SUMMARY
Defining the mechanisms underlying the control of mitochondrial fusion and fission is critical to understanding cellular adaptation to diverse physiological conditions. Here we demonstrate that hypoxia induces fission of mitochondrial membranes, dependent on availability of the mitochondrial scaffolding protein AKAP121. AKAP121 controls mitochondria dynamics through PKA-dependent inhibitory phosphorylation of Drp1 and PKA-independent inhibition of Drp1-Fis1 interaction. Reduced availability of AKAP121 by the ubiquitin ligase Siah2 relieves Drp1 inhibition by PKA and increases its interaction with Fis1, resulting in mitochondrial fission. High AKAP121 levels, seen in cells lacking Siah2, attenuate fission and reduce apoptosis of cardiomyocytes under simulated ischemia. Infarct size and degree of cell death were reduced in Siah2−/− mice subjected to myocardial infarction. Inhibition of Siah2 or Drp1 in hatching C. elegans reduces their life span. Through modulating Fis1/Drp1 complex availability, our studies identify Siah2 as a key regulator of hypoxia-induced mitochondrial fission and its physiological significance in ischemic injury and nematode life span.
INTRODUCTION
Mitochondria constitute a major cellular energy source, and their activity is controlled by normal cellular homeostasis, including nutrient availability and cell cycle status, and in response to physiological stress such as DNA damage or hypoxia. A key aspect of mitochondrial function is the dynamic balance of fusion and fission, events which alter mitochondria morphology, biogenesis, and activity and consequently influence embryonic development, metabolism, and apoptosis (Chan, 2006; Suen et al., 2008). Notably, control of mitochondrial fusion and fission is evolutionarily conserved, and their deregulation is implicated in pathological conditions, including neuropathological and cardiovascular disorders (Chan, 2006; Knott et al., 2008; Ong et al., 2010).
Mitochondrial fusion is controlled by Mitofusion 1 (Mfn1) and Mitofusion 2 (Mfn2), GTPases anchored in the mitochondrial outer membrane (MOM) and whose interactions tether two distinct MOMs to promote fusion (Santel and Fuller, 2001; Koshiba et al., 2004). Mitochondrial inner membrane fusion is controlled by a third GTPase, OPA1, which is implicated in control of cristae structure (Olichon et al., 2003). The fission process is regulated by dynamin-related protein 1 (Drp1), a cytosolic GTPase recruited to mitochondria. Drp1 self-assembly and subsequent GTP hydrolysis are the driving force for mitochondrial membrane fission (Smirnova et al., 2001; Roux et al., 2006). As such, Drp1 is subject to extensive posttranslational modification, including phosphorylation (Taguchi et al., 2007; Chang and Blackstone, 2007; Cribbs and Strack, 2007). Fis1, a small protein anchored on MOM, is required for mitochondrial fission (Yoon et al., 2003; James et al., 2003). Although the underlying mechanisms remain elusive, Fis1’s interaction with a Drp1-containing fission complex is likely essential for mitochondrial fission (Lackner and Nunnari, 2009). Reduced oxygen levels seen under hypoxic conditions affect mitochondrial function by increasing glycolysis and lactate production. At the molecular level, hypoxia stabilizes hypoxia-inducible factor (HIF), which controls transcription of a wide range of genes, including factors implicated in regulation of mitochondrial energy metabolism such as Glut1 and PDK1 (Ebert et al., 1995; Iyer et al., 1998; Papandreou et al., 2006). The ubiquitin ligase Siah2 controls HIF1α availability through its regulation of the stability of prolyl hydroxylases (PHDs) 1 and 3 under physiological hypoxic conditions (Nakayama et al., 2004). Siah’s contribution to mitochondria function has been noted previously through its regulation of the stability of A-kinase anchoring protein 121 (AKAP121) (Carlucci et al., 2008a). As a scaffold protein located at the mitochondrial membrane, AKAP121 is implicated in transmitting signaling cues to the mitochondrial microenvironment, thereby affecting oxidative phosphorylation, steroidogenesis, and cell survival (Carlucci et al., 2008b). Despite evidence of a functional link between oxygen availability and mitochondrial function, the impact of hypoxia on mitochondrial morphology is largely unexplored. Recent studies of cardiomyocytes that were maintained under low oxygen and glucose-deprived conditions in vitro (i.e., simulated ischemia) exhibited enhanced mitochondrial fragmentation (fission). The link between mitochondrial dynamics and cell death was also documented in cardiomyocytes that were subjected to ischemic injury in vivo, although the molecular mechanism linking ischemia and mitochondrial fission remains unclear (Ong et al., 2010; Wang et al., 2011).
Altered mitochondria activity was reported to affect longevity of various model organisms, including C. elegans (Balaban et al., 2005), pointing to the possibility that mitochondrial dynamics might influence the life span of nematodes. Notably, the majority of the mitochondrial biogenesis in C. elegans occurs during larval development accompanied by a high degree of fission/fusion events.
Our studies reveal that the ubiquitin ligase Siah2 occupies a critical point in the control of mitochondrial fission under hypoxia. Through its regulation of mitochondrial dynamics, Siah2 increases apoptosis of cardiomyocytes subjected to ischemia while prolonging the life span of C. elegans. These observations provide a causal link between Siah2, mitochondrial fission, and key physiological processes.
RESULTS
Hypoxia Induces Mitochondrial Fission
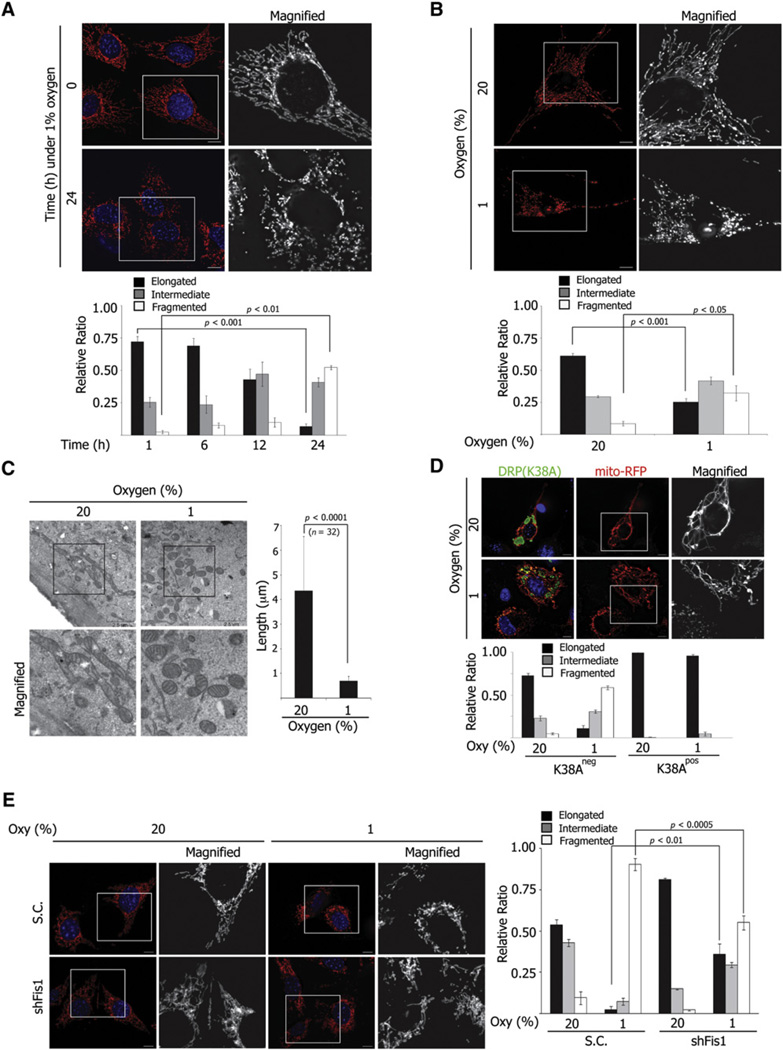
We set out to determine whether hypoxia affects mitochondrial dynamics and the possible role of Siah2 in this process. To this end, NIH 3T3 mouse fibroblast cells were maintained under normoxic or hypoxic conditions, and mitochondrial morphology was observed. Under normoxia, mitochondria appeared as a mixture of elongated tubules, short fragments, and puncta (Figures 1A and 1B). When maintained for 24 hr under hypoxia, mitochondria were less elongated (normoxia versus hypoxia, 72.2% ± 4.16% versus 6.8% ± 2.1%, p < 0.01) and more fragmented (normoxia versus hypoxia, 2.4% ± 1.2% versus 52.3% ± 1.58%, p < 0.001), suggesting that hypoxia induces mitochondria remodeling (Figure 1A). Similarly increased mitochondrial fragmentation was observed in human skin fibroblast cells (normoxia versus hypoxia, 8.3% ± 1.8% versus 32.0% ± 5.9%, p < 0.05) maintained under hypoxic growth conditions (Figure 1B and see Figure S1A available with this article online). Consistent with these observations, electron microscopy analysis confirmed shorter (0.70 ± 0.20 µm) and higher incidences of rounded forms of mitochondria under hypoxia, compared with elongated (4.37 ± 2.19 µm) forms seen under normoxia (Figure 1C and Figure S1B). These findings suggest that hypoxia, a normal physiological process, induces mitochondrial fragmentation, which may provide a possible mechanism for mitochondrial adaptation to low-oxygen conditions.
