Abstract
Super Elongation Complexes (SECs) contain two different transcription elongation factors, P-TEFb and ELL1/2, linked by the scaffolding protein AFF4 or AFF1. They stimulate the expression of both normal and disease-related genes, especially those of HIV or involved in leukemogenesis. Among all SECs subunits, ELL2 is stoichiometrically limiting and uniquely regulated at the level of protein stability. Here we identify the RING domain protein Siah1, but not the homologous Siah2, as the E3 ubiquitin ligase for ELL2 polyubiquitination and proteasomal degradation. Siah1 cannot access and ubiquitinate ELL2 bound to AFF4, although at high concentrations, it also degrades AFF4/1 to destroy SECs. Prostratin and HMBA, two well-studied activators of HIV transcription and latency, enhance ELL2 accumulation and SECs formation largely through decreasing Siah1 expression and ELL2 polyubiquitination. Given its importance in formation of SECs, the Siah1 ubiquitination pathway provides a fresh avenue for developing strategies to control disease-related transcription.
Keywords: Siah1, ELL2, Super Elongation Complexes (SECs), AFF4, P-TEFb, transcriptional elongation, ubiquitination, proteasome degradation
Introduction
For most of the past three decades or so, attention of the transcription field had been largely focused on the initiation and promoter clearance stages of eukaryotic transcription. This was motivated by the observations that the recruitment of RNA polymerase (Pol) II to a few model gene promoters is a key rate-limiting step for expression of these genes (Kuras and Struhl, 1999; Ptashne, 2005). However, recent years have witnessed a major paradigm shift in the field with the demonstrations that the subsequent stage of the transcription cycle, i.e. elongation by Pol II, is also strongly regulated. In fact, for a large number of metazoan genes that play important roles in cell growth, renewal and differentiation, the control of Pol II pausing and elongation is the primary regulatory point for their expression (Core and Lis, 2008; Levine, 2011).
Transcriptional elongation is controlled by a set of positive and negative factors. Shortly after initiation, Pol II is paused at a promoter-proximal region by negative elongation factors NELF and DSIF, whose phosphorylation by positive elongation factor b (P-TEFb), a heterodimer of CDK9-cyclin T, is essential to overcome this block to early elongation. P-TEFb also phosphorylates the Pol II C-terminal domain (CTD), and this event is critical for elongation-coupled mRNA processing. In addition to pausing near the transcription start site, Pol II also experiences pervasive pausing and backtracking throughout the body of genes (Churchman and Weissman, 2011). As such, productive elongation by Pol II requires constant stimulatory actions of several additional elongation factors such as ELL1/2, TFIIS, TFIIF, and the Elongins, all of which enhance the processivity of Pol II through mechanisms different from that of P-TEFb (Sims et al., 2004).
The recent identification of the Super Elongation Complexes (SECs), a set of closely related complexes that contain at least two well-characterized elongation factors, P-TEFb and ELL1/2, within the same complex, indicates that different classes of elongation factors can coordinate their actions on a single polymerase enzyme to synergistically stimulate elongation (He and Zhou, 2011; Smith et al., 2011). Within each SEC, AFF4 (likely also the homologous AFF1 existing in a separate SEC, (Biswas et al., 2011)) functions as a scaffolding protein and uses dispersed sites along its flexible axis to attract different SEC components into the complex (He et al., 2011).
SECs were initially identified through their interactions with the HIV-1 Tat (He et al., 2010; Sobhian et al., 2010) or fusion proteins containing the N-terminal portion of the mixed lineage leukemia (MLL) protein (Lin et al., 2010; Mueller et al., 2009; Yokoyama et al., 2010), both of which serve as sequence-specific recruitment factors to target SECs to the HIV-1 LTR and the MLL target genes, respectively. However, SECs can clearly exist in cells that are free of Tat and MLL translocations and are required for transcription of many non-HIV and non-MLL target genes (He et al., 2011; Lin et al., 2011; Lin et al., 2010). Our recent data indicate that ENL and AF9, which are homologous proteins existing in separate SECs of similar but non-identical functions, use their conserved YEATS domains to interact with the Polymerase-Associated Factor complex (PAFc), and through PAFc, the elongating Pol II (He et al., 2011). Besides this PAFc-mediated interaction, SECs could also be recruited to Pol II through binding to MED26 of the Mediator complex (Takahashi et al., 2011).
Among all SEC subunits, ELL2 plays an especially important role in SECs’ function (He et al., 2010). First, just like P-TEFb, ELL2 is essential for both basal and Tat-activated HIV-1 transcription. Reflecting its functional interactions with the scaffolding protein AFF4 and HIV Tat both inside and outside of SECs, ELL2 can synergize with these two proteins to greatly stimulate transcriptional elongation, a task that cannot be accomplished by its close homologue ELL1. Under normal conditions, approximately 40% of ELL2 in HeLa cells are sequestered in SECs, whereas less than 5% of ELL1 is found there (He et al., 2010). Interestingly, ELL2 but not ELL1 shows a unique expression pattern that is controlled at the protein stability level (He et al., 2010). For example, RNAi-mediated AFF4 knockdown reduces the cellular ELL2 level, and this effect is due to AFF4’s promotion of the half-life of ELL2. Similarly, both the expression of HIV Tat and treatment of cells with the proteasome inhibitor MG132 also markedly stabilize ELL2.
In an effort to determine the mechanism that controls ELL2 stability, we show here that ELL2 but not ELL1 is a polyubiquitinated protein. The ubiquitination is carried out both in vivo and in vitro by the RING domain protein Siah1, which is identified as an E3 ubiquitin ligase for ELL2. Notably, Siah2, a close homologue of Siah1, fails to modify ELL2 due to sequence variations between the RING domains of these two proteins. Our data indicate that AFF4 (likely also AFF1) protects ELL2 from degradation through binding to and sequestering ELL2 away from Siah1 thereby inhibiting ELL2 polyubiquitination. Finally, we show that prostratin and HMBA (hexamethylene bisacetamide), two chemical activators of HIV-1 transcription currently under intense investigation for their potential to reactivate HIV latency, significantly enhance ELL2 accumulation and SECs formation largely through decreasing Siah1’s expression and polyubiquitination of ELL2.
Results
ELL2 is a polyubiquitinated protein
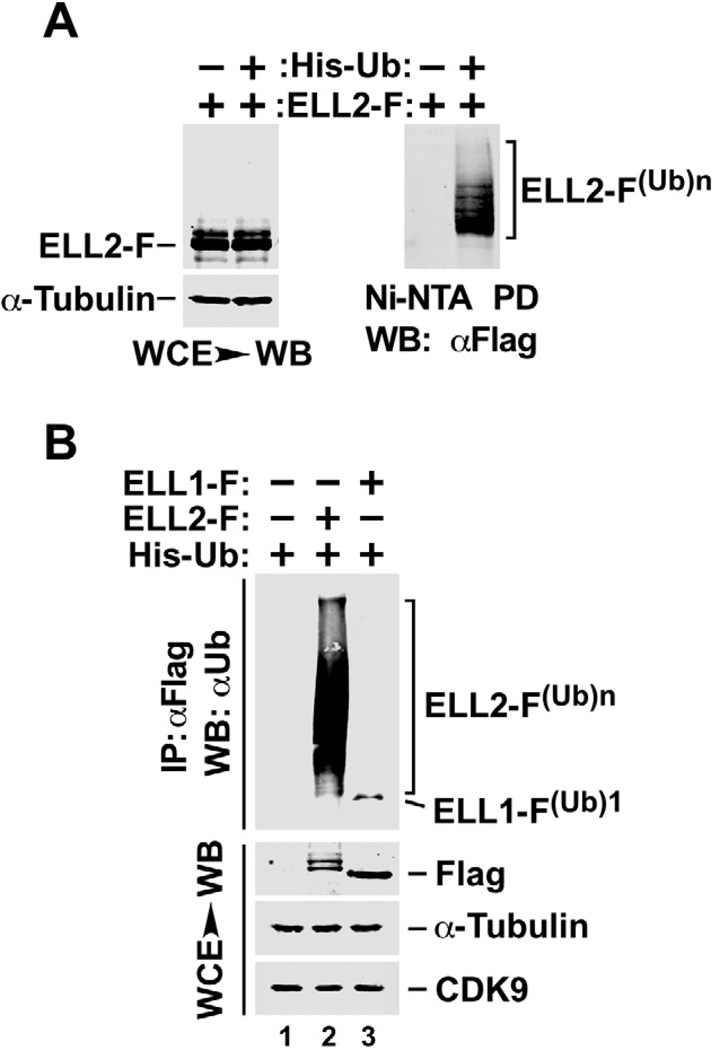
We have previously shown that ELL2 is a short-lived protein, whose stability can be significantly enhanced through inhibiting the 26S proteasome with MG132 (He et al., 2010). Since polyubiquitination is a posttranslational modification that frequently marks a protein for targeted proteolysis by the proteasome, we asked whether ELL2 is polyubiquitinated. Using a standard assay that involves the co-expression of Flag-tagged ELL2 (ELL2-F) with Histidine-tagged ubiquitin (His-Ub) in cells (Jin et al., 2008), we indeed detected the presence of polyubiquitinated ELL2, ELL2-F(Ub)n, by anti-Flag or anti-Ub western blotting of precipitates bound to the Ni2+-NTA (Fig. 1A) and the anti-Flag beads (Fig. 1B), respectively. Interestingly, unlike ELL2, the homologous ELL1 protein, which was shown to be very stable (He et al., 2010), was only mono- (ELL1-F(Ub)1) but not poly-ubiquitinated (Fig. 1B).
