Abstract
Racial/ethnic disparities in access to and outcomes of liver transplantation are an important topic given the increasing diversity in the United States. Most reports on this topic predate the advent of allocation based on the Model for End-stage Liver Disease (MELD). For many patients with a variety of lethal conditions, liver transplantation is the only effective therapy, signifying the importance of equitable access to care. Racial/ethnic disparities have been described at various steps of the liver transplant process, including liver disease prevalence and treatment, access to a transplant center and its waitlist, receipt of a liver transplant, and post-transplant outcomes.
The purpose of this mini-review is to critically evaluate the published literature on racial/ethnicity-based disparities in liver disease prevalence and treatment, transplant center referral, transplant rates, and post-transplant outcomes. We identify the shortcomings of previous reports and detail the barriers to completing properly constructed analyses, particularly emphasizing deficits in requisite data and the need for improved study design. Understanding the nature of race/ethnicity-based disparities in liver transplantation is necessary to improve research initiatives, policy design, and serves the broader responsibility of providing the highest quality care to all patients with liver disease.
Keywords: liver transplantation, race, ethnicity, access, outcome
Introduction
The transition from chronic liver disease to lifesaving liver transplantation requires the successful navigation through a complex process from referral, to evaluation, to waitlisting, to transplantation (Figure 1). In kidney transplantation, African Americans have been shown to be less likely than whites to complete each comparable step (1, 2). Similar racial/ethnic disparities in liver transplantation may represent the cumulative effect of structural, process, socioeconomic, cultural, and biological barriers that may arise at many stages, suggesting opportunities for remediation.
Figure 1. A Conceptual Model of Disparities in the Liver Transplant Process.
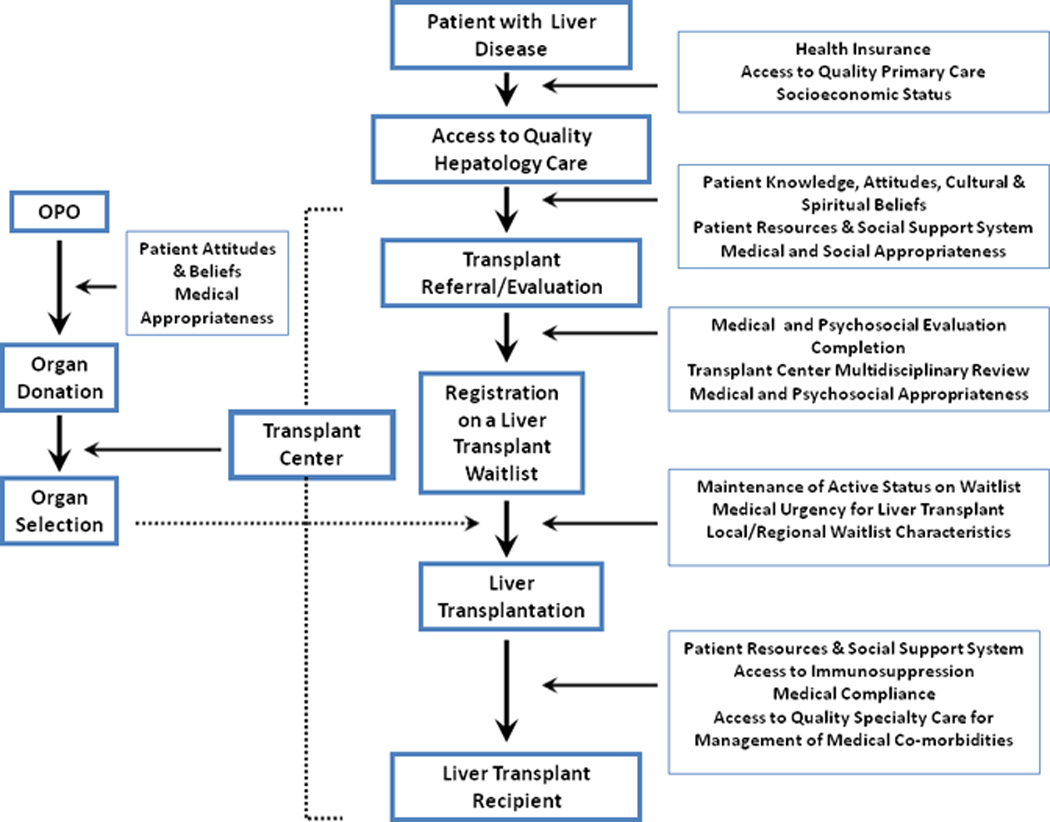
The patient with end-stage liver disease must navigate through several complex steps in order to successfully undergo liver transplantation if indicated. Several factors affect the likelihood that a patient will be able to successfully complete each step in the process. This mini-review aims to critique the available literature from each step in order to better understand racial/ethnic disparities in liver transplantation along a continuum of care.