Figure 1. Hypoxia Induces Mitochondria Fission.
(A) NIH 3T3 cells were cultured under hypoxia for the indicated times and visualized with anti-Tom20 antibody. Mitochondria morphology was assessed from 300 cells of three different slides at each time point and quantified (see Figure S1A).
(B) BJ cells expressing mito-RFP were cultured under normoxia (upper) or hypoxia (lower) for 24 hr.
(C) Ultrastructure of mitochondria in NIH 3T3 cells cultured under normoxia (20%) or hypoxia (1%). The length of mitochondria was measured from four different images (number of mitochondria counted, n = 32). Scale bars indicate 2.5 µm.
(D) NIH 3T3 cells transduced with retroviral mito-RFP (red) were transfected by Drp1(K38A) (green) and cultured under the indicated oxygen concentration for 24 hr. Mitochondrial morphology was assessed and quantified from cells with positive or negative green (Drp1K38A) signal.
(E) MEFs were transduced with scrambled or shFis1 lentivirus and cultured under normoxia or hypoxia for 24 hr. Mitochondria were visualized with anti-Tom20 antibody. For (A)–(E), data are presented as mean ± SD (standard deviation). Scale bars indicate 10 µm unless specified otherwise.
Given the role of Drp1 and Fis1 in mitochondrial fission, we next determined whether hypoxia-induced changes in mitochondrial morphology are mediated by fission machinery. To this end, we tested whether a Drp1 mutant (K38A, lacking GTPase activity and hence serving as a dominant negative) can affect mitochondrial fragmentation under hypoxia (Smirnova et al., 2001). Most cells expressing mutant DRP1 (96.8%) showed long tubular mitochondria and an interconnected mitochondrial network, which was no longer affected by exposure to hypoxic conditions. By contrast, control, nontransfected cells within the same field as transfectants, showed mitochondrial fragmentation under hypoxia (Figure 1D). Reduced expression of the fission factor Fis1 significantly attenuated hypoxia-mediated mitochondrial fragmentation (scramble versus shFis1, 90.49% ± 3.68% versus 55.06% ± 4.21%, p < 0.0005) and maintained tubular network (scrambled versus shFis1, 2.26% ± 1.96% versus 36.01% ± 6.08%, p < 0.01) (Figure 1E and Figure S1C). No significant cell death was recorded in NIH 3T3 or MEF cells maintained under 1% oxygen or in medium containing serum and glucose (data not shown). These data suggest that morphological changes seen in mitochondria under hypoxia reflect fission of the mitochondrial membrane, changes that require the conventional fission machinery.
Siah Is Required for Hypoxia-Mediated Mitochondrial Fission
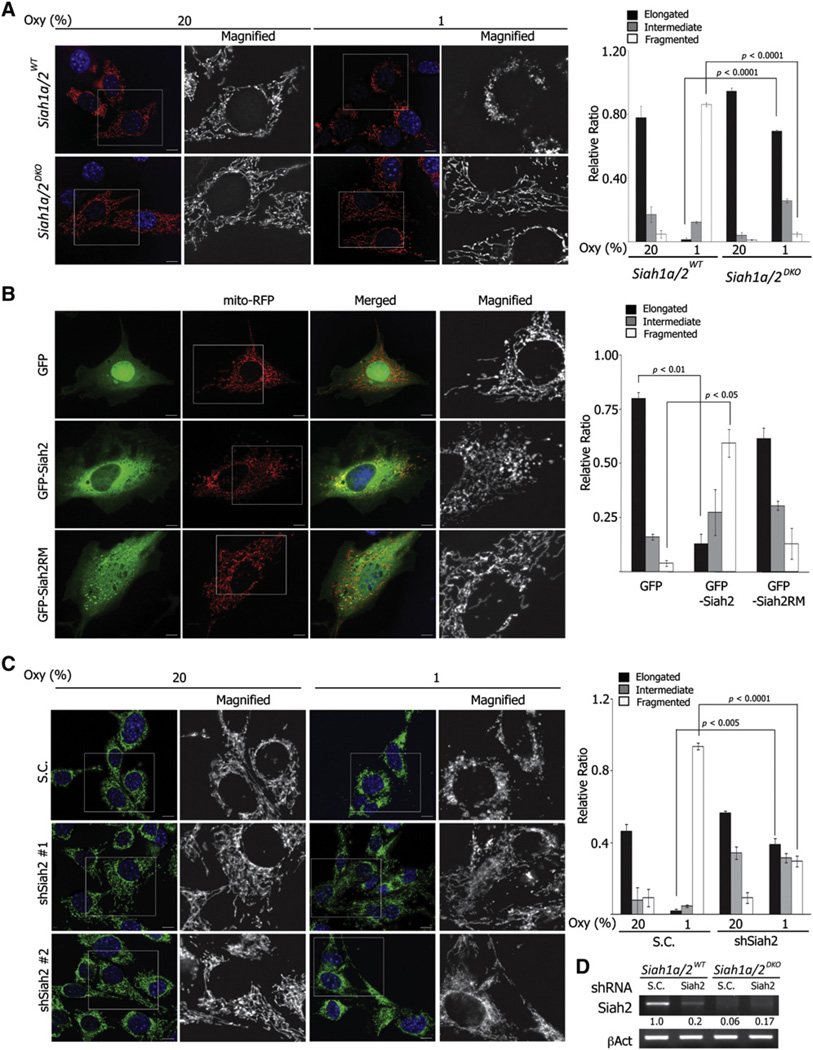
To directly evaluate the possible role for Siah2 in hypoxia-induced mitochondrial fission, we compared the effect of hypoxia on mitochondrial morphology in Siah1a/2 wild-type (WT) versus Siah1a/2 double knockout (DKO) immortalized MEF cell lines. Significantly, while Siah1a/2WT MEFs exhibited fragmented mitochondria (86.21% ± 1.25%) under hypoxic conditions, Siah1a/2DKO MEFs did not (4.82% ± 1.37%, p < 0.0001). Conversely, most Siah1a/2DKO MEFs maintained elongated mitochondrial tubules after hypoxic culture (normoxia versus hypoxia, 94.67% ± 1.89%, 69.35% ± 1.01%, p < 0.0001), implying that hypoxia-mediated mitochondrial fission is impaired in the absence of Siah1a/2 expression (Figure 2A). To exclude the possibility that these observed differences were attributable to clonal variations that may have occurred during immortalization, we tested primary MEFs isolated from Siah1a+/+/Siah2+/− or Siah1a+/−/Siah2−/− embryos (Figure S2A). Consistent with the observations in immortalized cells, lack of Siah1a/2 expression significantly attenuated hypoxia-mediated mitochondria fission under hypoxia (Siah1a+/+/Siah2+/− versus Siah1a+/−/Siah2−/−, 41.0% ± 2.0% versus 14.2% ± 2.4%, p < 0.001) (Figure S2B). To confirm a possible role for Siah2 in mitochondrial fission, we questioned whether ectopic expression of Siah2 phenocopies hypoxia-induced mitochondrial fragmentation. We overexpressed GFP-tagged WT Siah2 (GFP-Siah2) or a RING mutant (GFP-Siah2RM), which lacks E3 ligase activity in NIH 3T3 cells under normoxia. Expression of GFP-Siah2 reduced the appearance of elongated mitochondrial tubules (GFP versus GFP-Siah2, 80% ± 2.8% versus 13% ± 4.24%, p < 0.01) and increased the fraction of fragmented puncta (4% ± 1.41% versus 59.5% ± 6.36%, p < 0.05) (Figure 2B). Conversely, mitochondrial morphology in GFP-Siah2RM-expressing cells was comparable to GFP-expressing control cells (Figure 2B), suggesting that Siah2 E3 ligase activity is required for mitochondrial fragmentation. Lastly, Siah2 knockdown (80% efficiency) significantly impaired hypoxia-mediated mitochondrial fragmentation (scrambled control shRNA versus shSiah2, 93.8% ± 1.7% versus 29.7% ± 2.9%, p < 0.001) (Figures 2C and 2D). Taken together, these data strongly suggest that the ubiquitin ligases Siah1a/2 are required for hypoxia-induced mitochondrial fission.
