Abstract
We report the isolation and characterization of arylpalladium cyanide complexes that undergo reductive elimination to form arylnitriles. The rates of reductive elimination from a series of arylpalladium cyanide complexes reveal that the electronic effects on the reductive elimination from arylpalladium cyanide complexes are distinct from those on reductive reductive eliminations from arylpalladium alkoxo, amido, thiolate, and enolate complexes. Arylpalladium cyanide complexes containing aryl ligands with electron-donating substituents undergo faster reductive elimination of aromatic nitriles than complexes containing aryl ligands with electron-withdrawing substituents. In addition, the transition state for the reductive elimination of the aromatic nitrile is much different from that for reductive eliminations that occur from most other arylpalladium complexes. Computational studies indicate that the reductive elimination of an arylnitrile from Pd(II) occurs through a transition state more closely related in structure and electronic distribution to that for the insertion of CO into a palladiumaryl bond.
The palladium-catalyzed cyanation of aryl halides has become a common method for the synthesis of arylnitriles.1 Since the first report by Takagi in 1973,2 a large number of transition metal-catalyzed cyanations of aryl halides have been reported, and recently developed protocols occur under conditions suitable for commercial production.1,3 Despite these advances, little information on the productive steps of the catalytic cycle has been reported. Grushin and coworkers observed anionic palladium(0) and palladium(II) cyanide complexes from displacement of the phosphine ligand with cyanide and have shown that these complexes do not undergo the oxidative addition and reductive elimination steps proposed to occur during the catalytic cycle.4 Subsequently, Beller and coworkers demonstrated the advantageous effect of diamines on the oxidative addition of aryl halides to Pd(PPh3)4 in the presence of excess cyanide ion and on the rate of transmetallation proposed to form an arylpalladium cyanide that undergoes reductive elimination. However, the arylpalladium cyanide complex was not observed.5
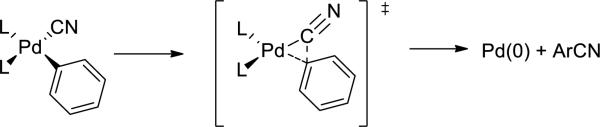
Thus, no reports have described the isolation of an arylpalladium cyanide complex or evaluated its propensity to undergo reductive elimination. Metal-cyanide linkages are well known to be thermodynamically stable,6 and the facile formation of anionic palladium(II) cyanide complexes4 underscores that palladium forms strong bonds with cyanide ligands. However, the absence of an observed arylpalladium(II) cyanide complex suggests that such complexes could be unstable; they could degrade in the presence of excess cyanide to form anionic species, or the sp2-sp carbon-carbon bond-forming reductive elimination to form arylnitrile could be fast. If arylpalladium cyanide complexes could be isolated and induced to undergo reductive elimination (Scheme 1), then one could gain information on the factors controlling the rates of this process. Moloy and Nolan proposed that reductive elimination of alkyl nitriles occurs by a mechanism related to that for CO insertion based on relative rates that coincided with those for CO insertion into metal-alkyl bonds. An accelerating effect of Lewis acids on the reductive elimination further supported a mechanism related to insertion. However, coupling of a cyano ligand with an aryl group could occur by a process much different than that for coupling with an alkyl group, and elimination of an aryl nitrile allows the electronic effects on the this class of reductive elimination to be probed in the absence of steric effects.7,8
Scheme 1.
Reductive elimination of aryl nitriles from a proposed arylpalladium cyanide complex.
Here we report an isolated arylpalladium cyanide complex and reductive eliminations from this complex and its congeners to form aromatic nitriles. The experimental and computational data we report assess the relationships between this reductive elimination and related reductive eliminations from palladium(II) complexes or migratory insertions of carbon monoxide. The effects of the electronic properties of the aryl ligand on the rates and thermodynamics for reductive elimination to form arylnitriles contrast those of reductive eliminations from palladium(II) to form other types of C-C bonds9 or to form C-N,10 C-O,11 or C-S12 bonds.13 Instead, our data and computational studies imply that the transition state maps closely onto that for insertion of CO into a metal-aryl bond.
