Abstract
Background
Addictive disorders are heritable, but the search for candidate functional polymorphisms playing an etiological role in addiction is hindered by complexity of the phenotype and the variety of factors interacting to impact behavior. Advances in human genome sequencing and neuroimaging technology provide an unprecedented opportunity to explore the impact of functional genetic variants on variability in behaviorally relevant neural circuitry. Here, we present a model for merging these technologies to trace the links between genes, brain, and addictive behavior.
Methods
We describe imaging genetics and discuss the utility of its application to addiction. We then review data pertaining to impulsivity and reward circuitry as an example of how genetic variation may lead to variation in behavioral phenotype. Finally, we present preliminary data relating the neural basis of reward processing to individual differences in nicotine dependence.
Results
Complex human behaviors such as addiction can be traced to their basic genetic building blocks by identifying intermediate behavioral phenotypes, associated neural circuitry, and underlying molecular signaling pathways. Impulsivity has been linked with variation in reward-related activation in the ventral striatum (VS), altered dopamine signaling, and functional polymorphisms of DRD2 and DAT1 genes. In smokers, changes in reward-related VS activation induced by smoking abstinence may be associated with severity of nicotine dependence.
Conclusions
Variation in genes related to dopamine signaling may contribute to heterogeneity in VS sensitivity to reward and, ultimately, to addiction. These findings illustrate the utility of the imaging genetics approach for investigating the neurobiological basis for vulnerability to addiction.
Keywords: neuroimaging, genetics, reward, dopamine, impulsivity, nicotine
In the past several decades, a wealth of research has begun to elucidate the neural mechanisms underlying acute drug effects, as well as the long-term neuroadaptations mediating the transition to drug dependence and compulsive drug-taking behavior (Kalivas and Volkow, 2005). However, not all individuals exposed to a drug of abuse go on to become dependent, highlighting the importance of identifying the factors which contribute to individual variability in this process. Individual differences in personality, temperament, or trait affect which help to shape complex behaviors and influence an individual’s interactions with the environment—including exposure to drugs of abuse—may ultimately confer vulnerability to or protection from the neural processes underlying drug dependence. Accordingly, identifying the biological mechanisms that give rise to individual trait differences affords a unique opportunity to develop a deeper understanding of addiction liability and, ultimately, prevention and treatment.
1. The case for integrating genetics and neuroimaging in the study of addiction
Behavioral genetics approaches have identified a strong heritability component to substance abuse and substance dependence (Agrawal and Lynskey, 2008; Han et al., 1999; Kendler et al., 2003). Recent advances in outlining the human genome have made identification of the genetic sources of variance in drug dependence a compelling and exciting avenue of research. However, isolating specific genetic contributors to the manifestation of a disease state has proven challenging. A single genetic polymorphism is likely to explain only a very small percentage of variance in complex behavioral outcomes such as addiction. This disconnect, between the heritability of addiction and the difficulty establishing specific effects of gene variants, may be a result of many intervening variables and levels of function that likely mask the more fundamental effects of genetic variation. In order to begin to bridge the gap between the basic building blocks of genetic variants and the complex, distal disease state of addiction, an integrated multilevel approach is needed to trace the neurobiological pathways which may contribute to inter-individual variability in trait-like behaviors, which may in turn confer risk for psychopathology, including addiction (see Figure 1).
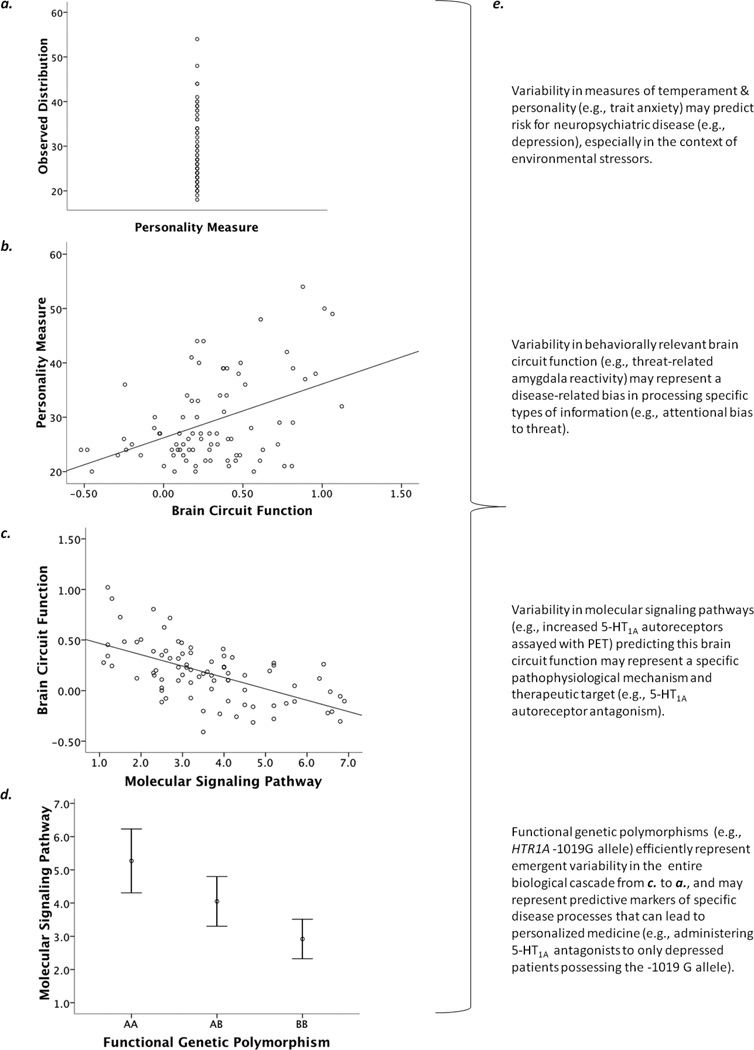
Figure 1.