While “race” has some biological implications, both “race” and “ethnicity” represent social constructs that overlap and are combined for the purposes of this paper. Race can be considered along both biological and social lines, and ethnicity is completely a social construct. If we consider race purely biologically, then differences between racial groups would be solely attributable to genetic differences. Instead, we considered race as a social classification based on the phenotypic characteristics that create differential contact with social factors that affect health status. Race and ethnicity each contribute to health practices, behavioral risks, and environmental exposures. In addition, race/ethnicity affects how patients interact with the health care system and may shape perceptions in health care encounters (3).
The Health Resource and Services Administration defines disparity as a “population-specific difference in the presence of disease, health outcomes, or access to health care” (4). Biological determinants, such as differential susceptibility to developing chronic liver disease, may contribute to racial/ethnic variation in disease despite adequate access to care. Non-biologic factors play a crucial role, such as provider bias, poor physician-patient communication, and an inability to get to the doctor. In evaluating inequities described in the liver transplant process, we have highlighted both health disparities and biological differences in order to guide future research.
As outlined in Table 1, this mini-review critiques the existing literature while focusing on each of the steps from liver disease to transplant: racial/ethnic disparities in liver disease prevalence and treatment, access to the transplant waiting list, access to transplantation once waitlisted, and variation in post-transplant outcomes (5).
Table 1.
Oxford Centre for Evidence-Based Medicine Levels of Evidence for Reports on Racial/Ethnic Disparities in the Liver Transplant Process†(5)
| Stage of Transplant Process |
Study | Year of Publication |
Evidence of Racial/Ethnic Disparities†† | |||
|---|---|---|---|---|---|---|
| African American (vs Whites) |
Hispanic (vs Whites) |
Asian (vs Whites) |
Other (vs Whites) |
|||
| Level of Evidence |
Level of Evidence |
Level of Evidence |
Level of Evidence |
|||
| Liver Disease Prevalence* | Armstrong et al. (6) | 2006 | B | - | - | - |
| Weston et al. (7) | 2005 | - | B | - | - | |
| Lin et al. (8) | 2007 | - | - | B | - | |
| Jim et al. (18) | 2008 | - | - | - | B | |
| Wong et al. (16) | 2008 | B | B | B | B | |
| Treatment of Liver Disease / Cirrhosis / HCC | Jacobson et al. (12) | 2007 | A | - | - | - |
| Nguyen et al. (14) | 2007 | B | B | - | - | |
| Sonnenday et al. (15) | 2007 | B | B | - | B | |
| Rodriguez-Torres et al. (13) | 2009 | - | A | - | - | |
| Access to a Transplant Center | Eckhoff et al. (20) | 1998 | C | - | - | - |
| Julapalli et al. (19) | 2005 | C | - | - | - | |
| Access to the Waiting List and Liver Transplantation | Reid et al. (9) | 2004 | B | - | - | - |
| Siegel et al. (26) | 2008 | B | B | B | - | |
| Moylan et al. (10) | 2008 | B | - | - | - | |
| Robbins et al. (27) | 2008 | - | - | B | - | |
| Post-Liver Transplant Outcome Graft and/or Patient Survival | Neff et al. (28) | 2007 | B | - | - | - |
| Ananthakrishnan et al. (29) | 2008 | B | - | - | - | |
| Freeman et al. **(30) | 2008 | B | B | B | B | |
Oxford Centre for Evidence Based Medicine Levels of Evidence (5): Level A - consistent randomized controlled clinical trial or cohort study; Level B - consistent retrospective cohort, exploratory cohort, outcomes research, case-control study, or extrapolations from level A studies; Level C - case-series study or extrapolations from level B studies; Level D - expert opinion without explicit critical appraisal
Most studies only address a single minority group compared to whites. The dashes in the table indicate that these groups were not addressed by the study in question.