Figure 1. ELL2 but not the homologous ELL1 is a polyubiquitinated protein.
A. HeLa cells were transfected with constructs expressing Flag-tagged ELL2 (ELL2-F) or/and Histidine-tagged ubiquitin (His-Ub) as indicated. Whole cell extracts (WCE) were prepared and subjected to analysis by Western blotting (WB) for the presence of the indicated proteins (left panel) or precipitation with Ni2+-NTA beads to pull down (PD) His-Ub, whose covalently bound ELL2-F was detected by anti-Flag WB (right panel). B. WCE were prepared from HeLa cells transfected with the indicated expression constructs and analyzed by WB directly (bottom panels) or anti-Flag immunoprecipitation (IP), which was followed by WB with anti-Ub antibody (top panel). ELL2-F(Ub)n and ELL1-F(Ub)1 indicate polyubiquitinated ELL2 and monoubiquitinated ELL1, respectively.
Siah1 depletion suppresses ELL2 polyubiquitination and increases ELL2 stability
Given that ELL2 is a polyubiquitinated protein, we would like to identify the E3 ubiquitin ligase responsible for this modification. Since ELL2 is a key component of SECs, we performed extensive literature search for any E3 ubiquitin ligase(s) that has previously been linked to either ELL2 or another subunit of SECs. This has led to two reports describing physical interactions identified through the yeast two-hybrid screen between the SECs component AFF1 and the RING domain E3 ubiquitin ligases Siah1 (seven in absentia homolog 1) and Siah2 (Bursen et al., 2004; Oliver et al., 2004).
Human Siah proteins can trace their evolutionary origin to the Drosophila protein Sina, which is required for R7 photoreceptor cell differentiation within the sevenless pathway (Carthew and Rubin, 1990). Like their Drosophila counterpart, Siah1 and Siah2 bind ubiquitin-conjugating enzymes (E2s) via an N-terminal RING domain and target a specific set of proteins for degradation (Hu et al., 1997; Tanikawa et al., 2000).
Although the overexpression of Siah1 has been shown to cause the degradation of transfected AFF1 ((Bursen et al., 2004; Oliver et al., 2004) and also confirmed here, see below), it remains to be demonstrated whether cellular levels of endogenous AFF1 as well as other subunits of SECs can be affected through manipulating the expression of endogenous Siah1 protein. Toward this goal, we selected shRNAs that can silence the expression of Siah1 (Fig. 2A), and performed Siah1 depletion using a combination of two effective shRNAs, shSiah1 #4 and #6, with a non-effective shRNA #3 serving as a negative control (Fig. 2B). To our surprise, the loss of Siah1 failed to produce the expected effect of elevating the endogenous AFF1 level. Rather, among all the SECs subunits that include CDK9, CycT1, AF9, ENL, AFF1, AFF4, ELL1 and ELL2, as well as the key 7SK snRNP component HEXIM1, the only protein that showed a significant shSiah1-induced accumulation is ELL2 (Fig. 2B).
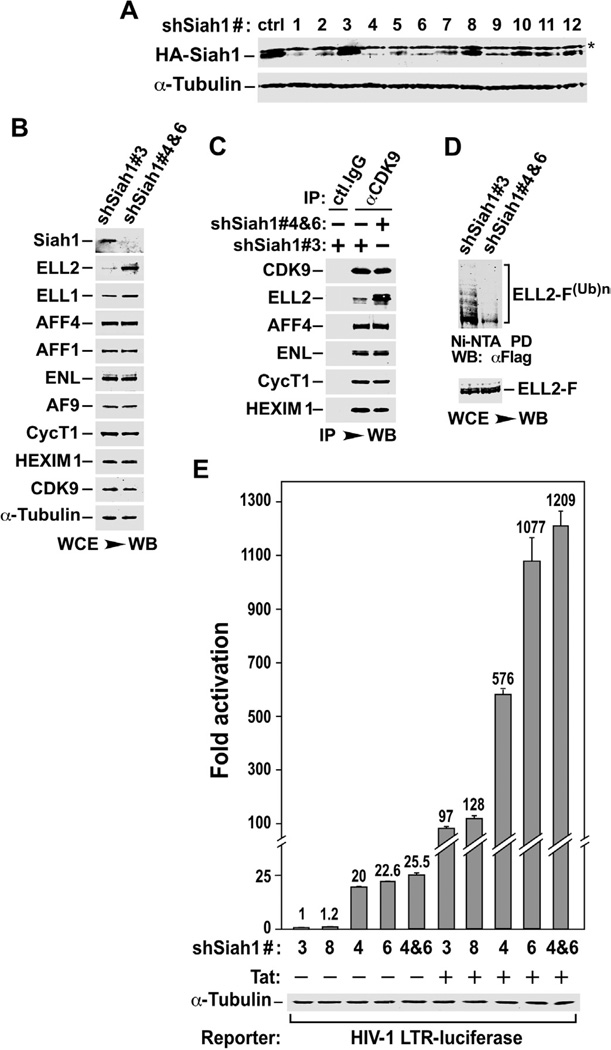
Figure 2. Siah1 depletion suppresses ELL2 polyubiquitination and promotes ELL2 stability, SEC formation and SEC-dependent HIV-1 transcription.
A. The HA-Siah1 expression plasmid was co-transfected into HeLa cells with an empty vector (ctrl) or plasmids expressing the indicated shRNAs targeting Siah1. HA-Siah1 and α-tubulin present in cell lysates were examined by Western blotting. B. Whole cell lysates (WCE) of cells expressing the indicated shSiah1 sequences were analyzed by Western Blotting (WB) for the indicated proteins. C. WCE examined in B were subjected to immunoprecipitation with either anti-CDK9 Ab or total rabbit (ctl.) IgG and immunoprecipitates were analyzed by WB for the indicated proteins. D. Polyubiquitinated ELL2-F(Ub)n isolated by pull-down with Ni2+-NTA beads and total ELL2-F in WCE of cells expressing the indicated shSiah1 sequences were examined by anti-Flag WB. E. Luciferase activities were measured in extracts of cells co-transfected with the indicated shSiah1-expressing constructs, the HIV-1 LTR-luciferase reporter gene, and a vector expressing Tat or nothing. The activity in cells expressing the non-effective shSiah1 #3 but not Tat was set to 1. The error bars represent mean +/− SD from three independent sets of transfection.
Siah1 depletion promotes SECs formation and SECs-dependent HIV transcription
The shSiah1-mediated increase in ELL2 level also caused more ELL2 to associate with the immunoprecipitated endogenous CDK9, indicating an enhanced formation of SECs in Siah1 knockdown cells (Fig. 2C). Furthermore, consistent with the notion that Siah1 is specifically involved in polyubiquitination of ELL2, the expression of effective shSiah1 #4 and #6 but not the ineffective shSiah1 #3, resulted in a drastic reduction in the level of polyubiquitinated ELL2(Ub)n, when loading of the two samples was adjusted to make the total ELL2 at a similar level (Fig. 2D).