Figure previously published in Annual Reviews of Neuroscience (Hariri, 2009). Integration of complementary technologies can be used to reveal the neurobiology of individual differences in complex behavioral traits. (a) Individual differences in personality and temperament are critical to shaping complex human behaviors and may serve as important predictors of vulnerability to neuropsychiatric disorders. (b) Neuroimaging technologies, especially BOLD fMRI, can identify links between variability in brain circuit function and individual differences in personality and temperament. (c) Multimodal PET/fMRI (or pharmacological fMRI) can map individual differences in behaviorally relevant brain circuit function to variability in specific molecular signaling pathways. (d) Variability in specific molecular signaling pathways can be mapped to functional genetic polymorphisms, which inform their ultimate biological origins and can be used to model efficiently how such emergent variability impacts behaviorally relevant brain function. (e) Each level of analysis can potentially inform clinically relevant issues, provide guiding principles for the development of more effective and personalized treatment options, and represent predictive risk markers that interact with unique environmental factors to precipitate disease.
1.1 Refining the addiction phenotype
The weak relationship between single polymorphisms and addiction may be due, in part, to the complexity of the addiction phenotype. Both theoretical and operational definitions of addiction often include multiple dimensions that may function independently and have distinct underlying pathophysiology (e.g., tolerance, cue-reactivity, failure of inhibitory control, mood disturbances, attentional disturbances, etc.). This problem is not adequately circumvented by focusing on clinically meaningful, but distal, endpoints, such as days of sustained abstinence following a quit attempt or categorical differences between subjects diagnosed as drug dependent and non-using controls. Indeed, detecting a significant effect using such a distal endpoint is likely to require sample sizes in the hundreds, and effects are often weak and inconsistent, probably due to variability in the determinants of chronic drug use. Furthermore, even when sufficient sample sizes are employed to allow such effects to be detected, the findings give little insight into the mechanisms by which such genes increase biological risk for disease.
To understand the role of genetic variation in risk for addiction, it may be necessary to refine the behavioral phenotype such that it reflects more fundamental characteristics of the disease state. Multiple cognitive, affective, and behavioral processes underlie addiction and likely contribute to a particular endpoint, such as days of abstinence following treatment. Isolating specific aspects of the disease process (e.g., cue reactivity) or the individual trait differences which may be associated with aspects of the disease process (e.g., impulsivity) marks an important step in beginning to trace the pathways of risk. This step is critical, because to the extent that addiction is a complex phenomenon subserved by multiple underlying processes (e.g., learning, memory, reward, attention), then variation in any one of these processes could augment or attenuate risk. Furthermore, targeting a specific, well-defined phenotype can pave the way for a mechanistic level of analysis, allowing researchers to investigate the biological underpinnings of variability in the phenotype.