Implies Level of Evidence that the minority group has higher prevalence than whites in the report
This represents the 2007 SRTR Report on the State of Transplantation, which reports trends in graft and patient survival by race/ethnicity. Previous similar reports from the SRTR were not included in the review.
Prevalence of Chronic Liver Disease in Minority Populations
According to data from the National Health and Nutrition Examination Survey and cohort studies, chronic viral hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease are more common in minority communities compared to whites (6–8). Minorities also present to physicians with more advanced disease (9, 10), and have significantly greater morbidity and mortality from cirrhosis compared to whites (11). The reasons for these disparities are likely protean and may be in part non-mutable, such as biological susceptibility to a clinical phenotype. However, modifiable risk factors such as intravenous drug use or hepatitis vaccination affect disease prevalence.
Establishing accurate epidemiology of liver disease in the U.S. is elusive, and thus appropriate risk assessment and timely diagnosis remain major public health challenges. Creation of a national data collection infrastructure to study liver disease prevalence, diagnosis, and treatment would be a herculean but invaluable achievement. Alternatively, racial/ethnic variation in liver disease prevalence could be estimated with a robust and validated sampling methodology. Unfortunately, prospects for funding these strategies are limited. Biased ascertainment of chronic liver disease prevalence is another problem, as patient and practitioner behaviors may lead to under-detection. Racial/ethnic minorities were 34% less likely to be tested for HCV despite having at least one risk factor, and whites were 1.8–2.2 times more likely to be referred to specialty care after an HCV diagnosis than African Americans or Hispanics (11).
Racial/Ethnic Variation in Treatment of Chronic Liver Disease
Substantial differences in treatment response and variability in the appropriateness of care between racial/ethnic groups has been reported, which may in turn affect the need for transplant evaluation. Prospective and retrospective studies indicate that African Americans have a 2.5-fold lower response to HCV antiviral therapy compared to whites (12). Latinos are also more likely to have a lower sustained virologic response to pegylated interferon alfa-2a/ribavirin therapy (13). Racial/ethnic variation in quality of care likely affect response to therapy and drop out. Translational studies are needed to address racial/ethnic variation in HCV treatment response. Further, racial/ethnic variation in therapy for non-viral liver diseases is not well studied.
Racial/ethnic variation in the management of cirrhosis and its complications has been reported. Minority patients were 30–60% less likely to receive a portosystemic shunt, 60% more likely to have delayed endoscopy for variceal bleeding, and more likely to die (14). This study employed the Nationwide Inpatient Sample (NIS), a database that includes 20% of U.S. hospital discharges. Since the unit of analysis was at the hospitalization level, more frequent hospitalizations among a few individuals within a given ethnic group may lead to misinterpretation. The diagnosis codes in NIS are derived from billing abstracts and are not validated by medical record review. The influences of geography and practice variation on cirrhosis management have not been clarified. Based on this study, it seems that minorities receive worse care for cirrhosis, but several sources of bias cloud potential conclusions.
HCC represents the fifth leading cause of cancer death worldwide, and may be cured with transplantation in patients who present at an appropriate stage (15). African Americans and Asians have demonstrated two- to four-fold higher HCC incidence than whites (16, 17). Native Americans and Alaskan Natives have increasing incidences of HCC (18). While some degree of the differential incidence may be biologically driven, lower utilization of surgical therapy (24–27% less often than white patients) exacerbates disparities in time to diagnosis (15). Given that HCC accounts for a growing proportion of liver transplants, metrics that suggest poor access to care for minorities with HCC are particularly concerning. Studies addressing provider bias or other systematic barriers to appropriate care for HCC are clearly warranted.