Given that Siah1 is very likely the E3 ubiquitin ligase for the SECs component ELL2, we would like to determine the impact of Siah1 depletion on basal and Tat-activated HIV-1 transcription, both of which have been shown to depend on SECs (He et al., 2010). Data in Fig. 2E indicate that shRNA depletion of endogenous Siah1 significantly activated the HIV-1 LTR-driven luciferase expression under both Tat(−) and (+) conditions. Correlating nicely with their abilities to reduce Siah1 expression (Fig. 2A), the two non-effective shRNAs, shSiah1 #3 and #8, failed to activate the LTR, whereas the effective shSiah1 #4 and #6 strongly stimulated HIV transcription when used either alone or in combination (Fig. 2E).
AFF1 and AFF4 are less susceptible to Siah1-induced degradation than ELL2
Providing further evidence in support of a key role for Siah1 in ELL2 degradation, ectopic expression of Siah1 in HeLa cells markedly suppressed the accumulation of ELL2 but not the other SECs subunits expressed from co-transfected plasmids (Fig. 3A). A reduction in the levels of co-expressed AFF1, and to a lesser degree, AFF4 was also detected, although this required the introduction of 4 to 12-times more Siah1 cDNA into cells (Fig. 3B). These results further underscore the notion that ELL2 is Siah1’s primary and most sensitive target among all SECs components. However, when Siah1 level is high, AFF1 and AFF4, which function as scaffolding proteins in SECs, can also be targeted to cause the destruction of SECs.
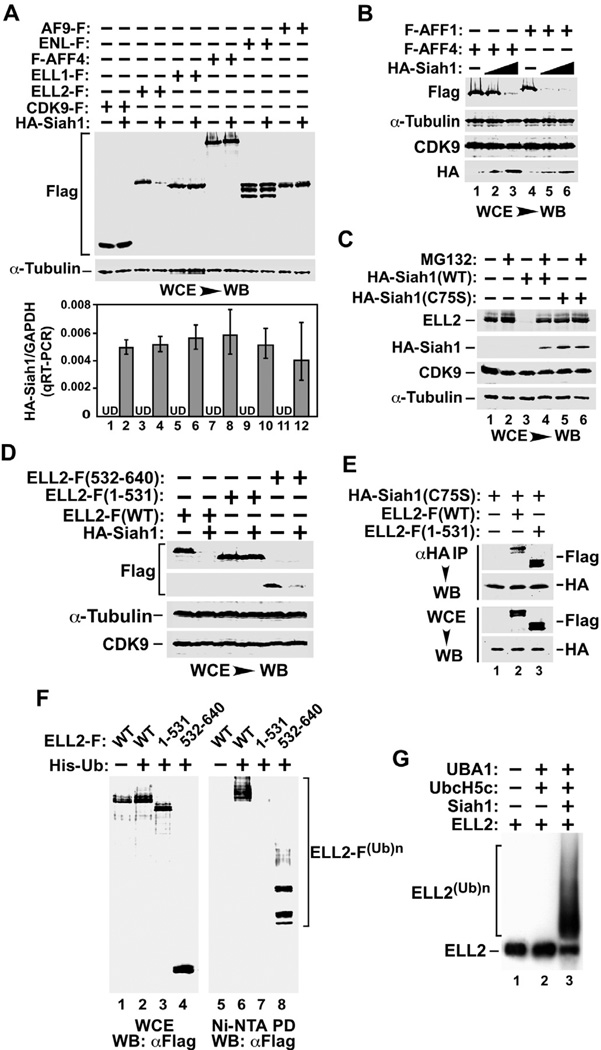
Figure 3. ELL2 is more susceptible than AFF1 and AFF4 to degradation induced by Siah1, an E3 ubiquitin ligase that directly polyubiquitinates the ELL2 C-terminal region in vivo and in vitro.
A. & B. HeLa cells were transfected with (+) or without (−) the indicated expression constructs and whole cell lysates (WCE) were examined by Western blotting (WB) with the indicated antibodies. The HA-Siah1 mRNA levels produced from the transfected plasmid were determined by qRT-PCR and the ratios over endogenous GAPDH mRNA were shown in the bottom panel of A. The error bars represent mean +/− SD from three independent experiments. UD: undetectable. The HA-Siah1 construct was transfected in 3-fold increment in B. C. HeLa cells were transfected with plasmids expressing either WT or the C75S mutant HA-Siah1 and then treated with (+) or without (−) MG132. WCE were examined by WB for the indicated proteins. D. WCE of transfected cells were analyzed by WB as in A. E. WCE of HeLa cells transfected with the indicated expression constructs and anti-HA IP derived from WCE were analyzed by anti-HA and –Flag Western blotting. F. HeLa cells were transfected with constructs expressing WT or truncated ELL2-F and His-Ub as indicated. WCE as well as polyubiquitinated ELL2 isolated from WCE by Ni2+-NTA pull down (PD) were analyzed by anti-Flag WB as in Fig. 1A. G. In vitro ubiquitination reactions containing the indicated components were performed and 35S-labeled WT ELL2 was detected by autoradiography.
The Siah1-mediated ELL2 degradation depended on the full catalytic activity specified by Siah1’s RING domain (Fig. 3C). While wild-type (WT) Siah1 was able to induce efficient ELL2 degradation, which was blocked by MG132 inhibition of the proteasome, the RING domain mutant C75S, which was shown to lack catalytic activity (Hu and Fearon, 1999), failed to cause ELL2 degradation (Fig. 3C). It is worth noting that Siah1 is known to cause self-polyubiquitination and degradation by the proteasome (Hu and Fearon, 1999). Consistently, the catalytically inactive C75S accumulated to a higher level than did WT Siah1 in the absence of MG132 (Fig. 3C, compare lanes 3 & 5).
As for ELL2, its C-terminal region encompassing the last 109 amino acids (aa532–640) was shown to confer high sensitivity to Siah1-induced degradation (Fig. 3D). In contrast, the C-terminally truncated ELL2 (aa1–531) lacking this fragment was completely insensitive to Siah1 and remained stable. Notably, ELL2(1–531) displayed nearly WT ability to bind to the immunoprecipitated HA-Siah1(C75S) (Fig. 3E; the catalytically inactive Siah1 mutant was used to avoid the complication of Siah1 degradation of WT but not the mutant ELL2). In the in vivo ubiquitination assay, the C-terminal 109 amino acids of ELL2 were found to be both necessary and sufficient for Siah1-mediated polyubiquitination of ELL2 (Fig. 3F). Together, these data indicate that the E3 ubiquitin ligase activity associated with the RING domain of Siah1 likely targets sites within the ELL2 C-terminal region for ubiquitination, although the C-terminal region itself does not appear to contribute prominently to the ELL2-Siah1 interaction.
Siah1 directly polyubiquitinates ELL2 in vitro
So far our data have implicated a critical role for Siah1 in inducing ELL2 polyubiquitination in vivo. In order to establish a direct enzyme-substrate relationship between these two proteins, we performed in vitro reactions to test the ability of recombinant HA-Siah1 to cause polyubiquitination of recombinant ELL2-F. Both proteins were produced in a cell-free protein synthesis system (Promega) and purified to homogeneity by anti-HA and anti-Flag affinity-purification, respectively. The reactions also contained human ubiquitin (Boston Biochem), the E2 ubiquitin-conjugating enzyme UbcH5c, and the E1 ubiquitin activating enzyme UBA1, all of which were purified recombinant proteins ((Jin et al., 2008); kind gifts from the Rape laboratory at UC Berkeley). As shown in Fig. 3G, the omission of UBA1, UbcH5c and Siah1 (lane 1) or just Siah1 (lane 2) from the reaction failed to induce ELL2 polyubiquitination. Prominent polyubiquitination was observed only when UBA1, UbcH5c and Siah1 were all present in the same reaction together with ATP and the ATP regeneration system (lane 3). Furthermore, in agreement with the in vivo result (Fig. 3F), the C-terminal 109 amino acids of ELL2 were also required for ELL2’s ubiquitination by Siah1 in vitro (Supplemental Fig. S1). Together, these results confirm ELL2 as a direct substrate of Siah1 both in vivo and in vitro.