Our studies began with efforts to isolate a stable arylpalladium cyanide complex. To do so, a variety of bisphosphine-ligated p-tolylpalladium bromide complexes were treated with sources of cyanide. The reactions of p-tolylpalladium(II) bromide complexes containing DPPF (DPPF = bis-1,1’-diphenylphospino ferrocene) and DiPPF (DiPPF = bis-1,1’-diisopropylphosphino ferrocene) with NBu4CN at 20 °C led to the complete conversion of the complexes, as determined by 31P NMR spectroscopy. 1H NMR analyses of the reaction mixtures indicated the formation of p-tolylnitrile in 89% and 98% yield, respectively, but the presumed arylpalladium cyanide complex was not observed directly. However, the Josiphos (CyPF-t-Bu)-ligated p-tolylpalladium bromide complex reacted with NBu4 13CN at -78 °C to form a new complex that was sufficiently stable to be characterized at -40 °C by 1H and 31P NMR spectroscopy. The two doublets of doublets in the 31P NMR spectrum confirmed binding of one labeled cyanide ligand to palladium (see supporting information for spectra). After warming the solution to room temperature, p-tolylnitrile was formed in 88% yield.
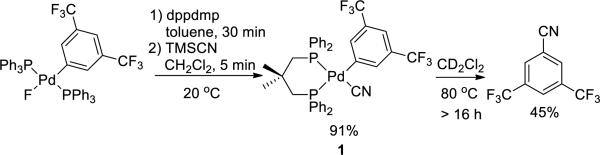
The arylpalladium cyanide complex (aryl = C6H4-p-Cl) containing the rigid bisphosphine ligand dppdmp (dppdmp = diphenyl-phosphine dimethylpentane) generated in situ from the reaction of (dppdmp)Pd(C6H4-p-Cl)(F) and TMSCN was even more stable. This complex persisted for hours at room temperature, as determined by 31P NMR spectroscopy, whereas the complex containing the more electron-rich para-anisyl group was unstable at room temperature. Ultimately, we found that complex 1 containing two trifluoromethyl groups on the palladium-bound aryl ligand was stable enough to be isolated in 91% yield (Scheme 2). Complex 1 was characterized by 1H, 31P NMR and IR spectroscopy. This complex undergoes reductive elimination at elevated temperatures over an extended time; heating a solution of 1 at 80 °C for 16 hours led to the conversion of >95% of 1 to form 3,5-trifluoromethyl benzonitrile in 45% yield, according to 1H NMR spectroscopy (Scheme 2).14
Scheme 2.
Synthesis and reductive elimination of arylpalladium cyanide complex 1.
These initial studies indicated that the arylpalladium cyanide complex containing an electron-poor aryl group is more stable toward reductive elimination than the analogous complex containing a more electron-rich aryl group. This relative reactivity contrasts that for reductive eliminations to form carbon-carbon bonds from arylpalladium(II) and arylplatinum(II) complexes, which occur faster from complexes containing electron-withdrawing substituents on the aryl group than from analogous complexes containing electron-donating substituents.9,13
 |
(2) |
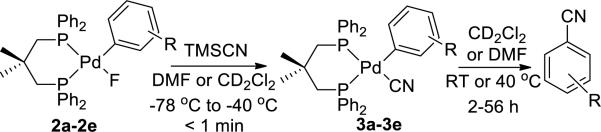
To investigate the effect of the electronic properties of the reacting aryl ligand quantitatively, a series of dppdmp-ligated arylpalladium cyanide complexes 3a-3e were prepared from fluoride complexes 2a-2e. Reaction of these fluoride complexes with TMSCN formed arylpalladium cyanide complexes (3a-3e, eq 2) that were characterized at low temperature by 1H and 31P NMR spectroscopy and by room temperature IR spectroscopy in some cases. Only the 3,5-trifluoromethyl-substituted complex 1 described above was stable enough to isolate.
Complexes 3a-3e underwent reductive elimination at 20 or 40 °C at convenient rates in DMF and CD2Cl2 to form the corresponding arylnitrile products in 80-95% yield (Scheme 3, Table 1). In DMF, the Pd(0) product was Pd(dppdmp)2 and was isolated in 72% yield by from heating 2a, TMSCN, and dppdmp at 80 °C in DMF for 30 min. In CD2Cl2, the palladium product (dppdmp)PdCl2 crystallized from the solution of complex 2e and TMSCN (Scheme 3, 3e), implying that the Pd(0) product reacts with the CD2Cl2 solvent.