Access to a Liver Transplant Center for Evaluation
Referral practice patterns from community physicians to transplant centers are a poorly studied area. One retrospective study has directly addressed racial/ethnic disparities in transplant referral. Julapalli et al. found that suitable African American veterans were 85% less likely than white veterans to have been referred for liver transplantation (19). This study was well-performed, but lacks generalizability due to the unique U.S. Veterans Administration transplant referral process. Eckhoff et al. reported that 14% of 844 liver transplant referrals were African American at a single center, despite a 25% African American population served by the hospital, demonstrating “under-representation” (20). Accurate measurement of racial differences in referral cannot assume a racially/ethnically-uniform distribution of liver disease in the parent population. The racial differences identified in these studies may not necessarily be a systematic disparity, and other factors may be affect referral including socioeconomic status (SES), geography, local organ procurement organization factors, physician practice variation, and provider perceptions (2, 20–22). Further, the paucity of studies on transplant referral may belie its decentralized nature (Table 1). Comprehensive evaluation of referral patterns are needed to inform policies aimed at improving minority access to transplant centers. Delays in referral carry important implications with regards to disease progression and mortality risk. Given the mortality burden associated with end-stage liver disease in the U.S., further study of referral patterns to transplant centers is particularly warranted.
Several studies have addressed the role of insurance status in transplant access (23). An estimated 30% of HCV-positive individuals are uninsured, and the insured are 1.5 times more likely to have public insurance than those who are HCV-negative. Medicaid enrollment, which is more common in minority communities, has also been associated with higher Model for End Stage Liver Disease (MELD) scores at transplant registration and worse post-transplant outcome (23). However, private insurance was not associated with improved minority access to transplant for hepatocellular carcinoma (HCC) in a single center retrospective study (17). Local variation in coverage under private insurance plans and state Medicaid programs further confounds racial/ethnic differences in access to care.
Insurance status is likely a surrogate of SES. Associations of insurance coverage and SES are not entirely collinear, so using one to represent the ot1her may bias secondary data analyses. Attempts to disentangle these factors analytically are flawed because the SES variables are usually based on geographic units (e.g., zip code) rather than on individual-level attributes, leading to misleading ecological inferences. The resulting spurious associations highlight the need for more granularity in data collection regarding insurance status and SES.
Access to the Liver Transplant Waitlist and from Waitlist to Transplant
Following referral, potential recipients must complete an intricate evaluation, and waitlist registration only occurs after review by a multidisciplinary team. No data exist regarding barriers to the completion of the liver transplant processor disparities in waitlisting rates. Barriers to transplant evaluation can be extrapolated from the ESRD literature in this context, but differences exist based on the acuity of liver failure and the lack of alternative therapies (24). Kidney and liver patients both undergo complex transplant evaluations, which require social support (25). Patients with cirrhosis may rely on social networks even more than potential renal transplant candidates because the evaluation process may have greater immediacy for patients with cirrhosis, but this has not been studied.
Disparities in waitlist mortality and transplant rates for minorities have received considerable attention. Reid et al. found a 33% lower transplant rate for African Americans compared to whites in the pre-MELD era (9), but the study had several limitations including lack of adjustment for geographic factors, center practices, and donor availability. In fact, similar rates of liver transplantation were found regardless of race when all follow-up was included in a time-to-transplant model. In their logistic regression model for waitlist dropout, African Americans were 50% more likely to become too sick for transplant or die. Potential statistical bias related to hospitalization status and waiting time for pre-MELD era patients may exist, since it is unknown if these modifiable factors affected the allocation rank order for minority candidates (9).
Recently, Moylan et al. reported on more than 45,000 African American and white patients, estimating liver transplant waitlist outcomes before and during the MELD era from Organ Procurement and Transplantation Network (OPTN) data (10). Hispanics, the largest ethnic minority in the U.S., were excluded, despite their rising incidence of liver disease, and higher mortality risk from liver disease compared to whites (10, 11). African Americans had a 25% lower transplant rate than whites pre-MELD, but displayed no difference in the MELD era within four arbitrary aggregates that grouped adjacent OPTN regions. Truncated follow-up and race-gender interactions may have produced biased results in this study. It is premature, therefore, to state that racial/ethnic disparities in access to transplantation from the waitlist have been eliminated in the MELD era.
