 |
| ≥50 | 24/155 | 14.8% (8.9%–20.8%) | 1.45 (0.55–3.81) | |
| Size | ||||
| ≤5cm | 7/68 | 9.7% (2.1%–17.2%) | Reference level | 0.186 |
| >5cm | 22/134 | 16.2% (9.6%–22.8%) | 1.75 (0.76–4.03) | |
| Site | ||||
| Lower | 22/150 | 15.3% (9.1%–21.4%) | Reference level | 0.861 |
| Upper | 7/52 | 9.9% (1.6%–18.2%) | 0.92 (0.4–2.14) | |
| Histology | ||||
| Myxofibrosarcoma | 17/114 | 14.6% (7.6%–21.6%) | Reference level | 0.594 |
| Leiomyosarcoma | 12/88 | 13.2% (5.8%–20.6%) | 0.82 (0.4–1.71) | |
| Depth | ||||
| Superficial | 5/53 | 9.2% (0.1%–18.2%) | Reference level | 0.233 |
| Deep | 24/149 | 15.8% (9.6%–21.9%) | 1.77 (0.68–4.59) | |
| Positive/Close margin | ||||
| No | 14/138 | 9.5% (4.3%–14.7%) | Reference level | 0.01 |
| Yes | 15/64 | 23.7% (12.5%–34.9%) | 2.55 (1.24–5.24) | |
| Negative Re-excision | ||||
| No | 25/152 | 16.4% (10.1%–22.7%) | Reference level | 0.157 |
| Yes | 4/50 | 6.4% (−0.7%–13.4%) | 0.48 (0.17–1.35) | |
| Adjuvant RT | ||||
| No | 12/64 | 20.1% (9.6%–30.6%) | Reference level | 0.185 |
| Yes | 17/138 | 11.1% (5.5%–16.7%) | 0.61 (0.29–1.27) | |
| Chemotherapy | ||||
| No | 27/179 | 14.6% (9.1%–20.1%) | Reference level | 0.426 |
| Yes | 2/23 | 9.1% (−3.2%–21.4%) | 0.57 (0.14–2.38) | |
| RT type | ||||
| EBRT | 12/101 | 11.6% (4.6%–18.6%) | Reference level | 0.925 |
| BRT | 5/37 | 11.1% (0.7%–21.5%) | 1.01 (0.35–2.94) |
CI indicates confidence interval; LR, local recurrence; CIF, cumulative incidence function; RT, radiotherapy; EBRT, external beam RT; BRT, brachytherapy
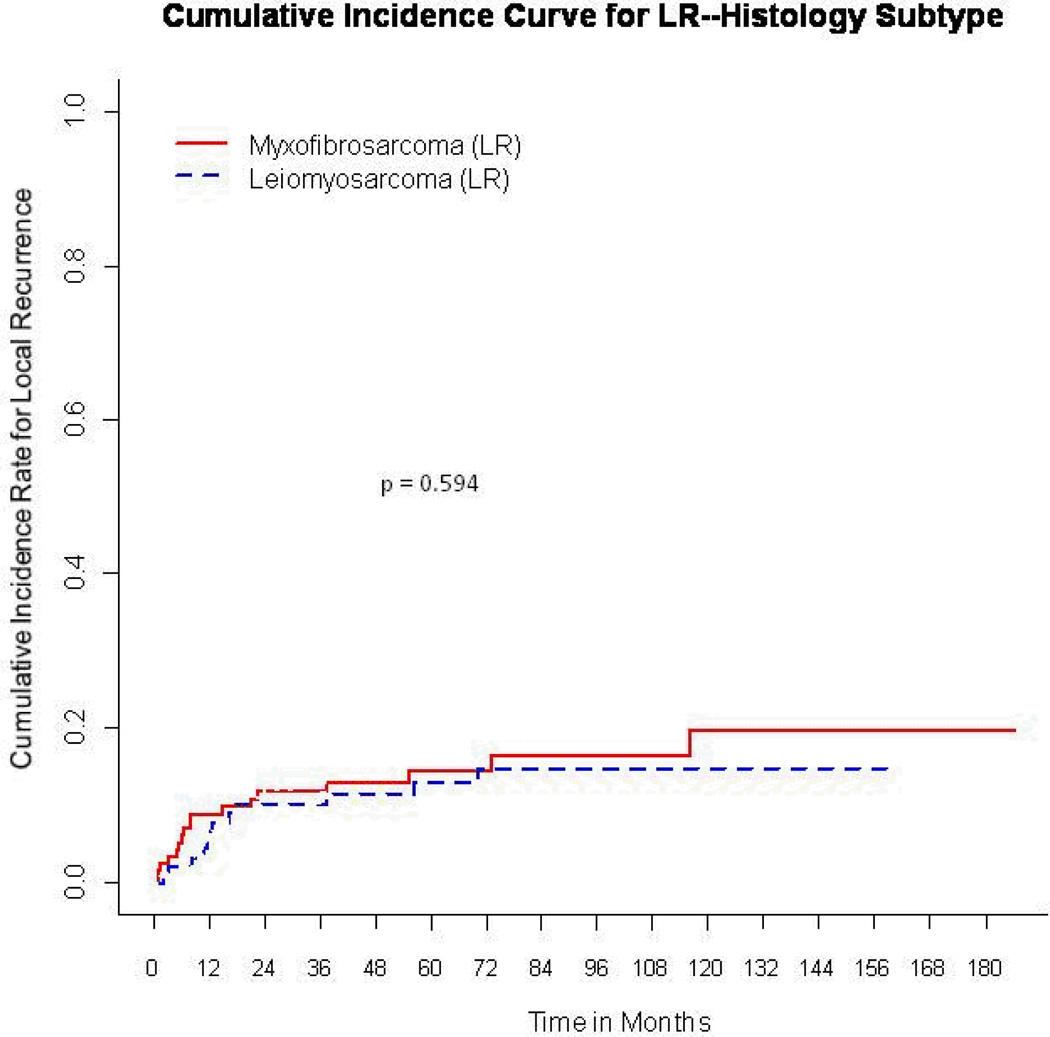
With a median follow-up of 53 months for event-free survivors, the 5-year cumulative incidence for LR was 14% (95% CI, 8.9%–19%). The LR rate was 14.6% (95% CI, 7.6%–21.6%) for myxofibrosarcoma, compared with 13.2% (95% CI, 5.8%–20.6%) for leiomyosarcoma, p = 0.47 (Fig. 1).
Figure 1.
Cumulative incidence curves for local recurrence (LR) according to histology.