Figure 2. Hypoxia-Induced Mitochondrial Fission Is Impaired in the Absence of Siah Expression.
(A) Siah1a/2WT and Siah1a/2DKO MEFs expressing mito-RFP were incubated for 24 hr under indicated oxygen concentrations. Mitochondria morphology was quantified from 300–600 cells from three different experiments.
(B) NIH 3T3 cells expressing mito-RFP were transfected with GFP, GFP-Siah2WT, or GFP-Siah2RM. Mitochondria morphology from 200 cells with positive GFP was quantified.
(C) Siah1a/2WT MEFs stably transduced with shSiah2 lentivirus were incubated under indicated oxygen conditions. Mitochondria were visualized with anti-Tom20 antibody and quantified. For (A)–(C), data are presented as mean ± SD. Scale bars indicate 10 µm.
(D) The efficiency of Siah2 shRNA is shown through corresponding RT-PCR bands. The ratio of band intensity was quantified using NIH Image J software and normalized with corresponding β-actin bands.
To assess whether HIF-1α expression is required for hypoxia-mediated changes in mitochondrial morphology, Hif1αΔ/Δ and control MEFs (Hif1αflox/flox) were maintained under normoxic or hypoxic conditions (Figure S3A). Compared to control MEFs, hypoxia-mediated mitochondrial fragmentation was slightly reduced in Hif1αΔ/Δ MEFs (Figure S3B). Notably, the relative difference in mitochondrial remodeling was less pronounced, compared with that seen between Siah1a/2WT and Siah1a/2DKO MEFs, suggesting that HIF1α cannot fully account for the changes seen in the absence of Siah. Correspondingly, expression of mutant HIF1α (P402A/P563A) (Figures S3C and S3D) under normoxia did not induce mitochondrial fragmentation to the degree seen upon expression of Siah2, suggesting Siah-dependent mitochondrial fragmentation under hypoxia is mostly independent of HIF1α activity (Figure S3E and Figure 2B).
Given that lack of HIF1α did not phenocopy the effects seen in the absence of Siah expression, we assessed other potential mechanisms that could underlie Siah2-dependent mitochondrial fission. Comparison between Siah1a/2WT and Siah1a/2DKO MEFs cultured under hypoxia did not identify notable changes in mitochondrial activities (ΔΨm, ATP generation, and ROS generation) that could account for mitochondrial fission under hypoxia (data not shown). Similarly, no differences were found in the expression levels of fusion/fission components between the Siah1a/2WT and Siah1a/2DKO MEF cell lines (data not shown). Altogether, these results indicate that impaired hypoxia-induced mitochondrial fragmentation in Siah1a/2 mutant cells is not due to differences in HIF activity, mitochondrial activity, or altered expression of factors controlling fusion/fission.
The Availability of AKAP121 Is Controlled by Siah and Associated with Mitochondria Dynamics
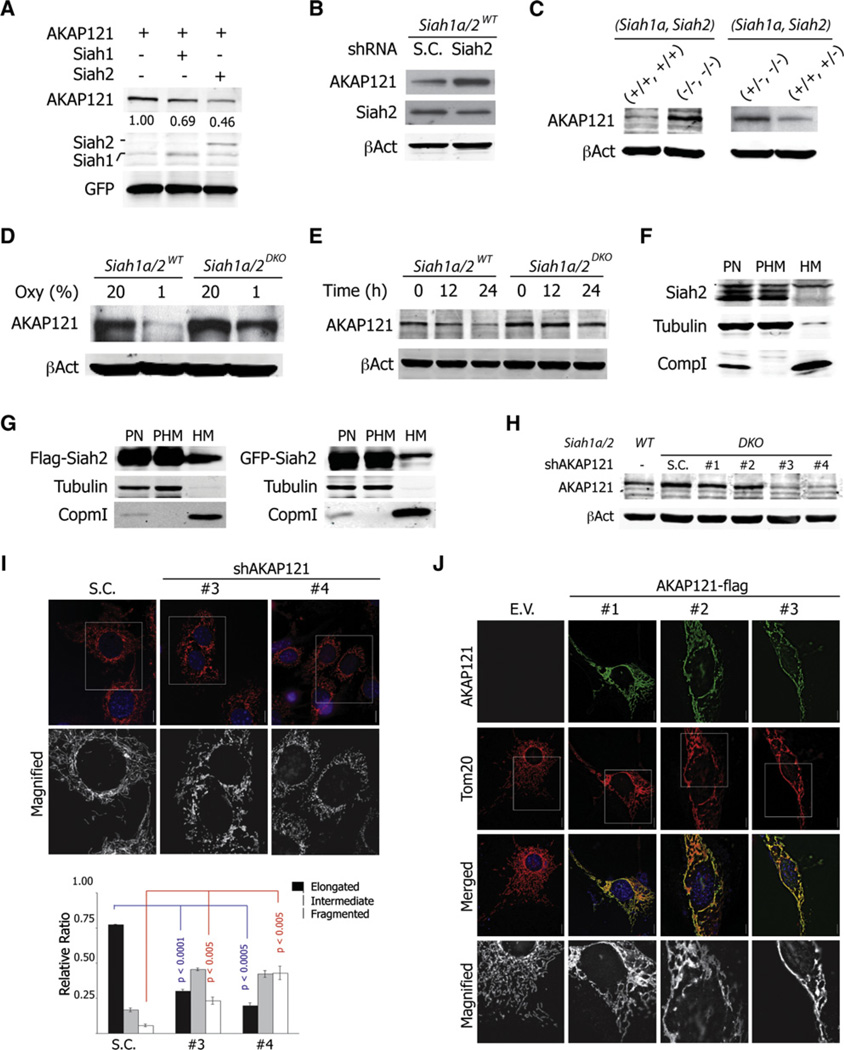
We next assessed the possibility that AKAP121, a Siah2 substrate (Carlucci et al., 2008a), plays a role in regulating mitochondrial morphology. Expression of either Siah2 or Siah1a resulted in efficient degradation of AKAP121, under normoxia (Figure 3A), in agreement with an earlier report (Carlucci et al., 2008a). In addition, reduced expression of Siah2 by shRNA increased levels of AKAP121 (Figure 3B). Notably, the expression level of AKAP121 was inversely correlated with the gene dosage of Siah1a and Siah2 (Figure 3C). Siah1a/2DKO MEFs expressed higher levels of AKAP121, which exhibited a longer half-life span under hypoxia (Figures 3D and 3E). Intriguingly, a portion of AKAP121 was still subjected to degradation in Siah1a/2DKO MEFs under hypoxia (Figures 3D and 3E), implying that additional pathways contribute to the regulation of AKAP121 expression. Lastly, we assessed whether Siah2 was localized to the mitochondria. Consistent with previous findings (Carlucci et al., 2008a), a portion of both ectopically expressed and endogenous Siah2 was found within the mitochondrial fraction (Figures 3F and 3G).
Figure 3. AKAP121 Regulates Mitochondrial Morphology.
(A) HEK293T cells were transfected with AKAP121-Flag, Flag-Siah1aWT, or Flag-Siah2WT, as indicated. Cell lysates were prepared and analyzed using indicated antibodies. GFP served as a control for equal transfection. Band intensity was quantified as indicated in Figure 2D.
(B) Siah1a/2WT MEF cells were transduced with scrambled or shRNA against Siah2.