Identification of racial/ethnic disparities in pre-transplant HCC care is particularly important, since curative therapies are available for small tumors, and access to liver transplant for HCC is enhanced under the MELD system with special allocation rules. Analyses of the National Cancer Institute’s Surveillance, Epidemiology, and End-Results (SEER) data have shown that some minorities with localized HCC utilized transplant less than whites, but the gap may be narrowing for Asians (26, 27). These data may have had measurement bias in tumor size and confounding bias due to lack of clinical detail. Moreover, these studies are unable to ascribe the disparity to a specific step in the transplant process, and other therapies offered or received are not accounted for methodologically. Moylan et al., however, did not find significant racial differences in transplant rates among HCC candidates who were waitlisted (10).
Research on racial/ethnic equity in access to liver transplantation can be improved. Most studies are observational due to the availability of OPTN data, which includes significant clinical detail on candidates, recipients, and donors. OPTN data quality improvement, however, needs to be a continuous process. More granular data on race, ethnicity, and SES are needed to understand these determinants. In order to determine the impact of geographically dissimilar waitlist outcomes, local/regional organ sharing, and organ availability on racial/ethnic disparities in transplant access, it is important to properly account for geographic variation within the context of allocation policy. With the advent of newer technologies to manage waitlisted patients with HCC, study designs should account for the receipt of ablative therapies prior to transplantation. Observational studies that inform medical decision making may have biased results if researchers fail to account for endogeneity in study cohorts. Finally, qualitative methods may be helpful in studying racial/ethnic differences in perception of the liver transplant process.
Post-Liver Transplant Outcomes
Data on racial/ethnic-based differences in post-transplant outcome are conflicting. Single center studies have reported equivalent graft and patient survival across racial/ethnic groups, but many are under-powered, and all are subject to pre- and post-MELD era effects. In studies of national registry data, African Americans had up to 10% worse overall survival and a two-fold higher risk of graft failure compared to whites at 10 years post-transplant (28, 29). Few attempts to model geographic or donor effects have been made in these studies, so the proposition that race has a large independent effect is speculative. The 2007 Scientific Registry of Transplant Recipients (SRTR) Report on the State of Transplantation provided comprehensive data indicating that African Americans had worse 3-month, 1-year, 3-year, and 5-year unadjusted graft survival rates when compared to whites (30). The true extent of racial/ethnic variation in major post-transplant events such as death or graft failure is difficult to discern from these data. Attempts to examine the effect of SES on post-transplant outcome are limited by inconsistent definitions and difficulties in obtaining socioeconomic data in national transplant registries (31). As a result, observations of racial/ethnic disparities on outcomes remain unexplained in terms of biological, social, or cultural factors.
In addition, minority liver recipients have been reported to experience higher rates of post-transplant infection and chronic rejection. Post-transplant HCV recurrence is nearly universal, and given that African Americans are more resistant to anti-HCV therapy in general, results are worse overall for this group of recipients (32). Among seropositive liver recipients, post-transplant CMV infection is three times more common in Hispanics (33). One small study reported a 15% higher incidence of chronic rejection among nonwhites versus whites (34).
Racial/ethnic differences in post-transplant outcomes have been described in recent OPTN/SRTR Annual Reports, which provide descriptive outcomes across racial/ethnic groups. More sophisticated analyses have not been performed, including needed studies evaluating center and geographic effects on racial/ethnic variation in post-transplant survival, graft failure, and health-related quality of life. In particular, equity in health-related quality of life has not been explored. Racial/ethnic variation in post-transplant outcome may be biologically driven, and in part related to race/ethnicity differences in immunosuppression pharmacokinetics and adherence, which have not been well-studied after liver transplantation, and only partially examined in renal transplantation (35).
Discussion
For minority groups, higher endemic rates of liver disease, greater morbidity and mortality from cirrhosis, and low referral rates to transplant centers confound our understanding of race/ethnicity in access to liver transplantation. Data on differences in waitlist event rates and post-transplant outcomes also are incomplete. At each step in the transplant process, however, racial/ethnic minorities appear to be disadvantaged despite the inherent analytical and statistical biases present in many of the reported studies. Table 1, which displays the highest level of evidence available for racial/ethnic disparities-related studies at each step in the liver transplant process, underscores the need for more prospective research. As emphasized in the Table, most of the available studies have focused on one race/ethnic group, and do not fully describe the issue of health disparities in liver transplantation.