On univariate competing-risk analysis, the only significant predictor of LR for the whole cohort of patients (n = 202) was positive/close margins, where the rate was 23.7% (95% CI, 12.5%–34.9%) vs 9.5% (95% CI, 4.3%–14.7%) for negative margins (p = 0.016). None of the other factors analyzed including age, tumor size, tumor site, depth, the use of chemotherapy, radiation therapy, or radiation-therapy type were significant predictors of LR. Patients who received adjuvant RT had a significantly higher rate of positive/close margins (39% vs 16%; p = 0.001), tumors >5 cm (82% vs 33%; p < 0.001), and deep location (86% vs 47%; p < 0.001), and were more likely to receive adjuvant chemotherapy (16% vs 2%; p = 0.003) than those who did not have adjuvant radiation therapy. In the myxofibrosarcoma group, the type of adjuvant radiation therapy did not significantly impact LR. The 5-year rate of LR was 17.6% for those treated with BRT, compared with 11.7% for those treated with EBRT (p = 0.359).
Patterns of Local Failure
Despite the lack of difference in the rates of LR according to histology, the patterns of LR did differ (Table 3). For those treated with surgery alone, an out-of-field LR was defined as a LR outside the tumor bed, and for those treated with adjuvant RT as a LR outside the radiation-therapy field. Out of 17 myxofibrosarcoma LRs, 8 (47%) were out of field. In comparison, just 1 of 12 (8%) leiomyosarcoma LRs occurred out of field (p = 0.04). Of the eight out-of-field LRs in myxofibrosarcoma, four occurred following EBRT, two following BRT, and two in patients who did not receive adjuvant RT.
Table 3.
The Patterns of Local Recurrence
| Variable | MFS (17/114) | LMS (12/88) | p Value |
|---|---|---|---|
| In field | 53% (9/17) | 92% (11/12) | p = 0.04 |
| Out of field | 47% (8/17) | 8% (1/12) |
p value is obtained by using Fisher’s exact test. MFS indicates myxofibrosarcoma; LMS, leiomyosarcoma.