1.2 Identifying the neural circuitry underlying the phenotype
Recent advances in human neuroimaging techniques have begun to reveal the neural substrates of inter-individual variability in complex traits, many of which are related to addiction. In particular, BOLD fMRI has the capacity to elucidate the systems-level neurocircuitry which may contribute to individual variability in behavioral phenotypes such as impulsivity (see Fig. 1b). A functional neuroimaging approach to describing the heterogeneity of complex disorders has several advantages. First, recent studies have established that BOLD fMRI measures represent temporally stable and relatively reliable indices of brain function (Johnstone et al., 2005; Manuck et al., 2007). Thus, much like their behavioral counterparts, patterns of brain activation can represent enduring, trait-like phenomena, which in and of themselves may serve as important markers of disease liability and pathophysiology. Second, the regional task-related BOLD response provides an objective measure of individual differences, thereby circumventing the traditional pitfalls encountered with self-report measures. Third, such markers have the benefit of being more proximal to the genetic sources of variance than complex behavioral outcomes. Since genes form the basic building blocks for life, genetic sequence variation impacting gene function can profoundly influence all levels of biology. The close relationship between genes and neural function enables researchers to assess the putative role of genetic variation even when the ultimate effects on more complex behaviors are difficult to determine because of imprecise definitions of the disease state, additional genetic and environmental moderators of the disease outcome, and measurement error. In addition, because any single polymorphism is likely to have only a small effect on complex behavior, assessing the impact of genetic variation on neural function affords increased power, enabling researchers to employ sample sizes in the tens rather than in the hundreds. For example, a recent meta-analysis demonstrated that functional variation in the promoter region of the serotonin transporter gene (5-HTTLPR) explained approximately 10% of the variance in amygdala reactivity to threatening stimuli (Munafo et al., 2008)—an effect size much higher than those observed for more complex behaviors. Finally, identifying how a functional polymorphism of a gene impacts neural activity at the systems level is much more informative than an association observed at the level of behavior because it places the findings within both a theoretical and empirical framework of neurobiological function. Characterization of the neural circuitry associated with trait-like behaviors then paves the way to a more systematic evaluation of the molecular substrates, which is ultimately necessary to understand the pathways through which genetic variance exerts its influence and ultimately confers risk for addiction or other disease states.
1.3 Linking regional activation to molecular signaling pathways
Although describing the relations between trait-like behaviors and regional activation patterns can be very informative, ultimately, the power of the imaging genetics approach lies in its ability to trace the pathway linking genes to variation in cellular processes and brain activation. Hence, an important next step is to identify the neurochemical mechanisms underlying variability in brain circuit function (Figure 1c). Both pharmacological neuroimaging and multi-modal (e.g. PET/fMRI) neuroimaging methodologies can help to further this aim. For example, recent neuroimaging studies employing pharmacological challenge paradigms targeting monoamine neurotransmission have revealed that even subtle alterations in dopaminergic, noradrenergic, and serotonergic signaling can have a profound impact on the functional response of brain circuitries supporting affect, personality, and temperament (Bigos et al., 2008). Similarly, multimodal neuroimaging approaches have provided evidence for directionally specific links between key components of monaminergic signaling cascades, assessed with radiotracer PET, and brain function, assessed with BOLD fMRI (Fisher et al., 2006). Collectively, these approaches are revealing how variability in behaviorally relevant brain activation emerges as a function of underlying variability in key brain signaling pathways. One logical next step is to identify the genetic sources of inter-individual differences in these signaling pathways that ultimately give rise to the neural and behavioral phenotypes of interest.
1.4 Identifying the genetic sources of variance in molecular signaling pathways
With recent advances in human molecular genetics, there is now tremendous potential for identifying common sequence variation in the genes that influence the functioning or availability of components of molecular signaling pathways (Figure 1d). Even subtle biases in molecular signaling can profoundly influence neural systems as the individual negotiates the environment, ultimately giving rise to complex behaviors and conferring risk for psychopathology. Because individual variation in DNA sequence is a significant source of variability in molecular signaling, understanding the links between genes and brain function may provide unique insight into behavior and a deeper understanding of the mechanistic foundation for individual differences in psychiatric diseases such as addiction. Furthermore, once a detailed, integrated pathway is characterized, such genetic polymorphisms have the potential to serve as highly informative markers for vulnerability or resilience to a disease state. Of course, basing predictive inferences for individuals on a strategy of genetic reductionism requires caution, particularly given the probabilistic nature of the biological impact of candidate functional polymorphisms whose likely effects are dynamically moderated by other genetic variants as well as environmental and epigenetic factors. However, even with the recognition that the impact of any one functional polymorphism on a disease process is not absolute, developing a comprehensive, detailed characterization of the neurobiological and, in turn, behavioral impact of functional genetic variants may allow researchers, and ultimately clinicians, to better understand the determinants of risk and target prevention or treatment interventions accordingly.
1.5 Summary of the imaging genetics approach and its application to the study of addiction
In sum, an integrated, multilevel framework for understanding individual variability in complex behaviors can be beneficial for understanding both vulnerability to addictive disorders among those not yet exposed to drugs of abuse, and the underlying pathophysiology contributing to individual differences in the manifestation of addictive phenotypes. Indeed, the disease of addiction has many characteristics that will likely make the imaging genetics approach a fruitful avenue for future research. Addiction is a heritable, but complex behavioral phenotype that demonstrates marked heterogeneity throughout the disease process (i.e., initiation, transition to regular use, emergence of dependence symptoms; Anthony et al., 1994; Cloninger et al., 1981; Donny and Dierker, 2007; Stulz et al., 2009). The field has made considerable progress identifying several core features of addiction (e.g., heightened incentive processing of drugs and drug related cues; reduced incentive properties of non-drug rewards) that can be specifically studied with greater precision than the ultimate, multidimensional disease state. The preclinical and clinical neuroimaging literatures have described the neural circuitry underlying many of these behavioral phenotypes. Finally, this neural response is highly variable across individuals in a manner that relates to variability in the disease state (see Section 3 below). These features compel the question – do functional polymorphisms implicated in regional patterns of neural activation underlie some of the heterogeneity in risk for drug use and dependence?