(C) Cell lysates from immortalized (left) or primary (right) MEF cells with indicated genotypes were analyzed.
(D and E) Cell lysates were prepared from Siah1a/2WT and Siah1a/2DKO MEFs incubated for 24 hr (D) or for the indicated time periods (E) at the indicated oxygen concentrations, followed by analysis using anti-AKAP121 antibody.
(F) HEK293T cells were fractionated into postnucleus (PN), post-heavy membrane (PHM), and heavy membrane (HM) fractions as described in experimental procedures. Of each fraction, 20 µg was analyzed. β-tubulin and Complex1 (Comp1) served as markers for PHM and HM fractions, respectively.
(G) HEK293T cells were transfected with Flag- or GFP-tagged Siah2 constructs, respectively. Cells were fractionated and analyzed as in (F).
(H) The efficiency of four different AKAP121 shRNAs is monitored by immunoblotting of cell lysates obtained from transduced Siah1a/2DKO MEFs.
(I) Mitochondria from control or AKAP121 knockdown Siah1a/2DKO MEFs were visualized by transducing mito-RFP viral particles and quantified. Data are presented as mean ± SD of three different slides.
(J) NIH 3T3 cells were transfected with empty vector (E.V.) or Flag-AKAP121. Mitochondria were visualized with anti-Tom20 antibody. All scale bars indicate 10 µm.
To evaluate a possible role for AKAP121 in regulating mitochondrial morphology, we assessed changes in Siah1a/2DKO MEFs in which AKAP121 expression was inhibited by the corresponding shRNA (shAKAP121). Significantly, efficient knockdown of AKAP121 in Siah1a/2DKO MEFs (Figure 3H) induced mitochondrial fragmentation (Figure 3I), suggesting that AKAP121 functions to regulate mitochondrial fusion/fission. To confirm this role, we overexpressed AKAP121 in NIH 3T3 cells and examined mitochondrial morphology. High levels of AKAP121 expression promoted highly interconnected elongation (50%), bundle-like aggregation (30%), perinuclear clustering (20%), and fragmentation (5%) of mitochondria, phenotypes similar to those seen following mitofusin overexpression (Figure 3J; Santel and Fuller, 2001). Collectively, these results suggest that Siah regulates the availability of AKAP121 at the mitochondria, implying a possible role for AKAP121 in the regulation of mitochondrial dynamics.
AKAP121 Facilitates the Phosphorylation of Drp1 at Mitochondrial Microenvironment
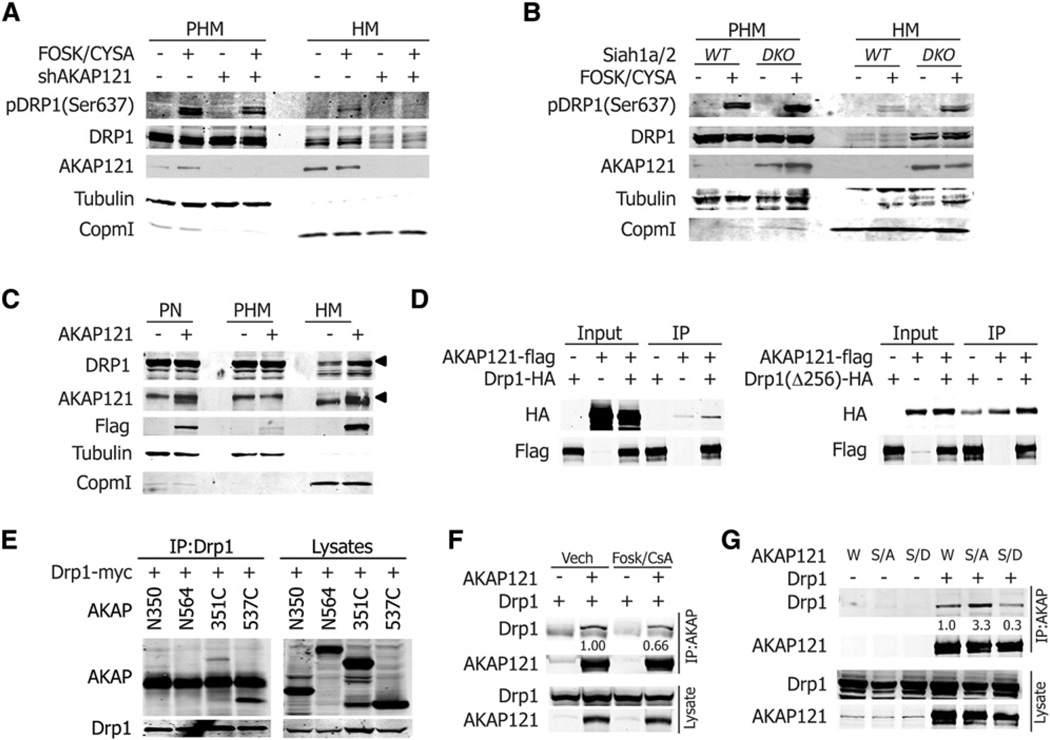
We next examined how the expression level of AKAP121 affects mitochondrial morphology. Given its subcellular localization and role in transducing PKA signaling to mitochondrial substrates, we tested possible changes in mitochondrial membrane potential, processing of OPA1, and inhibitory phosphorylation of Drp1, following attenuated AKAP121 expression (Carlucci et al., 2008b; Chang and Blackstone, 2007; Cribbs and Strack, 2007). Intriguingly, AKAP121 knockdown clones showed mitochondrial fragmentation without changes in ΔΨm or in OPA1 isotypes, suggesting that AKAP121 regulates mitochondrial morphology through a mechanism independent of ΔΨm and membrane potential-mediated processing of OPA1 (Figures S4A and S4B). Monitoring Drp1 phosphorylation in Siah1a/2DKO or Siah1a/2DKO MEFs expressing shAKAP121 prior to and following stimulation with forskolin/cyclosporine A revealed PKA- and AKAP121-dependent phosphorylation of Drp1 on Ser637 in the mitochondrial fraction, which resulted in the inhibition of Drp1 activity (Figure 4A). Consistent with these findings, Drp1 phosphorylation within the mitochondrial fraction of Siah1a/2DKO MEFs was more robust than that seen in Siah1a/2WT MEFs (Figure 4B), indicating the possible role of AKAP121 in inhibitory phosphorylation of Drp1, upon PKA stimulation at mitochondrial environment. In agreement, PKA activation protected mitochondria from hypoxia-mediated fission (Figure S4C). Interestingly, in addition to AKAP121-dependent Drp1 phosphorylation, we consistently detected more Drp1 protein in the mitochondrial fraction as AKAP121 levels increased (Figures 4A and 4B). Additionally, ectopic expression of AKAP121 in HEK293T cells increased Drp1 levels in the mitochondrial fraction (Figure 4C), suggesting that AKAP121 might serve as the docking site for Drp1 at the mitochondria membrane. Indeed, both full-length AKAP121 and its C-terminal domain interacted with Drp1 (Figures 4D and 4E). Interestingly, phosphorylation of Drp1 (pSer637) reduces interaction with AKAP121, as evidenced by reduced interaction of AKAP121 in the presence of PKA stimulation (Figure 4F) and by reduced interaction of phosphomimic Drp1 (S637D) (Figure 4G). Conversely, mutation of Drp1 (S637A), which abolishes PKA phosphorylation, enhanced the interaction with AKAP121 (Figure 4G). These observations suggest that AKAP121 recruitment of Drp1 to the mitochondrial membrane is inhibited upon Drp1 phosphorylation by PKA. Collectively our findings suggest that the presence of AKAP121 at the mitochondria membrane provides docking sites for Drp1 (C terminus), as well as PKA holoenzyme (central domain), facilitating inhibitory phosphorylation of Drp1 upon PKA activation, which results in inhibition of mitochondrial fission. Interestingly, despite the higher level of Drp1 in the mitochondrial fraction in Siah1a/2DKO MEFs, most cells did not show extensive mitochondrial fragmentation, as evidenced by mitochondria morphology of Siah1a/2DKO MEFs in the absence of PKA stimulation, suggesting that AKAP121 may regulate mitochondrial dynamics by additional mechanisms independent of PKA signaling.
Figure 4. AKAP121 Facilitates the Phosphorylation of Drp1 at Mitochondria Microenvironment.