Distant Control
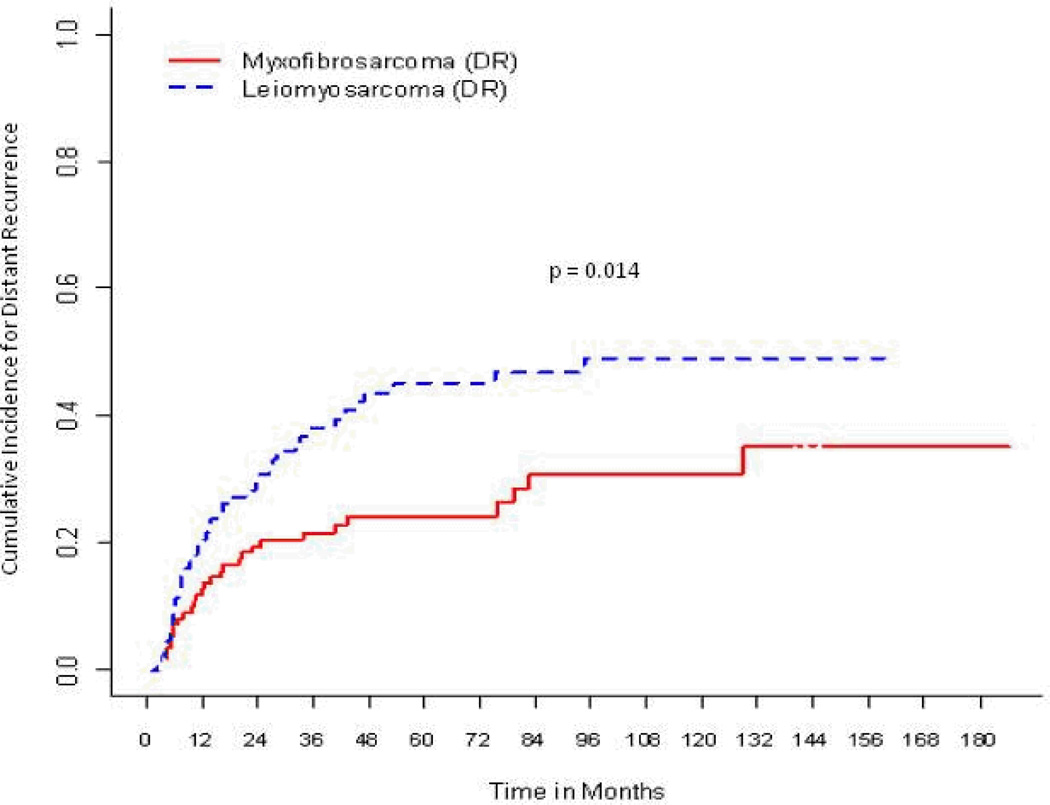
Distant metastasis developed in 69/202 (34%) of patients. With a median follow-up time of 55 months in the event-free survivors, the 5-year cumulative incidence function for distant recurrence was 34% (95% CI, 27%–41%). The influence of histology on distant metastasis was as follows (Fig. 2): for myxofibrosarcoma, the 5-year cumulative incidence was 24.3% (95% CI, 15.8%–32.8%) compared with 45.1% (95% CI, 34.2%–56%) for leiomyosarcoma (p=0.014). Other significant factors associated with distant recurrence on univariate competing-risks analyses (Table 4) were tumor size >5 cm (44.1% vs 14.7%; p < 0.001), deep location (37.9% vs 22.7%; p = 0.034), and positive/close margins (31.6% vs 18.4%; p = 0.024), whereas a negative pathologic specimen was associated with reduced risk of distant recurrence (21.8% vs 37.9%; p = 0.009). On multivariate analysis, tumor size >5 cm, with a hazard ratio of 4.05 (95% CI, 2.11%–7.78%; p < 0.001), and leiomyosarcoma histology, with a hazard ratio of 2.89 (95% CI, 1.78%–4.68%; p < 0.001), remained significantly associated with distant recurrence.
Figure 2.
Cumulative incidence curves for distant recurrence (DR) according to histology.
Table 4.