Addiction also has a unique feature, different from other psychopathologies, that makes the imaging genetics approach particularly compelling. Exposure to drugs of abuse represents a unique environmental perturbation; drugs of abuse directly target the brain signaling pathways that form the basis of regional activation patterns observed using functional neuroimaging. The effects of drug exposure on neural circuitry are not simply limited to the direct, acute actions of the drug; they also involve neuroadaptations in response to chronic drug use (Kalivas and Volkow, 2005) and are thought to form the physiological basis of addiction. Consequently, genetic variation related to brain signaling pathways may underlie not only stable trait-like neural responses, but also the degree to which acute drug effects, neuroadaptations, and drug withdrawal alter neural response. Characterizing the interplay between genetic variation in neural activation patterns and drug-induced changes in neural response may facilitate the development of both pharmacological and behavioral treatments targeting specific vulnerabilities.
In the following sections we present examples of the application of imaging genetics to addictive disorders. First, we discuss impulsivity, specifically a bias for immediate over delayed rewards, as a complex behavioral phenotype closely associated with vulnerability to addiction and present data tracing the neurobiological basis of inter-individual variability in this phenotype across multiple levels of analysis. Second, we present preliminary data demonstrating the relationship between neural response to reward and individual differences in nicotine dependence as an example of potentially important variation in neural phenotypes that likely have genetic underpinnings. Finally, we discuss additional considerations and future directions for the application of imaging genetics to addictive disorders.
2. An exemplar: Impulsivity, reward circuitry, and dopamine function
2.1 Delay discounting as a behavioral phenotype for the disease of addiction
Although the exact definitions and measures vary from study to study, impulsivity is thought to be a core feature of substance use disorders (Allen et al., 1998; Koob and Volkow, 2009). Indeed, impulsive traits have been shown to predict smoking reinforcement and reward (Perkins et al., 2008), initiation of drug use (Audrain-McGovern et al., 2009; Kollins, 2003), dependence once regular use is initiated (Sweitzer et al., 2008), and treatment response among those trying to quit (MacKillop and Kahler, 2009). The term “impulsivity” is often used to describe a variety of conceptually distinct processes, such as quickly responding to stimuli without adequate forethought (Moeller et al., 2001), a failure to inhibit a pre-potent response (Horn et al., 2003), and a tendency to place immediate gain ahead of long-term consequences (Logue, 1995). Each of these processes may have an important role in the addiction phenotype; however, the one that has arguably received the most attention is the tendency for behavior to be driven by immediate gains despite larger, long-term alternatives (i.e., delay discounting).
Most people will reliably choose a large reward over a small reward or an immediate reward over a delayed reward. However, individuals vary considerably in their degree of preference when a larger reward is delayed in time, such that some individuals will be more likely to sacrifice a larger nominal value for the sake of immediacy, while others will be more likely to delay gratification for the sake of profitability. The tendency to prefer immediacy over a delayed but larger reward can be easily measured through well-characterized inter-temporal choice procedures and is thought to provide an index of impulsivity (Bickel and Marsch, 2001; Green and Myerson, 2004). Preference for immediate over delayed rewards has been widely linked with abuse of substances including heroin (Kirby et al., 1999; Madden et al., 1997), cocaine (Coffey et al., 2003), alcohol (Petry, 2001; Vuchinich and Simpson, 1998), and tobacco (Mitchell, 1999; Reynolds et al., 2004), and even pathological gambling (Alessi and Petry, 2003). Although the causal direction and underlying mechanisms mediating this relationship have been difficult to identify, several recent studies suggest that delay discounting may increase vulnerability to addictive disorders (Anker et al., 2009; Audrain-McGovern et al., 2009; Diergaarde et al., 2008). Furthermore, although evidence is somewhat mixed, greater delay discounting has been observed among individuals with a positive family history for alcohol dependence and substance use disorders (Acheson et al, 2011; Petry et al, 2002; Mitchell, 2011), raising the possibility of a common genetic predisposition to both impulsivity and addiction. Consequently, a thorough characterization of the neurobiological pathways underlying individual variability in impulsive choice can be informative for understanding the mechanisms leading to not only normal variability in such behaviors but also the pathophysiology of addiction and related disorders.