Sequence variations in the RING domains of Siah1 and Siah2 prevent the latter from inducing ELL2 degradation
Human Siah1 and Siah2 are homologous proteins that display an overall 69.8% identity. However, unlike Siah1, ectopically expressed Siah2 failed to promote ELL2 degradation (Fig. 4A), despite the fact that both Siah proteins interacted with ELL2 similarly well (supplemental Fig. S2). This observation prompted us to focus our attention on the catalytic RING domain, which is known to assist the transfer of ubiquitin from E2s to the substrates (Ye and Rape, 2009), and we examined whether this domain could be responsible for the functional difference between Siah1 and Siah2 toward ELL2.
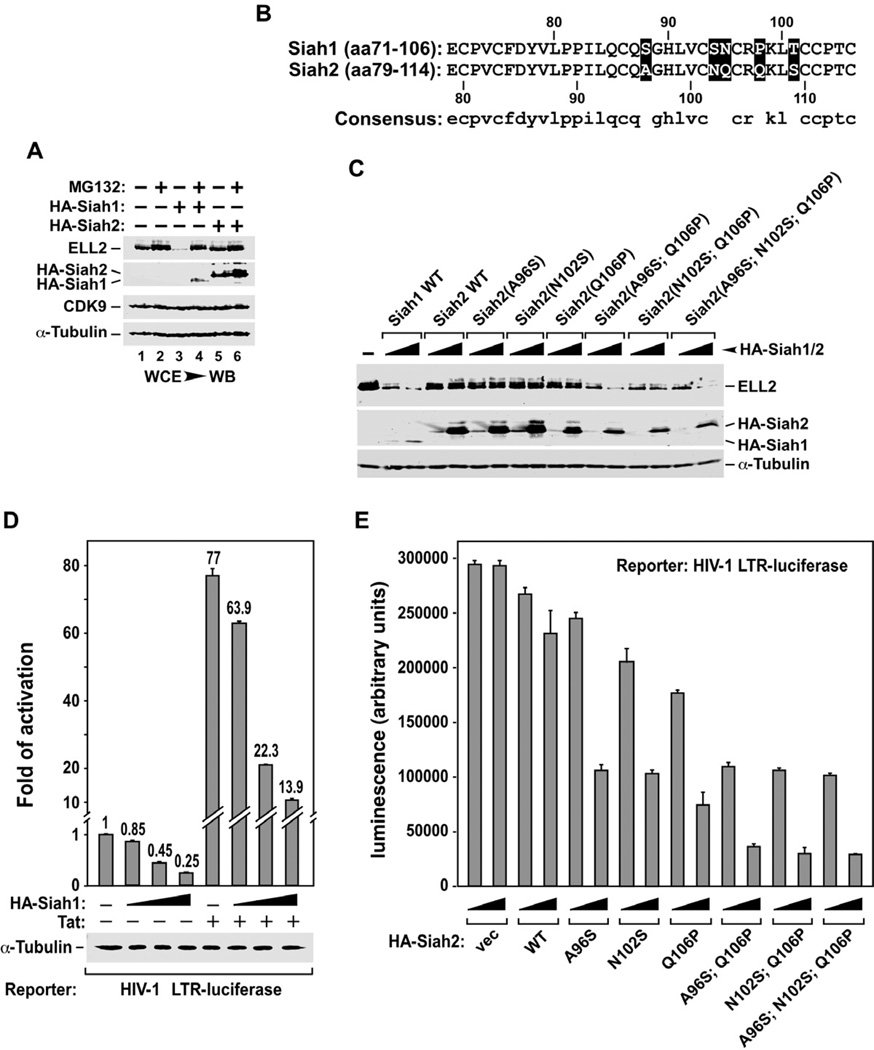
Figure 4. Sequence variations in the RING domains of Siah1 and Siah2 prevent the latter from inducing ELL2 degradation and inhibiting HIV transcription.
A. HeLa cells were transfected with plasmids expressing either HA-Siah1 or HA-Siah2 and then treated with (+) or without (−) MG132. WCE were examined by WB for presence of the indicated proteins. B. Sequence alignment of the RING domains of Siah1 and Siah2, with the amino acid differences highlighted. C. WCE of cells co-transfected with the indicated plasmids were examined by WB for the indicated proteins. The HA-Siah1/2 constructs were introduced in 4-fold increment. D. Luciferase activities were measured in extracts of cells co-transfected with the HA-Siah1-expressing plasmid (in 3-fold increment), the HIV-1 LTR-luciferase reporter gene, and a vector expressing Tat or nothing. The activity in cells expressing neither HA-Siah1 nor Tat was set to 1. The error bars represent mean +/− SD from three independent experiments. E. Cells were transfected with the indicated WT or mutant HA-Siah2 constructs in 3-fold increment and luciferase activities were examined and analyzed as in D.
Besides the two conserved amino acid changes (N at position 95 in Siah1 vs. Q at 103 in Siah2; T at 101 in Siah1 vs. S at 109 in Siah2; Fig. 4B), there are three non-conserved changes within the 36-aa segments that constitute the Siah1/2 RING domains. To determine whether the latter changes are responsible for Siah2’s failure to induce ELL2 degradation, we mutated the amino acids at these positions to those found at the corresponding positions in Siah1. Using WT ELL2 as a reference, changing only one residue at a time largely failed to induce ELL2 degradation by the altered Siah2 (Fig. 4C). Quantification of the data suggests that changing Q106 to P was slightly better than the other two mutations A96S and N102S in this assay. Based on this information, we created double and triple mutants that combined Q106P with A96S, N102S or both. Remarkably, these changes effectively converted Siah2 into an active enzyme for ELL2 degradation (Fig. 4C). Thus, despite the 85.7% identity shard between the Siah1 and Siah2 RING domains, a couple non-conserved amino acid variations within them (postions106 in Siah2 and 98 in Siah1 seem particularly important) render Siah1 a fully functional ubiquitin ligase for ELL2 and Siah2 a mostly inactive enzyme in the reaction.
Inhibition of HIV transcription by Siah2 mutants with altered RING domain
As expected from Siah1’s ability to polyubiquitinate ELL2 and decrease ELL2 level and SEC formation, ectopic expression of Siah1 in HeLa cells markedly inhibited the HIV LTR in a dose-dependent manner under both Tat(−) and (+) conditions (Fig. 4D), and this result recapitulates the effect of direct ELL2 KD on the LTR (He et al., 2010). In contrast, expression of WT Siah2 was largely unable to inhibit the LTR (Fig. 4E). While the Siah2 mutants A96S, N102S, and Q106P, which harbored only a single altered amino acid in the RING domain, were only partially active in this assay (Q106P was once again slightly more active than the other two), the double or triple mutants that contained Q106P together with changes at other non-conserved positions were significantly more active in suppressing the viral LTR (Fig. 4E). This result agrees well with the demonstrated effects of these Siah2 mutants on ELL2 stability as shown in Fig. 4C.