Scheme 3.
Reductive elimination from arylpalladium cyanide complexes 3a-3e.
Table 1.
Yields and rates of reductive elimination from 3a-3e.
| Complex | R | k (10-4 s-1)a | k (10-4 s-1)a,e | Yieldb |
|---|---|---|---|---|
| 3a | p-CF3 | 0.23 | - | 81g |
| 3a | p-CF3 | 1.8 | 0.73 | 94 |
| 3a | p-CF3 | 3.2e,f | 3.6f | 81 |
| 3b | p-Cl | 0.62d | - | 77h |
| 3b | p-Cl | 6.7e | 1.3 | 98 |
| 3c | H | 2.5d | 11 | 80 |
| 3d | p-OMe | 7.4c | 21 | 94 |
| 3e | o-Me | 5.9 | 22 | 93 |
First-order rate constants determined by monitoring the decay of the cyanide complex by 1H NMR spectroscopy (CD2Cl2) or 31P NMR spectroscopy (DMF).
Yield of the arylnitrile for the reductive elimination step determined by 1H NMR spectroscopy (in CD2Cl2).
Average of 3 separate runs.
Average of 2 separate runs.
At 40 °C.
With 2 equiv. B(C6F5)3.
Yield after 81% conversion of the cyanide complex.
Yield after 87% conversion of 3b.
The rate constants for reductive elimination were measured by monitoring the reaction by 1H and 31P NMR spectroscopy over a period of 2-56 h and are shown in Table 1. Indeed, complexes containing electron-withdrawing substituents on the aryl ligand (3a and 3b, Scheme 3) underwent reductive elimination more slowly than those containing unsubstituted aryl ligands or electron-donating substituents on the aryl ligand (3c and 3d,Scheme 3), and a ρ value of -2.00 in CD2Cl2 and DMF was obtained from a Hammett plot (See supporting information for the Hammett plots). This trend in electronic effects suggests that the aryl ligand acts as a nucleophile in the coupling with an electrophilic cyanide ligand. As a side note, complex 3e containing an ortho-substituted tolyl ligand reacted more rapidly than the less congested arylpalladium cyanide complexes.
Prior reductive eliminations to form alkyl nitriles from alkylpalladium cyanide complexes were accelerated by coordination of Lewis acids to the nitrogen of the cyanide ligand.7. Reactions of complex 3a with added B(C6F5)3 at 40 °C were only two to five times as fast as those without added B(C6F5)3, whereas the prior reactions of an alkylpalladium cyanide complex with added Lewis acids were one to two orders of magnitude faster than the reactions without an added Lewis acid. Although small, this effect of Lewis acid is consistent with a pathway in which the aryl group acts as a nucleophile and the cyanide ligand an electrophile.
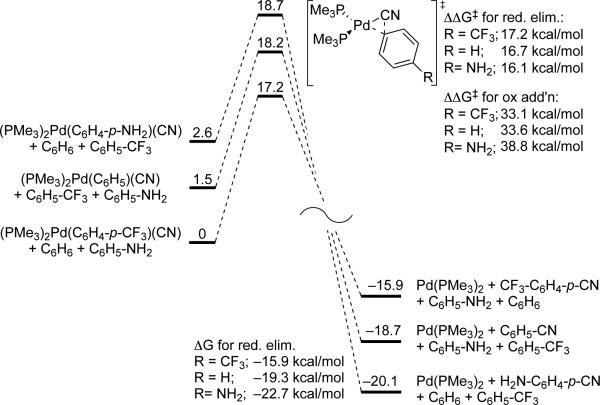
A series of computational studies were conducted to gain insight into the origin of the electronic effects on the reductive elimination of arylnitriles from arylpalladium(II) complexes. The ground-state and transition-state structures of three model PMe3-ligated arylpalladium cyanide complexes were calculated, and the results of these calculations are shown in Figure 1. The reductive elimination of benzonitrile from the phenylpalladium cyanide complex containing two cis-disposed PMe3 ligands was computed to have a barrier of 16.7 kcal/mol. The barrier to elimination from the complex containing the more electron-donating p-aminophenyl group was computed to be lower (16.1 kcal/mol) than that for elimination from the complex containing the more electron-poor p-trifluoromethylphenyl group (17.2 kcal/mol). These results agree with the experimental observation that reductive elimination from the more electron-rich arylpalladium cyanide complexes is faster than that from the more electron-poor analog.