2.2 Neural basis of delay discounting and processing of rewards
Because delay discounting involves the valuation and selection of rewards, the ventral striatum is a key structure likely to be recruited during delay discounting procedures. The ventral striatum (VS) is heavily interconnected with other subcortical (e.g. amygdala), cortical (e.g., orbitofrontal cortex; OFC) and brainstem (ventral tegmental area; VTA) structures, which together play a critical role in shaping reward processing and motivated behavior. Dopaminergic neurons projecting from the VTA to the VS fire in response to a motivationally salient event, serving to increase goal-directed approach behavior by attaching incentive salience to environmental cues associated with an obtained reward (Robinson and Berridge, 1993). VS activity increases both in anticipation of expected rewards and in response to the delivery of unexpected rewards, underscoring the putative role of the VS in prediction error and incentive motivation (Schultz, 2002; O'Doherty, 2004). Moreover, drugs of abuse all share the common property of increasing dopaminergic transmission to the VS. Thus, given the importance of its role in processing of both natural and drug rewards, individual differences in VS activity and its related circuitry may be important for understanding risk for substance abuse and dependence.
Several studies have implicated VS involvement in delay discounting, although theories differ in the attributions of its exact role. McClure and colleagues (McClure et al., 2004) have found increases in VS activation when participants weighed choices between a small, immediate reward and a larger, delayed reward. However, VS activation was only apparent when the smaller reward was available immediately; choices between two rewards of differing magnitudes available at different delay intervals did not recruit this region. This suggests that the VS may specifically respond to rewards available immediately, rather than abstract representations of rewards available at a future point. However, other theorists have suggested that activation of the VS during inter-temporal choice paradigms actually tracks the subjective value of the anticipated reward, such that delayed rewards may be equally represented but their subjective value diminished due to delay (Kable and Glimcher, 2007). Interestingly, both models have demonstrated that magnitude of VS activation to a potential reward choice corresponds to whether or not that choice is selected (Kable and Glimcher, 2007; McClure et al., 2004). This raised the intriguing but previously untested possibility that VS activation may not only be involved in delay discounting task performance, but may specifically be related to individual differences in the bias towards immediate reward. Indeed, because the VS exhibits an immediate response to rewards, variability in activation in this region could serve as an important neural marker of individual differences in impulsive choice and risk for substance use disorders.
To determine whether individual differences in delay discounting were related to neural activation in response to immediate reward, we utilized a blocked design monetary reward task designed to elicit activation in the VS and associated reward circuitry including regions of prefrontal cortex (Hariri et al., 2006). Subjects played a simple guessing game, in which they were required to guess whether they thought a number presented on a screen would be higher or lower than five. Subjects were told that if they got it right, they would win money, and if they got it wrong, they would lose money. The task was organized into blocks of mostly winning trials (75% positive feedback) and blocks of mostly losing trials (75% negative feedback). Analyses revealed that bias toward immediate over delayed rewards assessed outside of the scanner is positively correlated with the magnitude of VS activation, in response to both positive and negative feedback, as well as with differential reward-related VS activation in response to positive compared with negative feedback. Consistent with the moderate general correlation between delay discounting and traditional self-report measures of impulsivity (de Wit, 2007), we have also found that reward-related VS reactivity is positively correlated with scores from the Barratt Impulsiveness Scale (Forbes et al., 2009). Collectively, our results suggest that increased self-reported impulsivity as well as the preference for smaller immediate over larger delayed rewards, traits associated with drug use and dependence, reflect both a relatively indiscriminate and hyper-reactive VS circuitry. Similar variability in VS function has also been associated with more complex measures of incentive-based decision making (Knutson et al., 2007).
Importantly, dysregulation of VS response to reward is thought to contribute to addiction (Kalivas and Volkow, 2005). As such, inter-individual variability in VS reactivity to reward-related stimuli likely contributes to the emergence of differences in the intermediate behavioral risk factors for, and clinical expression of, addiction. For example, highly impulsive individuals may experience a bias toward immediate drug rewards due to a hyper-reactive striatal response, thereby motivating further drug use. Furthermore, chronic exposure to drugs of abuse directly alters reward circuitry. For example, repeated drug exposure leads to a sensitization of the striatal response to drug related cues and a heightened motivation to obtain the drug (Robinson and Berridge, 1993), while striatal response to non-drug rewards is blunted among chronic drug users (Volkow et al., 1997; Wrase et al., 2007). Similarly, chronic drug users demonstrate heightened activation in the OFC, a region closely interconnected with the VS, in response to recent drug exposure or drug-associated cues, but hypoactivation during protracted withdrawal (Volkow and Fowler, 2000). This dissociated pattern of activation is likely driven by compensatory neuroadaptations resulting from supraphysiological effects produced by drugs of abuse, and may mediate a pattern of compulsive drive to obtain drug reward along with a reduced motivation for non-drug rewards (Volkow et al., 2004).