The AFF4-ELL2 interaction stabilizes ELL2 through inhibiting ELL2 polyubiquitination
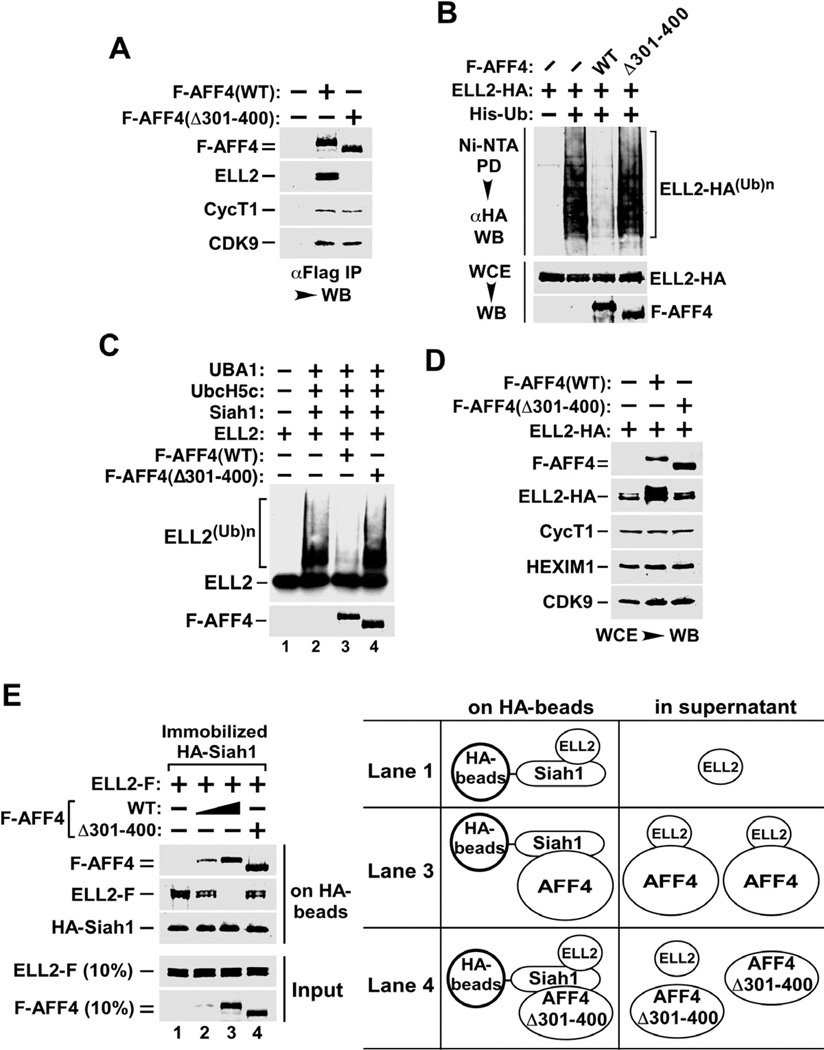
We have previously shown that a key function of the SEC component AFF4 is to significantly increase the half-life of ELL2, thereby promoting SEC formation (He et al., 2010). As a first step toward determining how AFF4 stabilizes ELL2 and whether the ELL2-AFF4 interaction is involved in this process, we generated an internally deleted AFF4 mutant (Δ301–400) lacking amino acids 301 to 400. Compared to WT AFF4, this mutant failed to bind to ELL2 but maintained normal interactions with CDK9 and CycT1 as indicated by Western analysis of co-immunoprecipitated proteins (Fig. 5A).
Figure 5. The AFF4-ELL2 interaction sequesters ELL2 away from Siah1 to inhibit Siah1-induced polyubiquitination and degradation of ELL2.
A. Anti-Flag immunoprecipitates (IP) derived from nuclear extracts of HeLa cells transfectd with the indicated F-AFF4 expression plasmids were analyzed by Western blotting (WB) for presence of the indicated proteins. B. Cells were transfected with the various expression plasmids as indicated at the top. Whole cell extracts (WCE) as well as His-Ub and the covalently bound ELL2-HA isolated from WCE by Ni2+-NTA pull down (PD) were examined by WB for the indicated proteins. C. In vitro ubiquitination reactions were performed with the indicated components. The 35S-labeled ELL2 and F-AFF4 proteins were detected by autoradiography and anti-Flag Western blotting, respectively. D. Cells were co-transfected with a constant amount of the plasmid producing ELL2-HA and the plasmid expressing WT F-AFF4, the Δ301–400 mutant or nothing. WCE were prepared and analyzed by WB for the indicated proteins. E. In vitro binding reactions contained constant amounts of HA-Siah1 immobilized on anti-HA beads and ELL2-F in solution. WT or Δ301–400 F-AFF4 were either not added (−) or added (+) in 3-fold increment into the binding reactions. The bound and input proteins were examined by Western blotting as indicated. The diagram on the right depicts the likely scenarios as encountered in the indicated reactions.
Next, polyubiquitinated ELL2 was isolated from cells expressing either WT AFF4 or AFF4(Δ301–400) and analyzed by Western blotting (Fig. 5B). When normalized for the total ELL2 levels, the presence of WT AFF4 was found to efficiently suppress the polyubiquitination of ELL2, whereas AFF4(Δ301–400) that is defective in ELL2-binding showed no effect. Importantly, the dependence on the AFF4-ELL2 interaction for inhibiting ELL2 polyubiquitination was also confirmed in vitro in ubiquitination reactions containing recombinant proteins (Fig. 5C). Since AFF4(Δ301–400) was even more sensitive to Siah1-induced degradation and thus a better substrate than AFF4(WT) (Supplemental Fig. S3), the inhibition of ELL2 ubiquitination by WT but not the mutant AFF4 was not caused by AFF4(WT) acting as a competitive substrate in the ubiquitination reaction. Finally, like AFF4, the homologous AFF1 protein also inhibited ELL2 polyubiquitination although with somewhat reduced efficiency (Supplemental Fig. S4).
Consistent with its effect on ELL2 polyubiquitination, WT AFF4 also caused more ELL2-HA to accumulate in cells than did AFF4(Δ301–400) on a per molecule basis, when the same amount of ELL2-HA cDNA was co-transfected with either of the two AFF4-expressing constructs (Fig. 5D). Together, these data reveal a strong correlation between AFF4’s inhibition of ELL2 polyubiquitination and its binding and stabilization of ELL2.
The AFF4-ELL2 interaction sequesters ELL2 away from Siah1 thereby inhibiting Siah1 ubiquitination of ELL2
How does the interaction between AFF4 and ELL2 lead to the inhibition of the latter’s polyubiquitination by Siah1? To answer this question, we examined the relationship among purified AFF4, ELL2 and Siah1 proteins (purity confirmed in Supplemental Fig. S5) in binding reactions. In the absence of AFF4, ELL2 bound readily to HA-Siah1 immobilized on anti-HA beads (Fig. 5E, lane 1), which is consistent with their direct substrate-enzyme relationship. The addition of increasing amounts of AFF4 into the binding reactions gradually decreased the levels of ELL2 bound to Siah1, and at the same time increased the amounts of AFF4 retained on the Siah1 beads (lanes 2 & 3). The direct Siah1-AFF4 interaction detected in these two reactions is consistent with the reported binding of Siah1 to AFF1 (Bursen et al., 2004; Oliver et al., 2004), which is highly homologous to AFF4 especially in the region predicted to contact Siah1 (84% similarity).
In contrast to WT AFF4, AFF4(Δ301–400), which is unable to bind ELL2, failed to disrupt the ELL2-Siah1 interaction despite its own efficient binding to Siah1 (Fig. 5E, lane 4). Thus, the AFF4-induced inhibition of the ELL2-Siah1 interaction, and in turn, suppression of ELL2 polyubiquitination was unlikely to be caused by a direct competition between AFF4 and ELL2 for binding to the same surface on Siah1. Rather, the AFF4-ELL2 interaction appeared to sequester ELL2 away, thereby preventing ELL2 from contacting Siah1 (Fig. 5E, diagram on the right). Since AFF4(Δ301–400) failed to bind ELL2, ELL2 was free to bind to Siah1 and became polyubiquitinated in the presence of this mutant (Fig. 5B & C), which likely contacted a different surface on Siah1 (Fig. 5E, lane 4).