Figure 1.
Ground state, transition state and overall free energies in kcal/mol for PMe3-ligated arylpalladium cyanide complexes undergoing reductive elimination of arylnitriles.
The effect of the electronic properties of the aryl group on the thermodynamic driving force for the reductive elimination was parallel to, but much larger, than that on the relative rates. Reductive elimination of aryl nitrile from p-aminobenzonitrile was computed to be more downhill (ΔG‡=-22.7 kcal/mol) than reductive elimination to form benzonitrile (ΔG‡=-19.3 kcal/mol), which was predicted to be more downhill than reductive elimination of p-trifluoromethylbenzonitrile (ΔG‡=-15.9 kcal/mol) (Figure 1). The large differences in driving forces arise from the contrasting electronic effects of the substituents on the arylpalladium cyanide and arylnitrile products. Electron-withdrawing substituents stabilize the arylpalladium species but destabilize the arylnitrile product.
This range in computed driving forces (6.8 kcal/mol) is much larger than the range (1.5 kcal/mol) in computed transition state energies. As a result, the barrier for reductive elimination is lower for complexes containing electron-rich substituents on the aryl ligand, but the barrier for the reverse oxidative addition is computed to be lower for complexes with electron-poor aryl groups.
In contrast to this trend, the magnitude of the effect of the aryl substituents on the transition state energies for reductive elimination of arylamines, which occur faster from complexes containing more electron-poor palladium-bound aryl groups, was computed previously to be similar (8.2 kcal/mol) to the effect on the ground state (8.9 kcal/mol).15 These effects are similar, because arylpalladium complexes and arylamines are both stabilized by electron-withdrawing substituents. Thus, we propose that the strong and opposite electronic effect of substituents on the stabilities of the starting palladium complexes and the nitrile products causes the unusual electronic effect on the rate of reductive elimination of arylnitriles.16
A second contribution to the observed electronic effect could stem from the relationship between the reductive elimination of arylnitriles and insertion of CO into a metal-aryl bond. As noted in the introduction, the reductive elimination of alkylnitriles from a neopentylpalladium cyanide complex was suggested to be related to the insertion of CO.8 Consistent with this proposed relationship, insertion of CO into a palladium-aryl bond is known to be faster for complexes containing electron-donating substituents on the aryl ligand,17 and CO insertion into an M-C bond in which the hydrocarbyl ligand is more electron-donating is more thermodynamically favored than CO insertion into an M-C bond in which the hydrocarbyl ligand is more electron-withdrawing.18
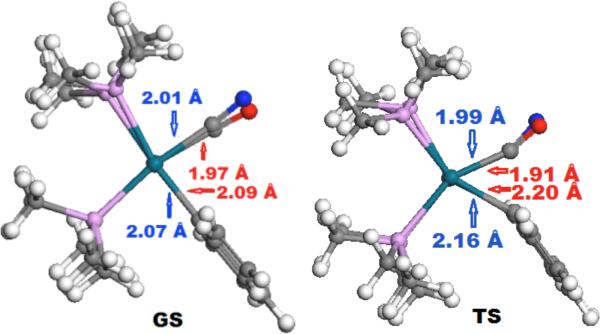
Computational data provide a more precise view of the relationships between these two reactions. The computed ground-state structures of neutral PMe3-ligated phenylpalladium(II) cyanide and cationic PMe3-ligated phenylpalladium(II)-CO complexes, as well as the transition states for reductive elimination of benzonitrile and insertion of CO into a palladium-phenyl bond are overlaid in Figure 2. The reaction coordinate for reductive elimination of aromatic nitriles resembles that for insertion of CO into a palladium-aryl bond. The C-N bond length in the ground-state cyanide complex and the C-O bond length in the ground-state carbonyl complex differ by only 0.03 Å. Likewise, the C-N and C-O bond lengths in the transition states differ by only 0.01 Å. The bond angles involving the CX (X=N, O) ligands and the phenyl ligand in the two complexes also change by a similar amount during the two reactions. The carbon-palladium-carbon angle changes from 85.7° in the ground state to 59.5° in the transition state (Δ=26.2°) during reductive elimination of the arylnitrile, while the carbon-palladium-carbon angle changes from 81.8° to 54.8° (Δ=27.0°) during insertion of CO into the palladium-aryl bond. The distortion from planarity reported for a related transition state involving oxidative addition of aromatic nitriles to Ni(0) was not observed for the second-row palladium system.19
Figure 2.