Univariate competing risks analysis for distant recurrence for all 202 patients
| Variable | N | 5-Year CIF for DR (95% CI) | Hazard Ratio | p Value |
|---|---|---|---|---|
| All | 69/202 | 34% (27%–41%) | ||
| Sex | ||||
| Male | 32/95 | 32% (22%–41.9%) | Reference level | 0.865 |
| Female | 37/107 | 35.8% (26%–45.7%) | 1.04 (0.65–1.66) | |
| Age | ||||
| <50 | 14/47 | 29.2% (15.5%–42.8%) | Reference level | 0.629 |
| ≥50 | 55/155 | 35.3% (27.3%–43.4%) | 1.6 (0.64–2.1) | |
| Size | ||||
| ≤5cm | 11/68 | 14.7% (5.7%–23.8%) | Reference level | <0.001 |
| >5cm | 58/134 | 44.1% (35%–53.2%) | 3.56 (1.1–6.64) | |
| Site | ||||
| Lower | 57/150 | 37.7% (29.4%–46%) | Reference level | 0.064 |
| Upper | 12/5 | 23% (10.8–35.3%) | 0.56 (0.3–1.05) | |
| Histology | ||||
| Myxofibrosarcoma | 29/114 | 24.3% (15.8%–32.8%) | Reference level | 0.014 |
| Leiomyosarcoma | 40/88 | 45.1% (34.2%–56%) | 1.82 (1.13–2.92) | |
| Depth | ||||
| Superficial | 12/53 | 22.7% (10.7%–34.6%) | Reference level | 0.034 |
| Deep | 57/149 | 37.9% (29.6%–46.2%) | 1.91 (1.04–3.51) | |
| Positive/Close margins | ||||
| No | 41/138 | 31.4% (23%–39.7%) | Reference level | 0.048 |
| Yes | 28/64 | 39.5% (26.9%–52.1%) | 1.63 (1.01–2.62) | |
| Negative Re-excision | ||||
| No | 60/152 | 37.9% (29.7%–46%) | Reference level | 0.009 |
| Yes | 9/50 | 21.8% (8.7%–34.8%) | 0.41 (0.21–0.82) | |
| Adjuvant RT | ||||
| No | 18/64 | 27.4% (15.7%–39.2%) | Reference level | 0.245 |
| Yes | 51/138 | 37% (28.4%–45.6%) | 1.37 (0.8–2.35) | |
| Chemotherapy | ||||
| No | 58/179 | 32.2% (24.9%–39.5%) | Reference level | 0.087 |
| Yes | 11/23 | 48.6% (25.6%–71.5%) | 1.78 (0.95–3.35) | |
| RT type | ||||
| EBRT | 36/101 | 37.9% (27.4%–48.4%) | Reference level | 0.828 |
| BRT | 15/37 | 36.9% (20.6%–53.1%) | 1.09 (0.58–2.02) |
CI indicates confidence interval; DR, distant recurrence; CIF, cumulative incidence function; RT, radiotherapy; EBRT, external beam RT; BRT, brachytherapy
Survival
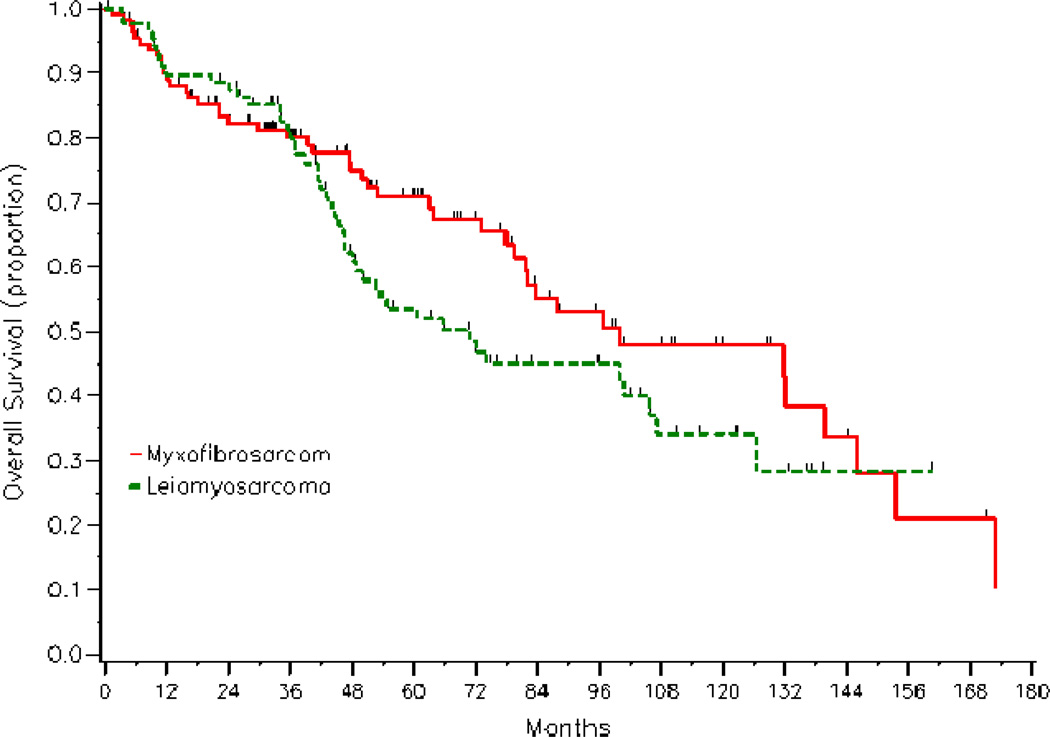
With a median follow-up of 48 months, 92 deaths were recorded among the 202 patients (45.5%) in the study. The 5-year survival rate for all patients was 62.4% (95% CI, 54.4%–69.3%). On univariate analyses, significant adverse prognostic factors for survival were tumor size >5 cm (53.4% vs 79.2%; p = 0.001), and positive/close margins (55.1% vs 65.8%; p = 0.034), whereas a negative pathologic specimen was associated with improved 5-year survival rate (73.1% vs 58.9%; p = 0.034). Histology did not impact survival (Fig.3), nor did patient age, tumor site, depth, sex, adjuvant radiation, and systemic chemotherapy (Table 5). On multivariate analyses, tumor size >5 cm, with a hazard ratio of 1.72 (95% CI, 1.03%–2.87%), remained a significant adverse feature.