(A and B) Siah1a/2DKO and Siah1a/2DKO MEFs stably expressing scrambled or shRNA against AKAP121 (A) or Siah1a/2WT and Siah1a/2DKO MEFs (B) were stimulated with forskolin (10 µM) and cyclosporine (10 µM) for 30 min. Cells were fractionated as described in the Experimental Procedures.
(C) HEK293T cells were transfected with AKAP121-Flag. Lysates from each fraction were analyzed with indicated antibodies.
(D) HEK293T cells transfected with indicates constructs. Lysates were immunoprecipitated with Flag (AKAP121) and immuoblotted with HA (Drp1 or Drp1Δ256 [lacking GTPase domain]).
(E) HEK293T cells transfected with Drp1-myc and indicated constructs of AKAP121, N350 (1–350), N564 (1–564), C351 (351–857), and C537 (537–857). Lysates were immunoprecipitated and blotted with indicated antibodies.
(F) Cells were transfected with indicated constructs and treated with forskolin (10 µM)/cyclosporineA (10 µM) for 30 min.
(G) Cells were transfected with AKAP121 and Drp1 wild-type (W), phosphormutant S637A(S/A), and phosphomimic mutant S637D (S/D).
(F and G) Intensity of immunoprecipitated Drp1 bands was quantified by normalizing with corresponding input for Drp1. The values in the control groups were set to 1.
Central Domain of AKAP121 Regulates Interaction between Drp1 and Fis1
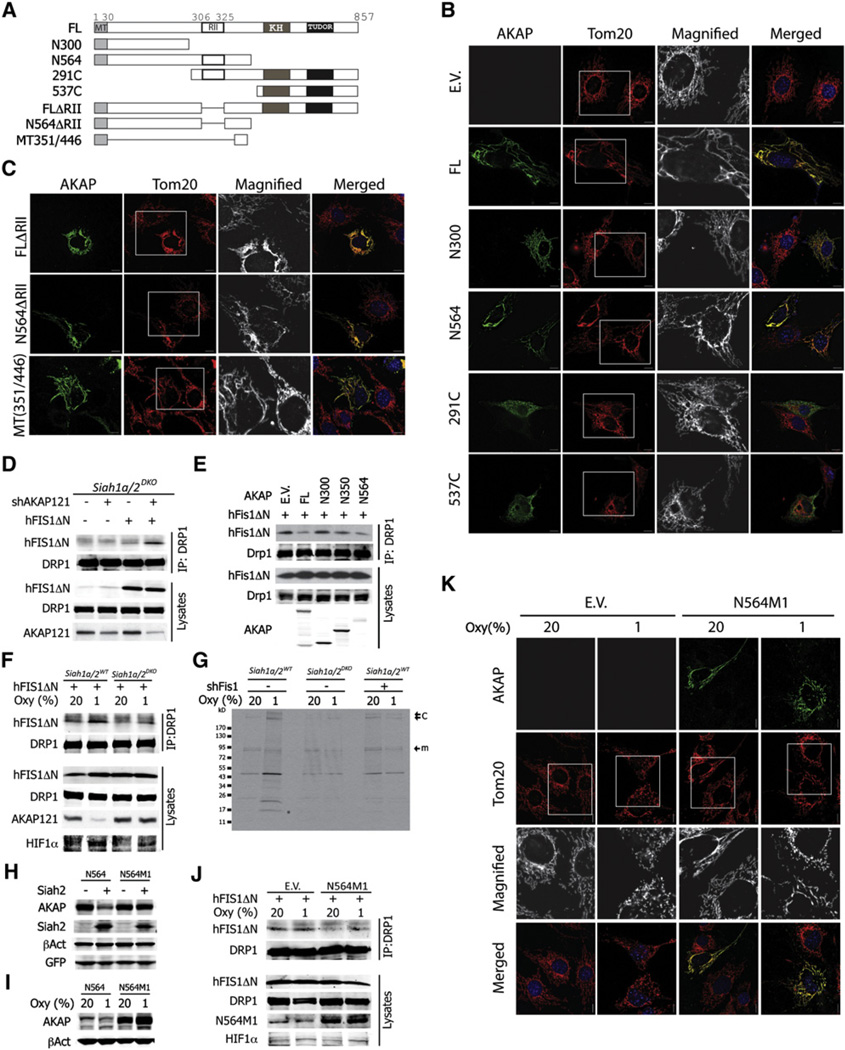
To elucidate the possible mechanisms underlying control of mitochondrial dynamics by AKAP121, we tested the contribution of AKAP121 protein domains to mitochondrial morphology (Figure 5A). This analysis revealed that both the mitochondrial targeting (MT) sequence located within the N terminus (aa 1–30) (Ma and Taylor, 2008) and the central domain (aa 301–546) were indispensable for regulating mitochondria morphology (Figure 5B). Notably, the domain required for recruiting PKA holoenzyme (RII, aa 306–325) (Feliciello et al., 1998) was dispensable for regulation of mitochondrial morphology, suggesting additional (or intrinsic) PKA-independent activity of AKAP121 in the regulation of mitochondrial dynamics (Figure 4C). Furthermore, expression of the MT domain (aa 1–30) fused with the central 96 amino acids (aa 351–446, named as mitochondria fission regulatory domain, MRD) was found sufficient to alter mitochondrial morphology in NIH 3T3 cells (Figure 4C). These data identify a minimal domain within AKAP121 that effectively controls mitochondrial dynamics.
Figure 5. Central Domain of AKAP121 Inhibits Drp1-Fis1 Interaction.
(A) Series of truncated constructs were generated as indicated.
(B and C) NIH 3T3 cells were transfected with empty vector (E.V.) or indicated AKAP121 constructs. Mitochondria were visualized with anti-Tom20 (red) and AKAP121 with anti-Flag (green).
(D) Siah1a/2DKO MEFs stably expressing control or shAKAP RNA were transfected with Fis1DN. Cell lysates were immunoprecipitated and immunoblotted with indicated antibodies.
(E) Siah1a/2WT MEFs were transfected with Fis1ΔN and indicated AKAP121 constructs. Cell lysates were analyzed as in (D).
(F) Siah1a/2WT and Siah1a/2DKO MEFs transfected with Fis1ΔN were grown for 24 hr under indicated oxygen concentrations. Lysates were analyzed as in (D).
(G) MEFs with indicated genotypes were cultured, labeled, and crosslinked as described in the Experimental Procedures. Lysates were immunoprecipitated with anti-Drp1 antibody. “m” and “c” indicate the expected molecular weight of Drp1 monomer and oligomer or complex, respectively. The asterisk indicates expected band of Fis1 around 17 kD.
(H) HEK293T cells were transfected with N564 and N564M1 constructs with or without Siah2.
(I) NIH 3T3 cells transfected with N564M1 were grown under normoxia (20%) or hypoxia (1%) for 24 hr.
(J) Siah1a/2WT MEFs transfected with Fis1ΔN and/or N564M1 were grown for 24 hr under indicated oxygen concentrations.
(K) Siah1a/2WT MEFs transfected with empty vector (E.V.) or N564M1 were grown under indicated oxygen concentration for 24 hr. AKAP121 (FL or N564M1) and mitochondria were visualized with anti-Flag and anti-Tom20 antibodies, respectively. All scale bars indicate 10 µm.
We next explored the possible mechanisms through which mitochondrial targeted AKAP121 is able to regulate mitochondrial morphology. Given the requirement for its mitochondrial localization, we first tested the possible effect of AKAP121 in regulating the formation of the fission complex, Drp1-Fis1, at the mitochondrial membrane. To overcome technical difficulties that hindered the detection of the Drp1-Fis1 complex, we used a Fis1 lacking its N-terminal domain (Fis1ΔN) (Yoon et al., 2003; Wells et al., 2007). Consistent with earlier reports, deletion of the first 31 amino acids increased the binding affinity of Drp1 to Fis1 (Yu et al., 2005; Figures S5A and S5B). Interestingly, overexpression of AKAP121 reduced interaction of Drp1 with Fis1 (Figure S5C). Conversely, reduced expression of AKAP121 by shRNA in Siah1a/2DKO increased Drp1-Fis1 interaction (Figure 5D). Notably, compared to Siah1a/2WT MEFs, Siah1a/2DKO exhibited less Drp1-Fis1 interaction (Figure S5D). Conversely, ectopic expression of AKAP121 WT or N564 (which retains its ability to affect mitochondrial dynamics) in Siah1a/2WT MEFs reduced Drp1-Fis1 interaction, whereas expression of N300 (which no longer affects mitochondrial dynamics) did not (Figures 5B and 5E). These findings suggest that the expression of AKAP121 at the mitochondrial membrane interferes with the interaction between Drp1 and Fis1. However, despite the ability to detect the interaction of AKAP121 with Fis1 or Drp1, this interaction does not appear to be direct, as neither AKAP121 nor N564 fragments exhibited direct association with these fission factors (data not shown). These observations suggest that additional, yet to be identified components are mediating the interaction between AKAP121 and the fission factors and thereby contributing to the regulation of fission by AKAP121.