Chemical activators of HIV transcription induce sustained ELL2 accumulation and SEC formation but only a transient disruption of 7SK snRNP
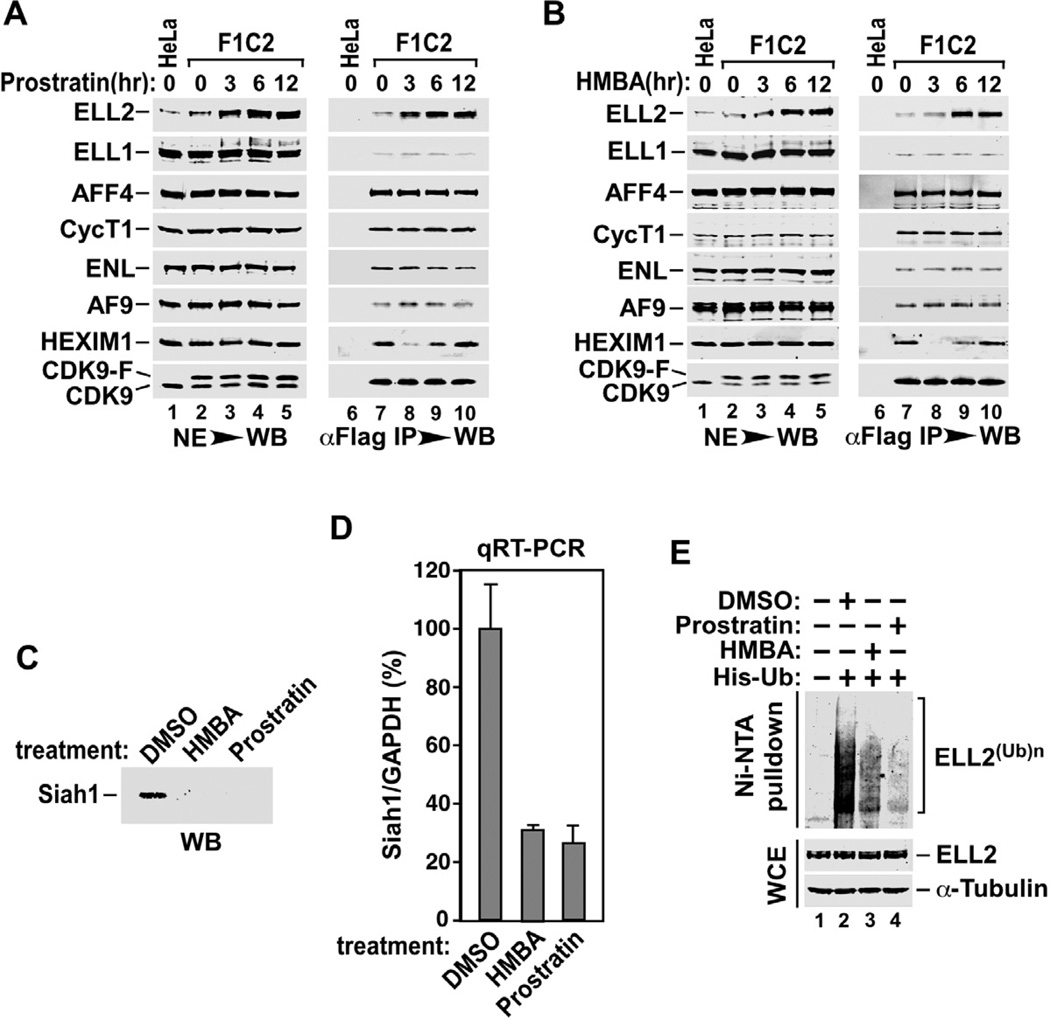
Several chemical compounds that can efficiently stimulate HIV-1 transcription are currently under active investigations for their potential to reactivate viral latency, which is considered a critical initial step toward implementing the so-called “shock and kill” strategy for eradicating the latently infected HIV reservoirs (Richman et al., 2009; Savarino et al., 2009). Because of the central role of SECs in promoting HIV transcription, we examined whether these activators could exert their effects through affecting SEC formation as well as Siah1-mediated control of ELL2 accumulation. First, we examined the cellular levels of SECs components and SECs formation in cells treated with prostratin or HMBA, two well-known chemical activators of HIV transcription and latency. Although the two compounds have completely distinct chemical structures and presumably very different mechanisms of action (e.g. HMBA is proposed to work by releasing P-TEFb from the 7SK snRNP (Contreras et al., 2007; He et al., 2006) and prostratin is known to activate the protein kinase C pathway and NF-κB (Williams et al., 2004), both display a common ability to gradually increase the level of ELL2 but not the homologous ELL1 or any other SECs components in F1C2 cells (Fig. 6A and 6B), a HeLa-based cell line stably expressing CDK9-F (Yang et al., 2001).
Figure 6. Activators of HIV transcription and latency promote ELL2 accumulation and SECs formation through inhibiting Siah1 expression and ELL2 polyubiquitination.
A. & B. F1C2 cells stably expressing CDK9-F were treated with prostratin (A) or HMBA (B) for the indicated periods of time. Nuclear extracts (NE) and anti-Flag immunoprecipitates (IP) derived from NEs were analyzed by Western blotting (WB) for the indicated proteins. C. Siah1 protein isolated from HeLa cells treated with the indicated compounds was analyzed by WB. D. The Siah1 mRNA levels relative to those of GAPDH were determined by qRT-PCR, and the ratio obtained in cells treated with DMSO was set to 100%. The error bars represent mean +/− SD from three independent experiments. E. HeLa cells expressing His-Ub or not were treated with the indicated chemicals for 6 hrs. Whole cell extracts (WCE) as well as His-Ub and the covalently bound ELL2 isolated by Ni2+-NTA pull down (PD) were analyzed by WB for the indicated proteins.
As expected from their promotion of ELL2 accumulation, both prostratin and HMBA also caused more ELL2 to bind to the immunoprecipitated CDK9-F, indicating an increased formation of the ELL2-containing SECs in drug-treated cells (Fig. 6A & 6B). Compared to ELL2, none of the other SEC components showed any increase in sequestration in SECs after the drug treatment, suggesting that ELL2 is stoichiometrically limiting in SECs under normal, untreated conditions.
It is interesting to note that besides the promotion of the ELL2-P-TEFb interaction, prostratin and HMBA also induced the dissociation of HEXIM1, the CDK9 inhibitor in the 7SK snRNP, from P-TEFb (lane 8, Fig. 6A & 6B). However, the effect was only transient (within the first 3 hrs of the treatment) and HEXIM1 became re-associated with CDK9 upon further exposure to the drugs. The transient disruption of 7SK snRNP is not consistent with the sustained induction of HIV gene expression and replication detected in the drug treated cells (Klichko et al., 2006; Vlach and Pitha, 1993). Thus, although prostratin and HMBA could exert multiple effects including the release of P-TEFb from 7SK snRNP, their promotion of sustained ELL2 accumulation and association with P-TEFb in SECs could very well be a predominant mechanism by which they activate HIV transcription and latency.
Prostratin and HMBA inhibit Siah1 expression and ELL2 polyubiquitination
In light of the above demonstrations that Siah1 is a specific E3 ubiquitin ligase for ELL2, we next examined whether prostratin and HMBA could affect ELL2 accumulation through controlling the expression of Siah1. Indeed, both compounds decreased Siah1 expression at both the protein (Fig. 6C) and mRNA (Fig. 6D) levels. Furthermore, they also markedly suppressed the polyubiquitination of ELL2 (Fig. 6E). Taking together, these data reveal an excellent correlation between the abilities of prostratin and HMBA to elevate the ELL2 level and their inhibition of Siah1 expression and Siah1-mediated polyubiquitination of ELL2.
Discussion
Although AFF1 can directly bind to Siah1 and its level decreased by Siah1 overexpression ((Bursen et al., 2004; Oliver et al., 2004) and also Fig. 3B), the data presented here indicate that this largest SECs subunit as well as its close homolog AFF4 are not the primary target of Siah1 present at normal physiological concentrations. Instead, another SEC component ELL2 is demonstrated as a direct substrate of the Siah1 E3 ubiquitin ligase both in vivo and in vitro. Among all known components of SECs, the only protein whose stability is significantly enhanced as a result of Siah1 knockdown is ELL2.
Importantly, in Siah1 knockdown cells, not only the levels of total ELL2 but also ELL2 sequestered in SECs were significantly elevated (Fig. 2B & 2C). Thus, although the SEC-free ELL2, which accounts for ~60% of total ELL2 in HeLa cells (He et al., 2010), is more susceptible to Siah1-induced polyubiquitination and proteasomal degradation than the AFF4-bound, SEC-sequestered ELL2 (Fig. 5), changes in the Siah1 cellular level and/or activity can ultimately affect both ELL2 populations as the SEC-bound ELL2 likely comes from the non-SEC pool.