Figure 3.
Kaplan-Meier overall survival curves according to histology.
Table 5.
Univariate Overall Survival Analyses for All Patients
| Variable | Events/N | 5-Year Survival Rate (95%CI) | Hazard Ratio (95%CI) |
p Value |
|---|---|---|---|---|
| All | 92/202 | 62.4% (54.4%–69.3%) | ||
| Sex | ||||
| Male | 45/95 | 65.4% (53.7%–74.8%) | Reference level | 0.576 |
| Female | 47/107 | 59.5% (48.2%–69.1%) | 1.13 (0.74–1.71) | |
| Age at primary surgery | ||||
| <50 | 16/47 | 66% (48.3%–79%) | Reference level | 0.13 |
| ≥50 | 76/155 | 61.3% (52.1%–69.2%) | 1.52 (0.88–2.6) | |
| Size | ||||
| ≤5cm | 22/68 | 79.2% (65.5%–88%) | Reference level | 0.001 |
| >5cm | 70/134 | 53.4% (43.5%–62.4%) | 2.2 (1.36–3.57) | |
| Site | ||||
| Lower | 73/150 | 58.2 (48.9%–66.4%) | Reference level | 0.217 |
| Upper | 19/52 | 74.8% (58.6%–85.4%) | 0.73 (0.4–1.21) | |
| Histology Subtype | ||||
| Myxofibrosarcoma | 46/114 | 70.8% (60.3%–79%) | Reference level | 0.157 |
| Leiomyosarcoma | 46/88 | 53.5% (41.6%–64%) | 1.35 (0.89–2.05) | |
| Depth | ||||
| Superficial | 20/53 | 67.2% (49.8%–79.7%) | Reference level | 0.106 |
| Deep | 72/149 | 60.6% (51.4%–68.6%) | 1.51 (0.91–2.5) | |
| Positive/Close Margin | ||||
| No | 57/138 | 65.8% (56.1%–73.9%) | Reference level | 0.034 |
| Yes | 35/64 | 55.1% (40.7%–67.3%) | 1.58 (1.03–2.41) | |
| Negative Re-excision | ||||
| No | 78/152 | 58.9% (49.8%–67%) | Reference level | 0.036 |
| Yes | 14/50 | 73.1% (55.5%–84.7%) | 0.55 (0.31–0.97) | |
| Adjuvant Radiation | ||||
| No | 26/64 | 61% (45.8%–73.2%) | Reference level | 0.497 |
| Yes | 66/138 | 62.9% (53.3%–71%) | 1.17 (0.74–1.85) | |
| Primary Chemotherapy | ||||
| No | 80/179 | 62.5% (54.1%–69.8%) | Reference level | 0.389 |
| Yes | 12/23 | 62.1% (35.9%–80.1%) | 1.31 (0.71–2.4) | |
| RT Type | ||||
| EBRT | 46/101 | 61.7% (49.9%–71.5%) | Reference level | 0.18 |
| BRT | 20/37 | 65.4% (47.1%–78.7%) | 0.68 (0.39–1.2) | |
CI indicates confidence interval; CIF, cumulative incidence function; RT, radiotherapy
DISCUSSION
The LR rates for STS of the extremity from previously reported experiences of pooled histological subtypes ranges from 10% to 30%.14, 18, 23–26 In the current study, the 5-year incidence of LR of 114 HG myxofibrosarcoma patients was 14.6% (95% CI, 8.9%–19%), which was not significantly different (p = 0.59) from the LR rate of the 88 HG leiomyosarcoma patients treated during the same era (13.2%; 95% CI, 5.8%–20.6%). The lack of difference in LR was observed despite significantly more adverse features in the myxofibrosarcoma group, where significantly more patients had positive/close margins (42 vs 18%; p < 0.001) and tumors >5 cm (78% vs 51%; p < 0.001) than the leiomyosarcoma group. Others, however, have reported substantially higher LR rates for myxofibrosarcoma, ranging from 32% to 60%.10–12, 27, 28