Computed structures of a PMe3-ligated phenylpalladium cyanide complex (blue) and a PMe3-ligated arylpalladium CO complex (red) in the ground state (GS) and transition state (TS) prior to reductive elimination of benzonitrile or insertion of CO.
The difference between a more synchronous coupling of two ligands and a more asynchronous migratory bond formation has been proposed to be distinct forms of concerted reductive eliminations. Calhorda reported calculations of the transition state structures for C-C bond formation through these two pathways by EHT20 and proposed that that the more synchronous process involved lengthening of the two palladium-carbon bonds, while the “migratory reductive elimination” involved lengthening of the M-C bond of the migrating group and shortening of the palladium-carbon bond of the unsaturated ligand to which migration occurs. Data in more recent work by Morokuma et al21 revealed that the Pd-alkynyl bond distances were constant or shortened during reductive elimination to form an enyne or phenylacetylene (1.99 Å and 2.00 to 1.99 Å, respectively) from a vinyl- or arylpalladium acetylide complex, while the palladium-vinyl and palladium-aryl bonds lengthened (2.06 to 2.11 Å and 2.04 to 2.09 Å, respectively). For reaction of the carbonyl and cyanide complexes depicted in Figure 2, the palladium-carbonyl bond shortens from 1.97 Å to 1.91 Å, and the palladium-cyanide bond shortens from 2.01 Å to 1.99 Å from the ground to transition states, while the palladium-aryl bonds lengthen by 0.11 and 0.09 Å for the two reactions, respectively. These changes are consistent with a “migratory” reductive elimination.
In conclusion, a series of arylpalladium(II) cyanide complexes that undergo reductive elimination of arylnitriles have been characterized, and studies of these complexes by experimental and computational methods provided insight into the mechanism of reductive elimination of arylnitriles. In contrast to the vast majority of reductive eliminations from arylpalladium(II) complexes, the reductive elimination of aromatic nitriles from arylpalladium(II) cyanide complexes is accelerated by electron-donating substituents on the aryl ligand. This result stems from the large electronic effect on the thermodynamic driving force for the reductive elimination of arylnitriles.22 In addition, DFT calculations indicate that the transition state structure for the reductive elimination from an arylpalladium cyanide complex is more similar to that for insertion of CO into a palladium-aryl bond than to the transition state structure for reductive elimination to form alkylarenes. Future studies will seek to reveal the scope of this trend in transition state structure and in using this information to improve couplings of sp-hybridized groups.
Supplementary Material
ACKNOWLEDGMENT
We thank Giang Vo for the synthesis of dppdmp and the characterization of complex 2b. We thank the NIH (GM-58108) for support of this work and Johnson-Matthey for a gift of PdCl2.