At relatively low concentrations of Siah1 in cells, ELL2, especially the pool residing outside of SECs, is highly sensitive to Siah1-induced proteolysis, suggesting that the primary effect of Siah1 is to prevent the formation of new ELL2-containing SECs under such conditions. However, when Siah1 levels become high, it is conceivable that all remaining SECs can be efficiently destroyed due to Siah1-induced degradation of not only ELL2 but also AFF1 and AFF4, which are scaffolding proteins in SECs (He et al., 2011; He et al., 2010). The differential reactivity of Siah1 toward distinct SECs components establishes an effective mechanism by which Siah1 can respond to changes in the type and intensity of outside stimuli to exert a range of controls over cellular SECs levels for optimal gene expression.
Similar to AFF4, the HIV Tat protein also markedly increases the half-life of ELL2, resulting in the formation of more SECs in vivo (He et al., 2010). However, unlike AFF4, Tat does not appear to act by inhibiting ELL2 polyubiquitination (data not shown). One possibility is that Tat affects a step downstream of the Siah1-mediated ELL2 polyubiquitination to directly suppress proteasomal degradation of ELL2. Consistent with this idea, Tat has been shown to directly bind to the β subunits of the constitutive 20S proteasome thereby inhibiting the proteolytic activity of the proteasome in cells (Apcher et al., 2003). Interestingly, targeting and dissociating the 26S proteasome has been found to contribute to Tat activation of HIV-1 transcription in a manner that does not depend on the proteolytic activity of the proteasome (Lassot et al., 2007).
An alternative mechanism that could potentially be used by Tat to increase the half-life of ELL2 involves protein phosphorylation. While the Tat-mediated stabilization of ELL2 is correlated with a significant increase in ELL2 phosphorylation, the AFF4-dependent process is clearly not (He et al., 2010). Our data suggest that the P-TEFb kinase activity is likely to be required either directly or indirectly for Tat to promote ELL2’s accumulation (He et al., 2010). However, the exact mechanism by which this is accomplished is still unknown.
Although displaying a strong similarity to Siah1, Siah2 is much less efficient in inducing ELL2 degradation in HeLa cells. This functional difference is not a result of defective interaction with ELL2 by Siah2. Rather, it is caused by a couple of non-conserved amino acid variations within the catalytic RING domains of the two E3 ubiquitin ligases. Since the RING domain is known to participate in the transfer of ubiquitin from E2 ubiquitin-conjugating enzymes to the substrates (Ye and Rape, 2009), it is possible that different E2s or co-factors are used by Siah1 and Siah2 to catalyze polyubiquitination of their substrates and that the E2/co-factor that is specifically required by Siah2 may not be present or functional in HeLa cells. This hypothesis is supported by the observations that Siah1 binds to and works well with members of the UbcH5 family of E2 enzymes (Dimitrova et al., 2010; Matsuzawa and Reed, 2001), which is the most highly conserved E2 family and also the most commonly used in ubiquitination assays in vitro. In contrast, Siah2 does not interact with UbcH5 but binds selectively to UbcH8 (Wheeler et al., 2002), another E2 that is more abundant in neuronal cells (Chin et al., 2002).
Consistent with the idea that Siah2 may lack the appropriate E2/co-factor for its ubiquitin ligase activity in HeLa cells, ectopically expressed Siah2 does not undergo efficient autoubiquitination, which is drastically different from the case of Siah1. As a result, the Siah2 level can be 13-times higher than that of Siah1 when same amount of Siah1 and Siah2 cDNAs were transfected into HeLa cells (data not shown). These observations also raise the possibility that although Siah2 does not cause ELL2 polyubiquitination and degradation in HeLa cells, it may be able to do so in other cell types or under conditions where the required E2/co-factor is readily available.
The data presented in the current study also show that prostratin, a non-tumorigenic phorbol ester, and HMBA, a hybrid bipolar compound, are both able to enhance ELL2 accumulation and SECs formation despite their completely different structures and presumably distinct mechanisms of action (Contreras et al., 2007; He et al., 2006; Williams et al., 2004). The enhancement correlates well with their abilities to decrease Siah1 expression and ELL2 polyubiquitination, although additional mechanisms may be used to further enhance ELL2 expression. For example, the ELL2 mRNA level was found to increase by about 2-fold upon the treatment with prostratin but not HMBA (data not shown).
Besides the elevation of cellular ELL2 levels, prostratin and HMBA also transiently release P-TEFb from 7SK snRNP, which may not lead to a persistent activation of HIV transcription. At this point, these two compounds and their derivatives are under active investigation for their potential to reactivate latent HIV, which can be eliminated subsequently to provide a real cure of HIV/AIDS (Richman et al., 2009; Savarino et al., 2009). Our data suggest that although these compounds could have pleiotropic effects, their induction of ELL2 expression and association with P-TEFb to form functional SECs could be a common and predominant mechanism by which they reactivate HIV from latently infected cells. To improve the efficacy and reduce the toxicity of these and other latency activators and also to develop effective strategies for activating latent HIV, the link between the Siah1 ubiquitination pathway and SECs-dependent HIV transcription identified here will be highly valuable to achieve these goals.
Experimental Procedures
Antibodies
The rabbit polyclonal anti-Siah1 antibodies were purchased from Sigma. The mouse monoclonal antibody against ubiquitin was from Santa Cruz Biotechnology. Antibodies to ELL1/2, AFF1/4, ENL, AF9, CDK9, CycT1, and HEXIM1 were described previously (He et al., 2010; Yang et al., 2005; Yik et al., 2003).
In vitro ubiquitination assay
Human ubiquitin protein was from Boston Biochem. The ubiquitin-conjugating enzyme (E2) UbcH5c and the ubiquitin activating enzyme (E1) UBA1 were kindly provided by Michael Rape’s laboratory at UC Berkeley. Both are recombinant human proteins purified to homogeneity from recombinant E. coli and baculovirus infected Sf9 cells, respectively (Jin et al., 2008). HA-Siah1 and ELL2-F were produced using the TNT® Quick Coupled Transcription/Translation System (Promega) and affinity-purified using anti-HA and anti-Flag beads (Sigma), respectively. The reaction for synthesizing ELL2 also contained 35S-labeled methionine (2 ul of 10 mCi/ml in a 50 ul reaction).
The in vitro ubiquitination reactions were conducted as described (Jin et al., 2008) with minor modifications. The reactions contained Buffer UBAB (25 mM Tris/HCl, pH7.5, 50 mM NaCl, 10 mM MgCl2), 50 nM E1, 100 nM E2, 1 mg/ml ubiquitin, 1 mM DTT, and the energy mix (2 mM ATP, 15 mM creatine phosphate, 1 U creatine phosphokinase, 2 mM MgCl2, 0.2 mM EGTA). Upon incubation at 30°C for 1hr, the reaction products were analyzed by SDS-PAGE followed by autoradiography.
In vivo ubiquitination assay
HeLa cells co-transfected with ELL2-F and His-ubiquitin expression plasmids for 40 hours were incubated with 10 uM MG132 for 6 hours before harvesting. Cells were separated into two aliquots. One aliquot (4%) was used for Western blotting to confirm expression of the transfected proteins. The remaining cells were used for purification of His-tagged proteins by Ni2+-NTA beads. The cell pellet was lysed in buffer A (6 M guanidine-HCl, 0.1 M Na2HPO4/NaH2PO4, 10 mM imidazole). The lysate was sonicated shortly before incubating with 50 ul of Ni2+-nitrilotriacetate–agarose (NTA) beads (Qiagen) and rotating at room temperature for 3 hours. The beads were washed sequentially with buffer A, buffer B (1.5 M guanidine-HCl, 25 mM Na2PO4/NaH2PO4, 20 mM Tris-Cl [pH 6.8], 17.5 mM imidazole), and buffer TI (25 mM Tris-Cl [pH 6.8], 20 mM imidazole). Finally, the beads containing ubiquitin-conjugated proteins were boiled in 40 ul of 2× SDS-PAGE loading buffer containing 200 mM imidazole. Samples were analyzed by Western blotting.