Perhaps the greater propensity for LR of myxofibrosarcoma reported in the literature might be related to the infrequent use of adjuvant radiation despite the adverse clinical and pathologic features of myxofibrosarcoma commonly seen at presentation.25, 29 In one series from Sweden, just 10% of the 109 myxofibrosarcoma patients who underwent primary surgical treatment received adjuvant radiation. With a median follow-up time of >5 years, the overall LR rate was 52%.27 In a recently published large contemporary series of extremity STS from the National Tumor Institute in Milan Italy, myxofibrosarcoma histology was associated with the poorest local outcomes. The 10-year estimate for LR was 32%, and the hazard ratio for local relapse-free survival for myxofibrosarcoma was 2.60 (95% CI, 1.38%–4.88%) relative to leiomyosarcoma. While the authors did not report the percentage of myxofibrosarcoma patients who received adjuvant RT, just 45% of all patients in the study and only 56% of those with positive or close margins were irradiated. 10 In contrast, 80% of myxofibrosarcoma patients overall and 90% of myxofibrosarcoma with positive or close margins were treated with adjuvant radiation in the current study, yielding a 14.6% 5-year LR rate.
Although LR rates were similar for the myxofibrosarcoma and the leiomyosarcoma groups in the current study, the locations of the local relapse, in relationship to the surgical bed or RT field, were different. For those treated with surgery alone, an out-of-field LR was defined as recurrence outside the tumor bed, and for those treated with adjuvant RT it was defined as recurrence outside the radiationtherapy field. Of the 17 LRs in the myxofibrosarcoma group, 47% (n = 8) were out of field, compared with only 8% (1/12) in the leiomyosarcoma group (p = 0.04). Another unique feature of the LR of myxofibrosarcoma was that once LR developed, the chance of subsequent LR was high. In the current study, 35% (6/17) of patients with myxofibrosarcoma who recurred locally went on to develop subsequent and sometimes multiple LRs at the same site, compared with none in the leiomyosarcoma group (p=0.056). This explains, at least in part, why 41% (7 of 17) of myxofibrosarcoma patients that recurred locally eventually required amputation, compared with 8% (1 of 12) for leiomyosarcoma (p = 0.09). In addition, the 5-year rate of distant relapse was significantly less in the myxofibrosarcoma group compared with the leiomyosarcoma group (24.3% vs. 45.1%, respectively; p = 0.014), further highlighting the importance of establishing local control. In future studies, more homogeneous populations will be required to identify patients most likely to benefit from chemotherapy. Our results suggest that patients with leiomyosarcoma histology may have more potential than myxofibrosarcoma to benefit from novel strategies aimed at reducing systemic relapse.
Taken together, the results of the current study imply that radiation is an effective adjuvant treatment in patients with myxofibrosarcoma and should be considered in the local management of this disease. The question then is not whether myxofibrosarcoma is radioresistant, but rather why there is a distinct pattern of LR.