Footnotes
ASSOCIATED CONTENT
Supporting Information. All experimental procedures and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Sundermeier M, Zapf A, Beller M. Eur. J. Inorg. Chem. 2003:3513. [Google Scholar]
- 2.Takagi K, Okamoto T, Sakakibara Y, Oka S. Chem. Lett. 1973:471. [Google Scholar]
- 3.Ryberg P, Torborg C, Beller M. Org. Proc. Res. Devel. Adv. Synth. Catal. 2008;2009;12351:540, 3027. [Google Scholar]; Hatsuda M, Seki M. Tetrahedron. 2005;61:9908. [Google Scholar]
- 4.Dobbs KD, Marshall WJ, Grushin VV. J. Am. Chem. Soc. 2006;129:30. doi: 10.1021/ja066931d. [DOI] [PubMed] [Google Scholar]; Erhardt S, Grushin VV, Kilpatrick AH, Macgregor SA, Marshall WJ, Roe DC. J. Am. Chem. Soc. 2008;130:4828. doi: 10.1021/ja078298h. [DOI] [PubMed] [Google Scholar]
- 5.Sundermeier M, Zapf A, Mutyala S, Baumann W, Sans J, Weiss S, Beller M. Chem. Eur. J. 2003;9:1828. doi: 10.1002/chem.200390210. [DOI] [PubMed] [Google Scholar]
- 6.Bryndza HE, Domaille PJ, Tam W, Fong LK, Paciello RA, E. Bercaw J. Polyhedron. 1988;7:1441. [Google Scholar]; Halpern J, Guastalla G, Bercaw J. Coord. Chem. Rev. 1972;8:167. [Google Scholar]; Chapman KW, Chupas PJ, Kepert C. J. Am. Chem. Soc. 2006;128:7009. doi: 10.1021/ja060916r. [DOI] [PubMed] [Google Scholar]; Jones LHS, Basil I. Acc. Chem. Res. 1976;9:123. [Google Scholar]; Jones LH. Inorg. Chem. 1963;2:777. [Google Scholar]
- 7.Huang J, Haar CM, Nolan SP, Marcone JE, Moloy KG. Organometallics. 1999;18:297. [Google Scholar]
- 8.Marcone JE, Moloy KG. J. Am. Chem. Soc. 1998;120:8527. [Google Scholar]
- 9.Culkin DA, Hartwig JF. Organometallics. 2004;23:3398. [Google Scholar]
- 10.Driver MS, Hartwig JF. J. Am. Chem. Soc. 1997;119:8232. [Google Scholar]
- 11.Mann G, Incarvito C, Rheingold AL, Hartwig JF. J. Am. Chem. Soc. 1999;121:3224. [Google Scholar]
- 12.Mann G, Baranano D, Hartwig JF, Rheingold AL, Guzei IA. J. Am. Chem. Soc. 1998;120:9205. [Google Scholar]; Alvaro E, Hartwig JF. J. Am. Chem. Soc. 2009;131:7858. doi: 10.1021/ja901793w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwig JF. Inorg. Chem. 2007;46:1936. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]
- 14.1,3-triflouormethylbenzene is the major side product.
- 15.Hoi KH, Çalimsiz S, Froese RDJ, Hopkinson AC, Organ MG. Chem. Eur. J. 2011;17:3086. doi: 10.1002/chem.201002988. [DOI] [PubMed] [Google Scholar]
- 16.Consistent with the assertion that the properties of the cyano group lead to the observed electronic effect, Jones and co-workers have reported an example of similar thermodynamic control over the electronic effects on oxidative addition of arylnitriles by Ni(0). As we predict for these palladium complexes, arylnitriles with electron-donating substituents underwent slower oxidative addition to Ni(0), but data indicating faster reductive elimination from the arylnickel(II) cyanide species can be extracted from this study: Garcia JJ, Brunkan NM, Jones WD. J. Am. Chem. Soc. 2002;124:9547. doi: 10.1021/ja0204933.Garrou PE, Heck RF. J. Am. Chem. Soc. 1976;98:4115.
- 18.Anderson GK, Cross RJ. J. Chem. Soc. Dalton. Trans. 1979:1246. [Google Scholar]; Cross RJ, Gemmill J. J. Chem. Soc. Dalton Trans. 1981:2317. [Google Scholar]
- 19.Ateşin T. l. A., Li T, Lachaize S. b., García JJ, Jones WD. Organometallics. 2008;27:3811. [Google Scholar]
- 20.Calhorda MJ, Brown JM, Cooley NA. Organometallics. 1991;10:1431. [Google Scholar]
- 21.Ananikov VP, Musaev DG, Morokuma K. Organometallics. 2005;24:715. doi: 10.1021/om050255r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.This result also parallels the accelerating effect of electron-donating substituents on the aryl groups during reductive elimination from arylpalladium phosphonate complexes, although the origins of this effect were not determinined: Kohler MC, Grimes TV, Wang X, Cundari TR, Stockland RA. Organometallics. 2009;28:1193.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.