Given the original observation that the Siah2 effects on mitochondrial fission are primarily seen under hypoxia conditions, we extended the analysis of Drp1-Fis1 interaction under normoxia or hypoxia in Siah1a/2DKO or Siah1a/2WT MEFs. Corresponding to the expression level of AKAP121, Drp1-Fis1 interaction increased in Siah1a/2WT MEFs, but not in Siah1a/2DKO MEFs, under hypoxia (Figure 5F). To substantiate the AKAP121-dependent regulation of Drp1-Fis1 interaction under physiologically relevant conditions, we monitored interaction of endogenous Drp1 and Fis1. We first established experimental conditions for crosslinking of intracellular proteins and immunoprecipitation with Drp1 antibody (Figures S5E–S5H). To overcome difficulties in detecting Drp1-Fis1 interaction, we have labeled proteins in vivo using [35S]Met/[35S]Cys and monitored the fission complex under hypoxia. With comparable efficiency of protein labeling and crosslinking (Figures S5I and S5J), immunoprecipitation with anti-Drp1 antibody led us to detect a 17 kD band, the expected molecular weight of Fis1, only in hypoxia-treated Siah1a/2WT MEFs. Notably, Fis1 was no longer detected in a parallel experiment using Siah1a/2DKO or Siah1a/2WT expressing shRNA against Fis1 (Figure 5G and Figure S5K).
To substantiate the role of AKAP121 in hypoxia control of mitochondrial fission as in the Drp1-Fis1 interaction, we generated AKAP121 mutant that can no longer associate with—or degrade by—Siah2. Analysis of truncated AKAP121 mutants identified two putative degrons for Siah2 (Figures S5L and S5M), of which one (M1) was confirmed to function as the primary degron for Siah2 binding and degradation (Figure S5N). Notably, AKAP121 (N564M1), which was mutated in the degron, abolished degradation by Siah2 (Figure 5H) and was no longer deregulated under hypoxia (Figure 5I). Correspondingly, AKAP121 mutated in Siah2 degron attenuated the interaction of Drp1 and Fis1 and, subsequently, mitochondrial fragmentation under hypoxia (Figures 5J and 5K). All together, these data identify a central domain of AKAP121 with intrinsic activities toward the regulation of the Drp1-Fis1 interaction at the mitochondrial membrane. Thus, deregulation of AKAP121 under hypoxia by Siah2 reduces AKAP121 availability and the ability to inhibit the Drp1-Fis1 interaction, which enables the execution of mitochondria fission.
Siah2-AKAP121 Regulates Ischemia-Induced Cell Death in Cardiomyocytes through Regulation of Mitochondria Fragmentation
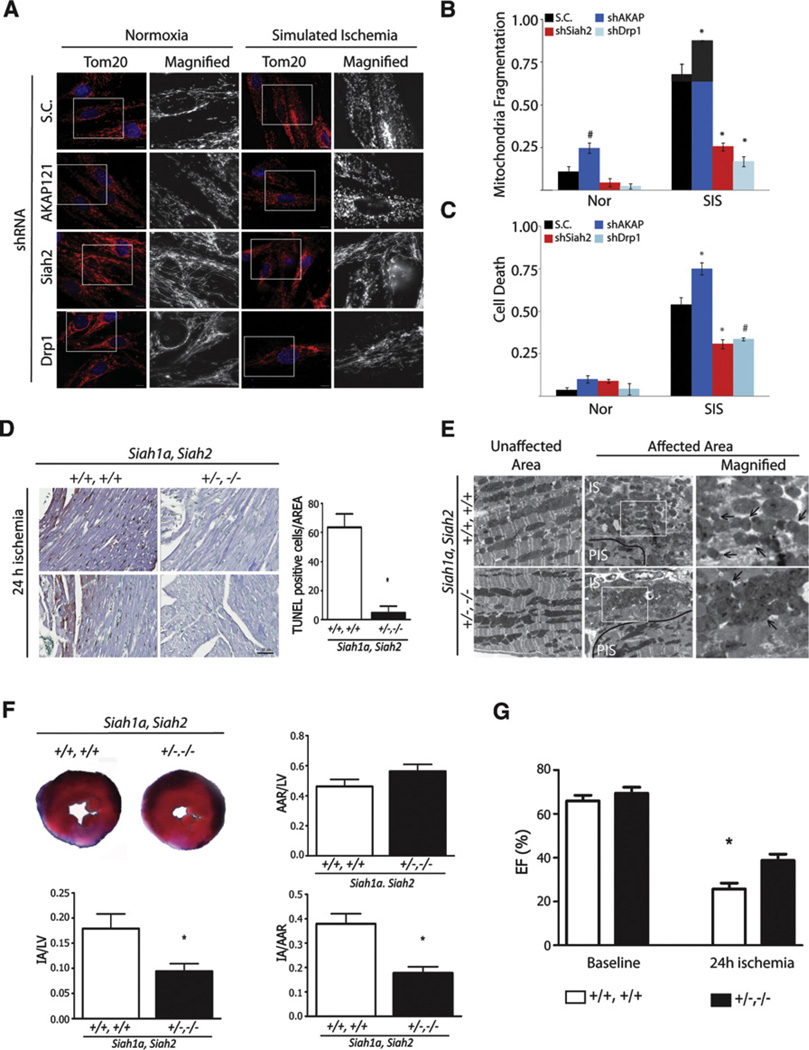
To determine the physiological significance of Siah2-regulated mitochondrial fission under hypoxia, we tested our hypothesis in cardiomyocytes, which were recently reported to exhibit mitochondrial fission following ischemia-regulated cell death (Ong et al., 2010; Wang et al., 2011). We first examined the effect of reduced Siah2, AKAP121, or Drp1 expression on mitochondrial fission and on cell death of H9C2, embryonic rat cardiac myoblast, cells that were subjected to simulated ischemia (SIS; anoxia in medium without serum and glucose). Simulated ischemia of cardiac myoblast H9C2 cells reduced AKAP121 expression, increased mitochondrial fragmentation, and increased cell death in a time-dependent manner (Figures S6A and S6B). Under ischemic conditions, reduced expression of Siah2 or Drp1 significantly attenuated mitochondria fragmentation and protected cardiac myoblasts from ischemia-induced cell death (Figures 6A–6C). Conversely, knockdown of AKAP121 significantly increased mitochondrial fragmentation and promoted cell death under ischemic conditions, thereby pointing to the physiological significance of Siah2-dependent regulation of AKAP121 availability, mitochondria fission, and cell death under ischemia (Figures 6A–6C). To substantiate the role of Siah2 in ischemia-induced cell death, we tested the response of the Siah mutant mouse to myocardial ischemia induced by ligation of the left anterior descending coronary artery, a surgical model of myocardial infarction. In agreement with the cell culture studies, analysis of heart tissues from Siah1a+/−Siah2−/− mice subjected to ischemia revealed that they are resistant to ischemic injury as compared with their Siah2 WT littermates. Correspondingly, cardiac function, measured as ejection fraction (EF), was significantly less impaired when compared to heart tissues of Siah2+/+ mice (Figure 6G, Movie S1 and Movie S2). A similar trend was observed for fractional shortening (data not shown). Heart tissue from Siah1a+/−Siah2−/− mice that were subjected to myocardial ischemia exhibited a significantly lower degree of impaired mitochondria morphology (Figure 6E), compared with unaffected areas of the same hearts. Further, the degree of cell death (Figure 6D) and myocardial infarct size (Figure 6F) were also significantly reduced compared to samples from the Siah1a+/+Siah2+/+ littermates. The area of hypoperfusion (area at risk, AAR) after myocardial infarction was indistinguishable in Siah1a+/−Siah2−/− and Siah1a+/+Siah2+/+ mice (Figure 6F), indicating that the cardioprotective effect is due to increased resistance to the ischemic injury. Collectively, data establish that through its regulation of AKAP121 stability and mitochondrial fission, reduced expression of Siah2 elicits a cardioprotective function under ischemia conditions.