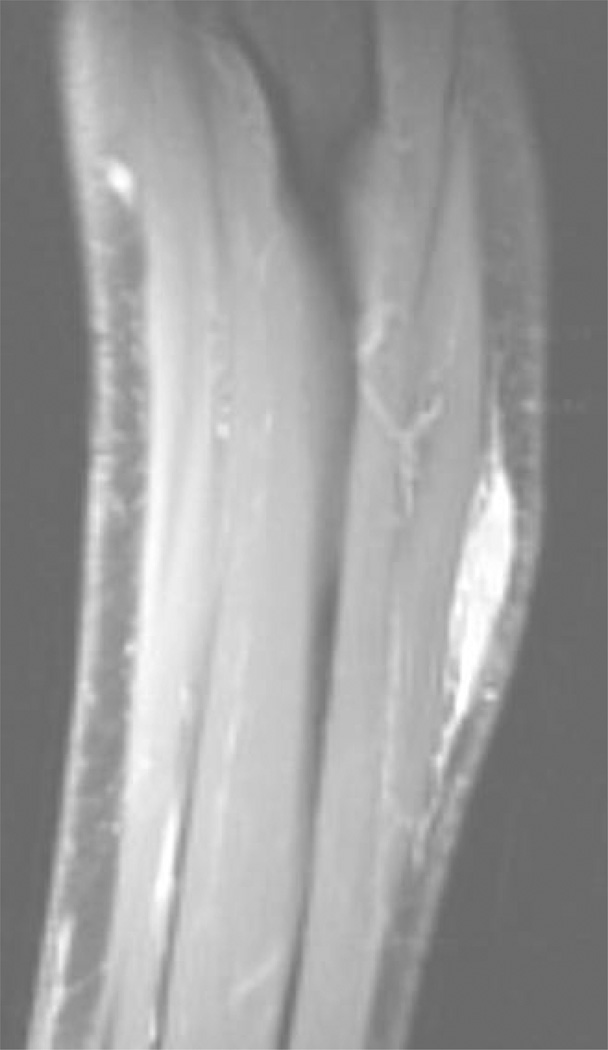
Some experts have suggested that the perceived high propensity of myxofibrosarcoma to recur locally is due to a characteristic infiltrative growth pattern, with extension along vascular and fascial planes.10, 11, 30–32 Authors have called for careful attention to easily overlooked tail-like extensions of myxofibrosarcoma disease on MRI,30 as shown in Fig. 4. In addition, some myxofibrosarcomas appear to be superficial on clinical examination or imaging, but they often involve the investing fascia, thus making them pathologically deep according to the AJCC guidelines. In surgical planning for resection of myxofibrosarcoma, it is a challenge to delineate the scope of the disease along fascial planes, as well as microscopic extension into the dermis and skeletal muscles.31, 33 Consequently, the entire extent of tumor infiltration may not be encompassed in the resection margin, resulting in high rates of positive margins, as reported here. Similarly, myxofibrosarcoma presents a targeting challenge for radiation oncologists attempting to ensure that all potential microscopic disease is encompassed in the planning target volume. Recently, there has been increasing interest in utilizing smaller radiation fields to treat STS of the extremity in an effort to decrease both acute and late radiation toxicity while preserving rates of local control.34, 35 These approaches have been facilitated by advances in the delivery of EBRT, particularly with the use of image-guided radiation therapy (IGRT).36, 37 Likely due to a greater number of adverse features, fewer myxofibrosarcoma patients received adjuvant brachytherapy compared with leiomyosarcoma in our study. Given the small number of highly selected myxofibrosarcoma patients (n=18) treated with brachytherapy and the smaller volume treated with that technique, we cannot rule out the possibility that the finding of a 6% absolute difference in LR in favor of EBRT, although not statistically significant, could have important clinical implications. The characteristic growth pattern and propensity of myxofibrosarcoma to recur out of field indicate that reduction in field margins may not be advisable in extremity myxofibrosarcoma. In the future, molecular profiling may enable better understanding of the patterns of growth and recurrence of this disease, which appears to represent unique tumor biology.38
Figure 4.
Sagittal magnetic resonance image of an extremity myxofibrosarcoma involving the forearm.
In conclusion, compared with leiomyosarcoma, myxofibrosarcoma commonly had adverse clinical and pathological characteristics, including a higher rate of positive margins following resection. Despite these differences, local control was comparable between the two groups, likely due to the frequent and effective use of adjuvant radiation. Our results suggest that extremity myxofibrosarcoma is not clinically inherently radioresistant and that adjuvant radiation should play an important role in the management of this histology.