Because of this direct impact and modification of reward circuitry by drugs of abuse, inter-individual variability in VS reactivity could interact with changes wrought by chronic drug exposure to contribute to variation in the expression of addiction. Identifying variability in neural signaling pathways, which contributes to individual differences in VS function, offers additional traction in the search for underlying biological mechanisms that may put some individuals at risk for addiction.
2.3 The role of dopamine signaling in VS response to rewards
Dopamine signaling within the midbrain is critical to reward processing, including learning environmental contingencies associated with prediction of future rewards and motivating appetitive behavior (Schultz, 2002). In addition, variability in dopamine signaling has been associated with impulsive behavior in animal models (Dalley et al., 2007; Diergaarde et al., 2008), and neuroadaptations in midbrain dopamine signaling are thought to be central to the development of addiction (Hyman et al., 2006; Robinson and Berridge, 1993). Multimodal and pharmacological neuroimaging studies of dopamine effects on brain function offer a unique opportunity to more directly evaluate underlying molecular mechanisms regulating this circuitry. A recent in vivo human study reported a direct positive link between striatal dopamine synthesis, assessed with PET, and positive affect-related brain activity, assessed with BOLD fMRI (Siessmeier et al., 2006). Acute increase of dopamine release via oral amphetamine has also been linked with a relatively increased extent of activation in the VS (Menon et al., 2007). More generally, an acute pharmacologic increase of dopamine in both healthy volunteers (Hariri et al., 2002) and patients with Parkinson disease (Tessitore et al., 2002) results in relatively increased BOLD fMRI-assessed activity in closely related limbic brain regions, namely the amygdala. Finally, consistent with the role of VS activation in studies of inter-temporal choice, a recent study demonstrated that administration of L-Dopa to healthy volunteers engaged in an inter-temporal choice task enhanced VS BOLD activation to immediate rewards but attenuated VS BOLD activation to delayed rewards—an effect paralleled by an increase in delay discounting (Pine et al., 2010).
Multimodal PET studies examining both ligand receptor binding and cerebral blood flow have also shown relations between striatal dopamine function and brain activation within substance dependent populations, including individuals dependent upon cocaine (Volkow and Fowler, 2000), methamphetamine (Volkow et al., 2001), and alcohol (Volkow et al., 2007). Specifically, reduced striatal dopamine D2 receptor levels have been associated with diminished regional activation in the OFC and anterior cingulate gyrus (Volkow et al., 1992a; Volkow et al., 1992b)—regions which are tightly interconnected with the VS and whose activation is associated with impulsivity and compulsive behavior (Baxter et al., 1987; Brown et al., 2006). While diminished activation in regions associated with compulsive behavior among substance dependent individuals initially seemed counterintuitive, other studies have since found that reductions in striatal D2 receptors are associated with increased OFC activation by drug related cues (Heinz et al., 2004; Volkow and Fowler, 2000). These findings suggest a common mechanism underlying the dissociation between heightened neural response to drug reward and attenuated neural response to non-drug rewards, which together may contribute to compulsive drug use. Furthermore, high levels of striatal D2 receptors have been shown to be protective against OFC dysregulation among those with a family history of alcoholism (Volkow et al., 2006), suggesting that variation in dopaminergic transmission—possibly mediated by genetic factors, influences more distal disease outcomes.
Together, these associations suggest a potential common pathway for vulnerability to both impulsive behavior and substance abuse, involving variability in dopaminergic function impacting reward-related VS and prefrontal activation. Given the importance of dopamine in modulating this behaviorally relevant neural circuitry, identifying factors that determine interindividual variability in dopamine signaling and its related impact on the reactivity of VS and associated behaviors will facilitate our understanding of the neurobiological mechanisms governing reward-related behaviors and augment efforts to improve the treatment and even prevention of pathological behaviors such as drug abuse and addiction.
2.4 The role of dopamine genes in regulating dopamine signaling and VS response to rewards
We and others have begun to explore how altered dopamine signaling resulting from common functional genetic variation contributes to inter-individual variability in reward-related VS activation (Dillon et al., 2010; Dreher et al., 2009; Forbes et al., 2009; Yacubian et al., 2007). For example, we have investigated the impact on VS reactivity of candidate polymorphisms shown in in vitro or in vivo assays to demonstrate significant impact on biological function related to dopamine neurotransmission (Forbes et al., 2009). This approach is consistent with the aim of identifying detailed, mechanistic pathways by which functional genetic variation ultimately contributes to manifestation of complex behavior and psychiatric disorder.