Figure 6. Siah2 Controls Ischemia-Mediated Remodeling of Mitochondria Dynamics and Cell Death of Cardiomyocytes In Vitro and In Vivo.
(A–C) H9C2 cells subjected to knockdown with scrambled (S.C.) or shRNA against indicated genes was incubated under normoxia or simulated ischemia for 6 hr. (A) The morphology of mitochondria was visualized with anti-Tom20 antibody and (B) quantified by counting cells (200–300 cells) with fragmented mitochondria in three independent experiments. Data are presented as mean ± SD (C) Cell death was assessed by propidium iodide staining. Death rate was quantified by counting the number of cells with PI-positive signal per total number of cells of ten different fields (100 cells) in three independent experiments. Data are presented as mean ± SD. The asterisks and fountains indicate p < 0.005 and p < 0.05, respectively.
(D) Representative images of histological sections of left ventricular myocardium from Siah1aWT/2WT and Siah1aHT/2KO mice 24 hr after myocardial infarction (MI). The TUNEL-positive cells in the area are quantified on three consecutive images of the AAR (n = 2 or 3 animals per Siah1aWT/2WT or Siah1aHT/2KO, respectively). Data are represented as mean ± SD. Scale bar indicates 50 µm.
(E) Ultrastructure of ventricular myocardium tissues from Siah1aWT/2WT and Siah1aHT/2KO mice exposed to 24 hr of myocardial infarction represent unaffected and affected areas. PIS and IS indicate peri-ischemic and ischemic regions, respectively. Scale bars indicate 5 µm. The arrows indicate mitochondria.
(F) (Upper left) Representative images of slices of ventricular myocardium from Siah1aWT/2WT and Siah1aHT/2KO mice 24 hr after myocardial infarction. The ratio of area at risk (AAR) to LV (left ventricle) (upper right), ratio of IA (infract area) to LV (lower left), and ratio of IA to AAR (lower right) were quantified.
(G) Ejection fraction percent (EF%) was calculated from echocardiographic images at 24 hr post-MI (see the Supplemental Experimental Procedures and the supplemental movies). Note the less impaired cardiac function of the double mutant Siah1aHT/Siah2KO relative to Siah1aWT/Siah2WT littermates post-MI. For (F) and (G), n = 3 animals per each genotype. Data are presented as mean ± SD. The asterisk indicates p < 0.05.
Regulation of Mitochondria Dynamics by Siah2 or Drp1 Affects Longevity in C. elegans
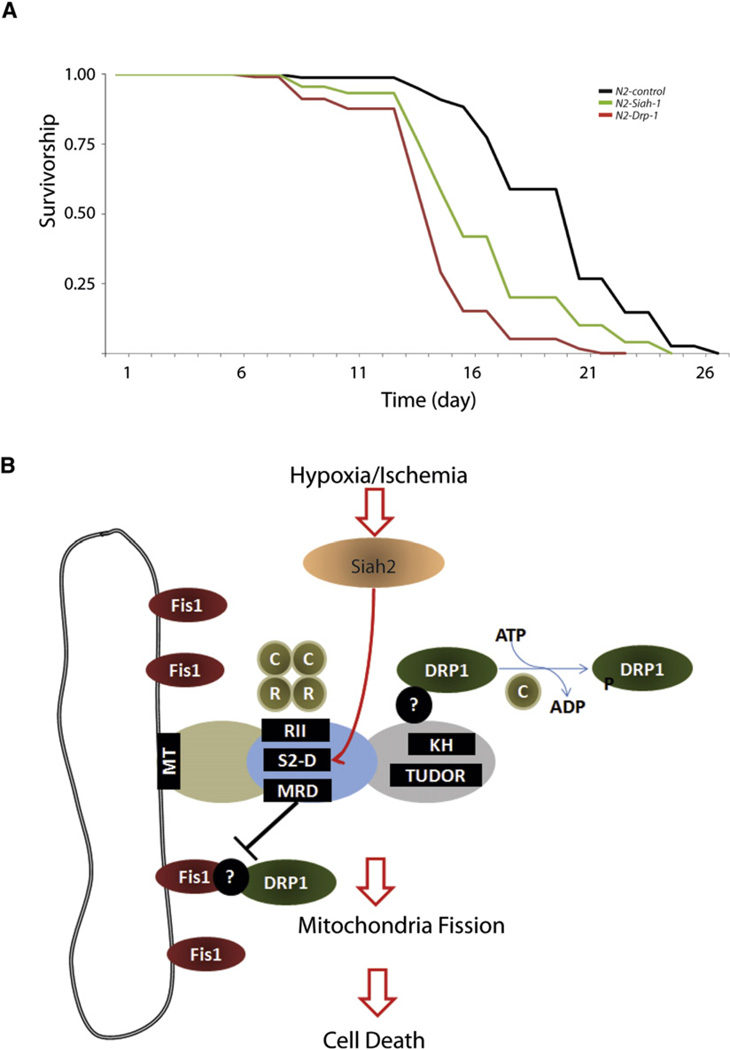
Although earlier studies pointed to the possible link between mitochondrial dynamics, senescence, and aging (Park et al., 2010; Mai et al., 2010), direct evidence supporting the role of mitochondrial fission in nematode life span is lacking. We predicted that impairing proper mitochondrial fission during nematode development would likely affect life span. To test this hypothesis we inhibited the expression of the C. elegans orthologs drp-1 and siah-1. Significantly, depletion of drp-1 and siah-1 resulted in shortened life span (Figure 7A). Crucially, this phenotype manifested itself only when the depletion was initiated from hatch (Figure 7A and Figure S7), underscoring the importance of proper fission at a time when mitochondrial biogenesis peaks and establishes a metabolic clock for the remainder of the organism’s life span.
Figure 7. Depletion of siah-1 or drp-1 from Hatch Decreases the Life Span of C. elegans.
(A) RNAi-mediated depletion of C. elegans drp-1 and siah-1 resulted in reduction of mean life span N2-control (black line, mean 20.1 ± 0.3 days), N2-drp-1 (red line, 15.5 ± 0.3 days), and N2-siah-1 (green line, 17.5 ± 0.4 days, log rank p < 0.0001 in each case). RNAi depletion was initiated from hatch.
(B) A proposed model. AKAP121 regulates mitochondria dynamics through (1) facilitating Drp1 phosphorylation via PKA binding domain (RII) (2) providing a docking site for Drp1 at C-terminal domains and (3) via inhibition of Drp1-Fis1 interaction by mitochondria fission regulatory domain (MRD) within central region. Upon hypoxia or ischemia, Siah2 reduces availability of AKAP121 via Siah degron (SD), which decreases AKAP121-mediated Drp1 phosphorylation and increases Drp1-Fis1 interaction, resulting in mitochondria fission and cell death of cardiomyocytes under myocardial ischemia. Question marks indicate an unidentified component(s) that is expected to be involved in AKAP121-mediated regulation of Fis1-Drp1 complex.
DISCUSSION
The present study establishes a previously undisclosed link between mitochondrial fission and mitochondrial adaptations to hypoxia. We demonstrate that hypoxia induces mitochondrial fragmentation, confirmed as fission, and that Siah2 functions in this process by regulation of AKAP121 levels under hypoxia. Our findings unveiled the function of AKAP121 in control of mitochondrial morphology by two complementary mechanisms—through inhibition of Drp1 and Fis1. While AKAP121 facilitates inhibitory phosphorylation of Drp1 by PKA signaling in the mitochondrial microenvironment, it also limits the formation of Drp1-Fis1-containing fission complex at the mitochondrial membrane by a PKA-independent mechanism (Figure 7B). Deregulation of AKAP121, as occurs upon increased Siah2 expression and activity under hypoxia (Nakayama et al., 2004), alleviates Drp1 phosphorylation and enables interaction of Drp1 with Fis1 on mitochondria membrane, resulting in mitochondrial fission. Notably, the first detection of endogenous Drp1 and Fis1 complex in hypoxia-treated Siah1a/2WT MEFs further substantiates regulatory roles in mitochondria fission under physiological stress condition.