Footnotes
The authors have no financial disclosures
References
- 1.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 2.Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25(7):571–576. doi: 10.1016/s0145-2126(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 3.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3(9):685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 4.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9291):1421–1423. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 5.Schwab JH, Boland P, Guo T, et al. Skeletal metastases in myxoid liposarcoma: an unusual pattern of distant spread. Ann Surg Oncol. 2007;14(4):1507–1514. doi: 10.1245/s10434-006-9306-3. [DOI] [PubMed] [Google Scholar]
- 6.Knight JC, Renwick PJ, Dal Cin P, Van den Berghe H, Fletcher CD. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995;55(1):24–27. [PubMed] [Google Scholar]
- 7.Chung PW, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115(14):3254–3261. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
- 8.Guadagnolo BA, Zagars GK, Ballo MT, et al. Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):760–765. doi: 10.1016/j.ijrobp.2007.07.2337. [DOI] [PubMed] [Google Scholar]
- 9.Pitson G, Robinson P, Wilke D, et al. Radiation response: an additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys. 2004;60(2):522–526. doi: 10.1016/j.ijrobp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506–511. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]
- 11.Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20(4):391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Angervall L, Kindblom LG, Merck C. Myxofibrosarcoma. A study of 30 cases. Acta Pathol Microbiol Scand A. 1977;85A(2):127–140. [PubMed] [Google Scholar]
- 13.Svarvar C, Bohling T, Berlin O, et al. Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer. 2007;109(2):282–291. doi: 10.1002/cncr.22395. [DOI] [PubMed] [Google Scholar]
- 14.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. Journal of Clinical Oncology. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 15.Massi D, Beltrami G, Mela MM, Pertici M, Capanna R, Franchi A. Prognostic factors in soft tissue leiomyosarcoma of the extremities: a retrospective analysis of 42 cases. Ejso. 2004;30(5):565–572. doi: 10.1016/j.ejso.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Erlandson RA, Antonescu CR. The rise and fall of malignant fibrous histiocytoma. Ultrastruct Pathol. 2004;28(5–6):283–289. doi: 10.1080/019131290882150. [DOI] [PubMed] [Google Scholar]
- 17.Brennan MF, Hilaris B, Shiu MH, et al. Local recurrence in adult soft-tissue sarcoma. A randomized trial of brachytherapy. Arch Surg. 1987;122(11):1289–1293. doi: 10.1001/archsurg.1987.01400230075014. [DOI] [PubMed] [Google Scholar]
- 18.Pisters PWT, Harrison LB, Leung DHY, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. Journal of Clinical Oncology. 1996;14(3):859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 19.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 21.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34(2):187. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 23.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. Journal of Clinical Oncology. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. Journal of Clinical Oncology. 1996;14(3):869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 26.Fleming JB, Berman RS, Cheng SC, et al. Long-term outcome of patients with American Joint Committee on Cancer stage IIB extremity soft tissue sarcomas. Journal of Clinical Oncology. 1999;17(9):2772–2780. doi: 10.1200/JCO.1999.17.9.2772. [DOI] [PubMed] [Google Scholar]
- 27.Merck C, Angervall L, Kindblom LG, Oden A. Myxofibrosarcoma-a Malignant Soft-Tissue Tumor of Fibroblastic-Histiocytic Origin - a Clinicopathologic and Prognostic Study of 110 Cases Using Multivariate- Analysis. Acta Pathologica Microbiologica Et Immunologica Scandinavica Section a-Pathology. 1983;91:1. [PubMed] [Google Scholar]
- 28.Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977;39(4):1672–1685. doi: 10.1002/1097-0142(197704)39:4<1672::aid-cncr2820390442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Alektiar KM, Brennan MF, Singer S. Influence of site on the therapeutic ratio of adjuvant radiotherapy in soft-tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2005;63(1):202–208. doi: 10.1016/j.ijrobp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Waters B, Panicek DM, Lefkowitz RA, et al. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol. 2007;188(2):W193–W198. doi: 10.2214/AJR.05.1130. [DOI] [PubMed] [Google Scholar]
- 31.Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum Pathol. 2004;35(5):612–621. doi: 10.1016/j.humpath.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Kaya M, Wada T, Nagoya S, et al. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skeletal Radiol. 2008;37(12):1085–1090. doi: 10.1007/s00256-008-0542-4. [DOI] [PubMed] [Google Scholar]
- 33.Mansoor A, White CR., JR Myxofibrosarcoma presenting in the skin: clinicopathological features and differential diagnosis with cutaneous myxoid neoplasms. Am J Dermatopathol. 2003;25(4):281–286. doi: 10.1097/00000372-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Krasin MJ, Davidoff AM, Xiong X, et al. Preliminary results from a prospective study using limited margin radiotherapy in pediatric and young adult patients with high-grade nonrhabdomyosarcoma softtissue sarcoma. Int J Radiat Oncol Biol Phys. 2010;76(3):874–878. doi: 10.1016/j.ijrobp.2009.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim B, Chen YL, Kirsch DG, et al. An effective preoperative three-dimensional radiotherapy target volume for extremity soft tissue sarcoma and the effect of margin width on local control. Int J Radiat Oncol Biol Phys. 2010;77(3):843–850. doi: 10.1016/j.ijrobp.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 36.Griffin AM, Euler CI, Sharpe MB, et al. Radiation planning comparison for superficial tissue avoidance in radiotherapy for soft tissue sarcoma of the lower extremity. Int J Radiat Oncol Biol Phys. 2007;67(3):847–856. doi: 10.1016/j.ijrobp.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 37.Alektiar KM, Hong L, Brennan MF, Della-Biancia C, Singer S. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: preliminary results. Int J Radiat Oncol Biol Phys. 2007;68(2):458–464. doi: 10.1016/j.ijrobp.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 38.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]