In vitro competition assay
Proteins used in the assay were affinity-purified under highly stringent conditions (1.0 M KCl plus 0.5% NP-40) to strip away their binding partners (He et al., 2010). The purity was confirmed by SDS-PAGE followed by silver staining. HA-Siah1 was immobilized on anti-HA-agarose beads (Sigma). The reactions (300 ul) contained 20 ng ELL2-F and immobilized HA-Siah1 isolated from 400 uL of WCE of transfected HEK293 cells in Buffer D0.1 (20 mM HEPES-KOH [Ph7.9], 15% glycerol, 0.2 mM EDTA, 0.2% NP-40, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluride, and 0.1M KCl). AFF4 (WT or MT) were added at 100 or 300 ng into the reactions to initiate the competition. After incubation at 4°C for 2 hours, the beads were washed and eluted as described (He et al., 2010).
shRNAs targeting Siah1
The two effective shRNA sequences:
shSiah1#4: 5’-GATCCCCCTGCTTTGACTATGTGTTATTCAAGAGATAACACATAGTCAAAGCAGTTTTTA-3’
shSiah1#6: 5’-GATCCCCTGAAGAGCTCTGTGAGTTTTTCAAGAGAAAACTCACAGAGCTCTTCATTTTTA-3’
The two non-effective shRNA sequences:
shSiah1#3: 5’-GATCCCCGATCCATTCGCAACTTGGCTTCAAGAGAGCCAAGTTGCGAATGGATCTTTTTA-3’
shSiah1#8: 5’-GATCCCCCATGTTAGTCTTAGAGAAATTCAAGAGATTTCTCTAAGACTAACATGTTTTTA-3’
All are cloned into the pSuper expression vector.
Highlights.
ELL2 but not the homologous ELL1 is polyubiquitinated for proteasomal degradation
The RING domain protein Siah1 is the E3 ubiquitin ligase for ELL2 ubiquitination
Siah1 cannot access and modify ELL2 bound to the SECs scaffolding protein AFF4
HIV latency activators suppress Siah1 ubiquitination of ELL2 to form more SECs
Supplementary Material
Acknowledgements
We thank Dr. Michael Rape for providing cDNA constructs, purified proteins, and technical assistance for setting up ubiquitination assays. This work is supported by grants (R01AI41757 and R01AI095057) from the National Institutes of Health to Q.Z., a postdoctoral fellowship (F10-B-201) from California HIV/AIDS Research Program to M.L., and a pre-doctoral fellowship (F31GM082156) from the National Institutes of Health to J.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Kruger E. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS letters. 2003;553:200–204. doi: 10.1016/s0014-5793(03)01025-1. [DOI] [PubMed] [Google Scholar]
- Biswas D, Milne TA, Basrur V, Kim J, Elenitoba-Johnson KS, Allis CD, Roeder RG. Function of leukemogenic mixed lineage leukemia 1 (MLL) fusion proteins through distinct partner protein complexes. Proc Natl Acad Sci USA. 2011;108:15751–15756. doi: 10.1073/pnas.1111498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursen A, Moritz S, Gaussmann A, Dingermann T, Marschalek R. Interaction of AF4 wild-type and AF4.MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene. 2004;23:6237–6249. doi: 10.1038/sj.onc.1207837. [DOI] [PubMed] [Google Scholar]
- Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- Chin LS, Vavalle JP, Li L. Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation. J Biol Chem. 2002;277:35071–35079. doi: 10.1074/jbc.M203300200. [DOI] [PubMed] [Google Scholar]
- Churchman LS, Weissman JS. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469:368–373. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Lis JT. Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science. 2008;319:1791–1792. doi: 10.1126/science.1150843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova YN, Li J, Lee YT, Rios-Esteves J, Friedman DB, Choi HJ, Weis WI, Wang CY, Chazin WJ. Direct ubiquitination of beta-catenin by Siah-1 and regulation by the exchange factor TBL1. J Biol Chem. 2010;285:13507–13516. doi: 10.1074/jbc.M109.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Chan CK, Sobhian B, Chou S, Xue Y, Liu M, Alber T, Benkirane M, Zhou Q. Human Polymerase-Associated Factor complex (PAFc) connects the Super Elongation Complex (SEC) to RNA polymerase II on chromatin. Proc Natl Acad Sci USA. 2011;108:E636–E645. doi: 10.1073/pnas.1107107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol Cell. 2010;38:428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Pezda AC, Zhou Q. Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Mol Cell Biol. 2006;26:7068–7076. doi: 10.1128/MCB.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Zhou Q. New insights into the control of HIV-1 transcription: when Tat meets the 7SK snRNP and super elongation complex (SEC) J Neuroimmune Pharmacol. 2011;6:260–268. doi: 10.1007/s11481-011-9267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Chung YL, Glover T, Valentine V, Look AT, Fearon ER. Characterization of human homologs of the Drosophila seven in absentia (sina) gene. Genomics. 1997;46:103–111. doi: 10.1006/geno.1997.4997. [DOI] [PubMed] [Google Scholar]
- Hu G, Fearon ER. Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol Cell biol. 1999;19:724–732. doi: 10.1128/mcb.19.1.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klichko V, Archin N, Kaur R, Lehrman G, Margolis D. Hexamethylbisacetamide remodels the human immunodeficiency virus type 1 (HIV-1) promoter and induces Tat-independent HIV-1 expression but blunts cell activation. J Virol. 2006;80:4570–4579. doi: 10.1128/JVI.80.9.4570-4579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- Lassot I, Latreille D, Rousset E, Sourisseau M, Linares LK, Chable-Bessia C, Coux O, Benkirane M, Kiernan RE. The proteasome regulates HIV-1 transcription by both proteolytic and nonproteolytic mechanisms. Mol Cell. 2007;25:369–383. doi: 10.1016/j.molcel.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145:502–511. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Garrett AS, De Kumar B, Smith ER, Gogol M, Seidel C, Krumlauf R, Shilatifard A. Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC) Genes Dev. 2011;25:1486–1498. doi: 10.1101/gad.2059211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. 2010;37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa SI, Reed JC. Siah-1 SIP Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Mueller D, Garcia-Cuellar MP, Bach C, Buhl S, Maethner E, Slany RK. Misguided transcriptional elongation causes mixed lineage leukemia. PLoS Biol. 2009;7:e1000249. doi: 10.1371/journal.pbio.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PL, Bitoun E, Clark J, Jones EL, Davies KE. Mediation of Af4 protein function in the cerebellum by Siah proteins. Proc Natl Acad Sci USA. 2004;101:14901–14906. doi: 10.1073/pnas.0406196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Regulation of transcription: from lambda to eukaryotes. Trends Biochem Sci. 2005;30:275–279. doi: 10.1016/j.tibs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Savarino A, Mai A, Norelli S, El Daker S, Valente S, Rotili D, Altucci L, Palamara AT, Garaci E. "Shock and kill" effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009;6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, 3rd, Belotserkovskaya R, Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 2004;18:2437–2468. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- Smith E, Lin C, Shilatifard A. The super elongation complex (SEC) and MLL in development and disease. Genes Dev. 2011;25:661–672. doi: 10.1101/gad.2015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhian B, Laguette N, Yatim A, Nakamura M, Levy Y, Kiernan R, Benkirane M. HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol Cell. 2010;38:439–451. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa J, Ichikawa-Iwata E, Kanei-Ishii C, Nakai A, Matsuzawa S, Reed JC, Ishii S. p53 suppresses the c-Myb-induced activation of heat shock transcription factor 3. J Biol Chem. 2000;275:15578–15585. doi: 10.1074/jbc.M000372200. [DOI] [PubMed] [Google Scholar]
- Vlach J, Pitha PM. Hexamethylene bisacetamide activates the human immunodeficiency virus type 1 provirus by an NF-kappa B-independent mechanism. J Gen Virol. 1993;74(Pt 11):2401–2408. doi: 10.1099/0022-1317-74-11-2401. [DOI] [PubMed] [Google Scholar]
- Wheeler TC, Chin LS, Li Y, Roudabush FL, Li L. Regulation of synaptophysin degradation by mammalian homologues of seven in absentia. J Biol Chem. 2002;277:10273–10282. doi: 10.1074/jbc.M107857200. [DOI] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC. Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414:317–322. doi: 10.1038/35104575. [DOI] [PubMed] [Google Scholar]
- Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol cell. 2003;12:971–982. doi: 10.1016/s1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.