Several functional polymorphisms have been identified which contribute to subtle alterations in the availability of components of dopamine signaling pathways, which could in turn lead to heightened VS reward-related activation. The dopamine transporter (DAT) is responsible for the active clearance of synaptic dopamine and, thus, plays a critical role in regulating the duration of postsynaptic dopamine signaling, especially in the striatum (Sesack et al., 1998). Accumulating evidence indicates that a 40-base-pair variable number of tandem repeats polymorphism (DAT1) in the 3’ untranslated region of the DAT gene (DAT1) influences the expression and availability of DAT (Bannon et al., 2001). Although investigators have not consistently observed a genotype effect across all studies (Martinez et al., 2001; Michelhaugh et al., 2001; Mill et al., 2005; van Dyck et al., 2005), several suggest that in comparison to the 9- repeat allele, the 10-repeat is associated with relatively increased levels of DAT both in vivo (Cheon et al., 2005; Heinz et al., 2000) and in vitro (Mill et al., 2002; VanNess et al., 2005). We hypothesized that there would be relatively greater VS reactivity associated with the 9-repeat allele, which is linked with reduced DAT expression and presumably greater striatal synaptic dopamine, in comparison with the 10-repeat allele.
The dopamine D2 receptor (DRD2) is located densely throughout the striatum and is thought to regulate extracellular dopamine release through an auto-inhibitory mechanism (Benoit-Marand et al., 2001). Somatodendritic DRD2 autoreceptors inhibit mesencephalic dopamine neuron firing rates, while activation of terminal DRD2 autoreceptors result in a blockade of neurotransmitter release (Hahn et al., 2006; Jomphe et al., 2006). Furthermore, postsynaptic DRD2 receptors also exert inhibitory effects via second-messenger signaling cascades (Sibley, 1993). An insertion/deletion single nucleotide polymorphism (SNP) in the 5’ promoter region (−141C insertion/deletion, Ins/Del) of the DRD2 gene alters DRD2 expression, with the deletion variant resulting in relatively reduced DRD2 expression (Arinami et al., 1997), and presumably enhanced dopamine release resulting from decreased inhibitory autoregulation, in comparison with the insertion variant. Thus, we hypothesized that the deletion variant would be associated with relatively greater VS reward-related activation relative to the insertion variant.
Consistent with our hypotheses, both the DAT1 9-repeat allele and the DRD2 −141C Del allele were associated with relatively greater VS reactivity and accounted for nearly 12% and 10% of inter-individual variability, respectively. Interestingly, the DRD2 gene exhibited the largest effect when restricting the analysis to only those voxels showing a significant correlation with self-reported impulsivity, demonstrating 33% overlap within this region. These and similar results from other studies (Dillon et al., 2010; Dreher et al., 2009; Yacubian et al., 2007) highlight an important role for genetic polymorphisms affecting striatal dopamine neurotransmission in mediating inter-individual differences in reward-related VS reactivity. They further suggest that altered VS reactivity may represent a key neurobiological pathway through which these polymorphisms contribute to variability in behavioral impulsivity and related risk for substance use disorders.
3. Individual differences in nicotine dependence and withdrawal-related changes in reward processing
In the previous sections we described an example of the application of the imaging genetics approach to understanding the neural basis of inter-individual variability in impulsivity; a behavioral phenotype closely related to addictive disorders. We demonstrated the development of a mechanistic framework in which variability in the phenotype of impulsivity could be traced through patterns of neural activity to genetically driven alterations in molecular signaling pathways. However, the above analyses were conducted in healthy, non-addicted adult populations, and thus likely speak only to the pathways associated with early processes (e.g., initiation of substance use). Although it is possible that the same mechanisms underlying impulsivity in healthy populations may also interact with drug exposure to confer risk for drug dependence, additional work is needed to dissect the processes acting in addicted populations. Such research would help to identify the pathways which may place some individuals at greater risk for neuroadaptations leading to dependence and relapse. In the next section, we turn to discussion of building upon this knowledge and applying this approach within substance dependent populations.
As described above, addiction is a complex, multidimensional disorder. As with other complex traits such as impulsivity, it is necessary to refine the behavioral phenotype to focus on specific dimensions or aspects of addictive disorders in order to begin to isolate the specific neural processing mediating inter-individual differences. Similarly, characterization of a neural phenotype which is associated with variability in behavior paves the way for further investigation of the genetic sources of variance and can be used to understand the pathways mediating ultimate clinical outcomes, such as treatment response. Given that research has begun to describe the long-term neuroadaptations resulting from chronic drug exposure, we can begin to examine how variability in these neuroadaptations may predict severity of addictive phenotypes, as well as identify the genetic sources of this variance. Here, we focus on variability in nicotine dependence and incentive processing of non-drug rewards.