Racial/ethnic disparities-related conclusions in transplant research could generate ethical issues relating to use of scarce donor resources, and equity is one of the competing goals of transplant allocation. Interventions designed to better understand and ameliorate the role of racial/ethnic barriers are needed and review of the available data has crystallized areas where information can be improved.
We have depicted the liver transplant process as a continuum, using an approach that has been applied to kidney transplantation (2, 24). The factors in play at each step in process may be exerted at multiple levels, and our model (Figure 1) represents a simplified representation. Looking at all studies addressing racial/ethnic disparities across this spectrum of care, we assert that racial/ethnic disparities and biological differences each affect access to care and outcomes for patients with liver disease. The weaknesses of many of these studies represent opportunities to improve our understanding. New research, conducted using a variety of methods including cohort studies, clinical trials, and translational methods, should address the problems noted here, accounting a priori for selection, measurement, and confounding biases present in current observational studies.
Policy makers and the transplant community cannot assess the degree of racial/ethnic disparity in access to transplantation without understanding which groups are at highest risk for liver failure. The available data likely underestimate the true burden of liver disease in the U.S., and perhaps the associated magnitude of disparity. In order to understand problems of access, it is necessary to understand the geographic, socioeconomic, and racial/ethnic distribution of those who may benefit from transplant. Diagnosis-based regional and national registries are used to track cancer and ESRD. An analogous registry for chronic liver disease could be envisioned that, in conjunction with linkage to the SRTR, would facilitate development of allocation policy through better estimates of end-stage liver disease incidence. Such data should provide a basis for continuous quality improvement. Similar analyses, performed regularly for ESRD and renal transplantation, have produced useful models.
Other clinical developments in liver transplantation may have profound implications on racial/ethnic disparities in access and outcome. The growing use of novel treatment strategies for HCC, expansion of the donor pool, and the use of living donors are examples of emerging practices that remain untouched in the context of racial/ethnic disparities research. Future research should focus on these issues, with careful attention to the equally important socioeconomic factors that may affect access to newer therapies.
Progress in overcoming the challenges to achieving racial/ethnic equity in liver transplantation is contingent on coupling high quality data with rigorous research methods in studies addressing this critical issue. Attention should be particularly paid to areas where current data are inadequate and in situations where clinical practices are in evolution. This approach may improve our understanding of racial/ethnic disparities in transplantation at large.
References
- 1.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280(13):1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 2.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant. 2009;9(4):669–674. doi: 10.1111/j.1600-6143.2009.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert PL, Sisk JE, Howell EA. When does a difference become a disparity? Conceptualizing racial and ethnic disparities in health. Health Aff (Millwood) 2008;27(2):374–382. doi: 10.1377/hlthaff.27.2.374. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg J, Hayes W, Huntley J. Understanding Health Disparities. 2004 Available from: http://www.healthpolicyohio.org/publications.html.
- 5.Phillips B, Ball C, Badenoch D, Straus S, Haynes B, Dawes M. Oxford Centre for Evidence-based Medicine Levels of Evidence. 2001 May [Google Scholar]
- 6.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 7.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 8.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46(4):1034–1040. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 9.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl. 2004;10(7):834–841. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 10.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kung H, Hoyert DL, Xu J, Murphy SL. Deaths: Final Data for 2005. National Vital Statistics Reports. 2008;56(10) [PubMed] [Google Scholar]