Our studies also identified the minimal domain on AKAP121 that is required for the regulation of Drp1-Fis1 interaction. Notably, this central domain does not bind either of these fission factors directly, pointing to one or more additional factors, yet to be identified, that mediate AKAP121’s effect on Fis1 and Drp1 association. Notably, our studies revealed the proximal sites of three functional domains, the PKA binding (RII), the Siah2 degron, and the MRD (domain inhibiting Drp1-Fis1 interaction) (Figure 7B), pointing to possible coregulation among factors that bind to these neighboring domains.
As AKAP121 serves as a docking platform for multiple signaling pathways, diverse protein kinases are likely to modulate Drp1-Fis1 interactions, consistent with the extensive posttranslational modifications known to control Drp1 function. Accordingly, AKAP121 itself is also likely subject to tight regulation, which is yet to be identified. Siah control of AKAP121 is one mechanism, which limits its availability with a concomitant effect on Drp1/Fis1 complex formation and mitochondrial fission. Other pathways could control AKAP121 availability and its ability to associate with distinct mitochondrial components.
The induction of Siah2/AKAP121-mediated mitochondrial fission in response to hypoxia provides a conceptual framework for understanding mechanisms underlying mitochondrial adaptation to low oxygen. Since there is a bidirectional relationship between mitochondrial morphology and bioenergetics, remodeling of mitochondrial morphology by Siah2 adds a new layer to the complex regulation of cellular adaptations to low oxygen conditions by this ubiquitin ligase. Siah2 control of mitochondrial fission is independent of its previously characterized role in the regulation of PHD and consequently HIFα availability and transcriptional activity, and its role in inhibition of oxidative phosphorylation by AKAP121 degradation (Nakayama et al., 2004; Carlucci et al., 2008a). Importantly, the effect of Siah2 on mitochondrial fission was largely independent of HIF1α. This newly identified role for Siah2 illustrates three distinct mechanisms that synergize to govern cellular adaptation to hypoxia.
Mitochondria are critical in determining cell survival, and their morphology is closely associated with the susceptibility to cell death signals (Suen et al., 2008). Our studies suggest a role of Siah2 in ischemia-induced cardiomyocyte cell death by regulating AKAP121 availability and subsequent mitochondria dynamics. Interestingly, in addition to previous mechanistic models of AKAP121 function on apoptosis, the newly identified role for AKAP121 in regulation of mitochondria dynamics and cell death explains the observation that delocalization of AKAP121 from mitochondria by competitor peptide increases apoptosis of cardiomyocytes (Perrino et al., 2010).
Our study also identifies the role of mitochondrial fission on nematode life span. Notably, the effect of Siah2 or Drp1 on nematode life span required their inhibition during larval development, a stage during which mitochondrial fission and fusion events are maximal. Underscoring this is the observation that mutation and/or RNAi depletion of mitochondrial electron transport chain genes results in a wide spectrum of defects in life span. Consistent with the extensive degree of fission/fusion during larval development, these defects manifest themselves only when RNA-mediated depletion is initiated from hatch, and not when initiated during adulthood (Dillin et al., 2002; Rea et al., 2007). Overall, our study identifies a molecular mechanism underlying control of mitochondrial fission in a physiological condition, namely hypoxia, thereby linking control of mitochondrial morphology with corresponding physiological readouts.
EXPERIMENTAL PROCEDURES
Animals
The Institutional Animal Care and Use Committee (IACUC) of Sanford-Burnham Medical Research Institute approved our study protocols. We prepared mice with various genotypes from 129 strain male and female mice with genotypes, Siah1aWT/Siah2HT and Siah1aHT/Siah2HT.
Immunocytochemistry
Mitochondria were stained as follows: cells expressing mito-RFP were fixed with 4% paraformaldehyde, cells were stained with MitoTracker red (20–40 nM; Invitrogen) and subsequently fixed as described, and cells were fixed as described and then permeabilized with 0.2% Triton X-100. After blocking with 3% bovine serum albumin (BSA) in PBS, mitochondria were stained with anti-Tom20 (1:300). The fixation of cells cultured under hypoxia was performed in interlock (anaerobic and room temperature) of hypoxia workstation.
Measurement of Mitochondrial Activities
For measuring mitochondrial membrane potential, tetramethylrhodamine ethyl ester (TMRE, 150 nM, Invitrogen) in culture medium was preconditioned under hypoxia (1% O2). For depolarized control, carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 50 µM, Sigma) was pretreated for 1 hr before adding TMRE. After addition of TMRE solution, cells were further incubated for 30 min, subsequently rinsed with warmed PBS, and trypsinized. Cells were washed with warmed PBS supplemented with 5% FBS and analyzed using FACSCanto. Data obtained were analyzed using WinMDI (version 2.9; TSRI).
Metabolic Labeling
MEFs were cultured under normoxia or hypoxia (1% oxygen) for 20 hr. After washing with labeling media (DMEM-Met/Cys-free, Invitrogen), cells were labeled by incubation for 4 hr in labeling media (DMEM-Met/Cys-free with of Expre35S35S protein labeling mix [50 µCi/ml], PerkinElmer).
Additional information is provided in the Supplemental Information, available online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Peter Ratcliffe (University of Oxford, UK), Craig Blackstone (National Institute of Neurological Disorders and Stroke [NINDS], National Institutes of Health [NIH]), David Chan (Caltech), and Olexander Korchynsky (University of Amsterdam) for generous gifts of DNA constructs and antibodies; Randall Johnson (University of California, San Diego) for HIF1α MEFs, and Ed Monosov (Sanford-Burnham Medical Research Institute) for assistance with electron microscopy. We thank members of the Ronai lab for discussions and Dr. Susan Taylor for critical comments. This work was supported by National Cancer Institute (NCI) grants CA1111515 and CA128814 (to Z.A.R.) and by National Heart, Lung, and Blood Institute (NHLBI) grants HL088266 and HL098053 and California Institute for Regenerative Medicine RC1-00132-1 (to M.M.). M.C.S. is a California Institute for Regenerative Medicine (CIRM) clinical fellow.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, two movies, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.molcel.2011.08.045.
REFERENCES
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Carlucci A, Adornetto A, Scorziello A, Viggiano D, Foca M, Cuomo O, Annunziato L, Gottesman M, Feliciello A. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J. 2008a;27:1073–1084. doi: 10.1038/emboj.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci A, Lignitto L, Feliciello A. Control of mytochondrial dynamics, oxidative metabolism by cAMP, AKAPs and proteasome. Trends Cell Biol. 2008b;18:604–613. doi: 10.1016/j.tcb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Jsu AL, Arantes-Oliveira J, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochndrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J. Biol. Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- Feliciello A, Rubin CS, Avvedimentoi EV, Gottesman ME. Expression of A kinase anchor protein 121 is regulated by hormones in thyroid and testicular germ cells. J. Biol. Chem. 1998;273:23361–23366. doi: 10.1074/jbc.273.36.23361. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Lackner LL, Nunnari JM. The molecular mechanism and cellular functions of mitochondrial division. Biochim. Biophys. Acta. 2009;1792:1138–1144. doi: 10.1016/j.bbadis.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Taylor SS. A molecular switch for targeting between endoplasmic reticulum (ER) and mitochondria. J. Biol. Chem. 2008;283:11743–11751. doi: 10.1074/jbc.M710494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondria fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiqutin ligase induces cellular senescence through dynamin-related protein 1 and mitofusion 1. J. Cell Sci. 2010;15:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Feliciello A, Schiattarella GG, Esposito G, Guerriero R, Zaccaro L, Gatto AD, Saviano M, Garbi C, Carangi R, et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovasc. Res. 2010;88:101–110. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondria dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- Wells RC, Picton LK, Williams SC, Tan FJ, Hill RB. Direct binding of the dynamin-like GTPase, Dnm1, to mitochondrial dynamics protein Fis1 is negatively regulated by the Fis1 N-terminal arm. J. Biol. Chem. 2007;282:33769–33775. doi: 10.1074/jbc.M700807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J. Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.