- 12.Jacobson IM, Brown RS, Jr, McCone J, Black M, Albert C, Dragutsky MS, et al. Impact of weight-based ribavirin with peginterferon alfa-2b in African Americans with hepatitis C virus genotype 1. Hepatology. 2007;46(4):982–990. doi: 10.1002/hep.21670. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, Rossaro L, Ankoma-Sey V, Hamzeh FM, et al. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360(3):257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen GC, Segev DL, Thuluvath PJ. Racial disparities in the management of hospitalized patients with cirrhosis and complications of portal hypertension: a national study. Hepatology. 2007;45(5):1282–1289. doi: 10.1002/hep.21580. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11(12):1636–1646. doi: 10.1007/s11605-007-0315-8. discussion 1646. [DOI] [PubMed] [Google Scholar]
- 16.Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525–531. doi: 10.1016/j.amjmed.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Kemmer N, Neff G, Secic M, Zacharias V, Kaiser T, Buell J. Ethnic differences in hepatocellular carcinoma: implications for liver transplantation. Dig Dis Sci. 2008;53(2):551–555. doi: 10.1007/s10620-007-9872-7. [DOI] [PubMed] [Google Scholar]
- 18.Jim MA, Perdue DG, Richardson LC, Espey DK, Redd JT, Martin HJ, et al. Primary liver cancer incidence among American Indians and Alaska Natives, US, 1999–2004. Cancer. 2008;113(5 Suppl):1244–1255. doi: 10.1002/cncr.23728. [DOI] [PubMed] [Google Scholar]
- 19.Julapalli VR, Kramer JR, El-Serag HB. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11(11):1370–1378. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 20.Eckhoff DE, McGuire BM, Young CJ, Sellers MT, Frenette LR, Hudson SL, et al. Race: a critical factor in organ donation, patient referral and selection, and orthotopic liver transplantation? Liver Transpl Surg. 1998;4(6):499–505. doi: 10.1002/lt.500040606. [DOI] [PubMed] [Google Scholar]
- 21.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 22.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM. Physicians' beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis. 2004;43(2):350–357. doi: 10.1053/j.ajkd.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Kemmer N, Zacharias V, Kaiser TE, Neff GW. Access to Liver Transplantation in the MELD Era: Role of Ethnicity and Insurance. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GC, Sehgal AR. Why hemodialysis patients fail to complete the transplantation process. Am J Kidney Dis. 2001;37(2):321–328. doi: 10.1053/ajkd.2001.21297. [DOI] [PubMed] [Google Scholar]
- 25.Clark CR, Hicks LS, Keogh JH, Epstein AM, Ayanian JZ. Promoting access to renal transplantation: the role of social support networks in completing pre-transplant evaluations. J Gen Intern Med. 2008;23(8):1187–1193. doi: 10.1007/s11606-008-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel AB, McBride RB, El-Serag HB, Hershman DL, Brown RS, Jr, Renz JF, et al. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998–2002. Am J Gastroenterol. 2008;103(1):120–127. doi: 10.1111/j.1572-0241.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 27.Robbins AS, Daily MF, Aoki CA, Chen MS, Jr, Troppmann C, Perez RV. Decreasing disparity in liver transplantation among white and Asian patients with hepatocellular carcinoma : California, 1998–2005. Cancer. 2008;113(8):2173–2179. doi: 10.1002/cncr.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neff GW, Kemmer N, Kaiser T, Zacharias V, Majoras N, Safdar K. Outcomes in adult and pediatric liver transplantation among various ethnic groups. Transplant Proc. 2007;39(10):3204–3206. doi: 10.1016/j.transproceed.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the MELD era. Am J Gastroenterol. 2008;103(4):901–910. doi: 10.1111/j.1572-0241.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 30.Freeman RB, Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant. 2008;8(4 Pt 2):958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. 2004;10(2):235–243. doi: 10.1002/lt.20069. [DOI] [PubMed] [Google Scholar]
- 32.Smallwood GA, Coffey G, Davis L, Martinez E, Stieber AC, Heffron TG. Hepatitis C treatment outcomes of African Americans following liver transplantation. Transplant Proc. 2002;34(8):3317–3318. doi: 10.1016/s0041-1345(02)03573-x. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transpl. 2005;11(6):700–704. doi: 10.1002/lt.20417. [DOI] [PubMed] [Google Scholar]
- 34.Freese DK, Snover DC, Sharp HL, Gross CR, Savick SK, Payne WD. Chronic rejection after liver transplantation: a study of clinical, histopathological and immunological features. Hepatology. 1991;13(5):882–891. [PubMed] [Google Scholar]
- 35.Chisholm-Burns MA, Kwong WJ, Mulloy LL, Spivey CA. Nonmodifiable characteristics associated with nonadherence to immunosuppressant therapy in renal transplant recipients. Am J Health Syst Pharm. 2008;65(13):1242–1247. doi: 10.2146/ajhp070630. [DOI] [PubMed] [Google Scholar]