As described above, neuroadaptations resulting from chronic exposure to drugs of abuse, including nicotine, are known to involve midbrain dopaminergic function, leading to sensitization of the dopamine response to drugs of abuse and heightened sensitivity to drug related cues (Robinson and Berridge, 1993). By contrast, behavioral evidence suggests that withdrawal from drugs of abuse leads to deficits in reward functioning. For example, intracranial self-stimulation (ICSS) experiments demonstrate increased reward thresholds during withdrawal from multiple drugs of abuse including cocaine (Markou and Koob, 1991), amphetamine (Cryan et al., 2003), alcohol (Schulteis et al., 1995), and nicotine (Epping-Jordan et al., 1998). Indeed, opponent-process theory posits that chronic drug exposure results in a compensatory alteration in reward processing in an attempt to correct the imbalance that is produced by constant stimulation of the reward pathways (Koob and Le Moal, 1997, 2001). Consistent with this framework, research with both animals and humans suggests that nicotine facilitates the reinforcing properties of other stimuli (Barr et al., 2008; Chaudhri et al., 2006; Donny et al., 2003; Olausson et al., 2003, 2004), while abstinence from nicotine appears to attenuate the value of other reinforcing stimuli (Weaver et al., 2012). Indeed, abstinent smokers experience diminished capacity for reward relative to both satiated smokers and non-smokers including less enjoyment from ordinarily pleasurable events and reduced response to financial reward (Dawkins et al., 2006; Powell et al., 2002; Powell et al., 2004). Compared with satiated smokers, abstinent smokers also demonstrate less interference from and report lower levels of happiness in response to positive or pleasure-related stimuli (Dawkins et al., 2006).
While some symptoms described above, such as loss of hedonic experience of pleasure, may be mediated by a variety of neurotransmitter systems including endogenous opioids or serotonin, other symptoms related to deficits in motivated behavior during nicotine withdrawal may be related to attenuation of dopamine related activation in the VS. Indeed, Martin-Soelch and colleagues (2003) reported that although there was a significant correlation between magnitude of monetary rewards and VS activity, this relation was not observed in smokers, suggesting they were much less responsive to even the largest rewards presented. Likewise, data from a PET study revealed a negative correlation between metabolic activity in the VS and abstinence-induced withdrawal, suggesting that a component of withdrawal may be a reduction in activation within this region (Rose et al., 2007). Together, these data support theories of addiction which suggest that adaptations in dopamine reward pathways due to chronic stimulant exposure, including nicotine, mediates the development of an abstinence-induced withdrawal syndrome characterized by a decreased incentive motivation (Koob and Le Moal, 1997, 2005, 2008; Volkow et al., 2004). Genetically driven variation in dopamine signaling and associated reward circuitry, in interaction with the changes wrought by chronic nicotine exposure, could contribute to individual differences in the extent of reward deficits observed during abstinence, and in turn, the manifestation of nicotine dependence in chronic smokers.
As an initial step in investigating this line of inquiry, we tested whether variation in nicotine dependence, previously shown to be associated with delay discounting (Sweitzer et al., 2008), was associated with magnitude of change in reward-related VS activation during abstinence compared with shortly after smoking. We utilized the same monetary reward task previously shown to elicit activation associated with impulsivity (Hariri et al., 2006). It is important to note that these data are from a small sample and hence should be viewed and preliminary and hypothesis-generating. Because these data have not previously been reported, we provide a brief summary of the methods below1.
3.1 Materials and methods
Complete data were available from 10 male subjects (mean age, 41.5 years +/− 6.6 SD). All subjects were non-treatment seekers who reported smoking between 10 and 30 cigarettes per day (mean CPD, 19.4 +/− 6.9 SD) for the past 10 to 30 years (mean years, 22.0 +/− 6.3), scored a minimum of 4 on the Fagerstrom Test for Nicotine Dependence (FTND), and had a baseline exhaled carbon monoxide (CO) level of 10 ppm or greater.
Subjects participated in an initial screening session plus two fMRI sessions separated by a minimum of five days. Prior to one fMRI session, subjects were instructed to smoke ad libitum up until the time of their appointment (non-abstinent condition). Prior to the other fMRI session, subjects were required to abstain from smoking for a minimum of 12 hours (abstinent condition). Compliance with instructions was verified using self-report and expired CO levels (abstinence verified as < 8 ppm or 50% of baseline). Order of sessions was randomly assigned and counterbalanced across subjects.
During the screening session, nicotine dependence was assessed using the Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004) and the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991). Procedures within each fMRI session were identical for both sessions. Subjects first completed a measure of nicotine withdrawal (Minnesota Nicotine Withdrawal Scale; MNWS) and were trained on the VS task to be completed in the scanner. After smoking a final cigarette (non-abstinent condition) or waiting an additional 10 minutes (abstinent condition), subjects were tested for expired CO level immediately prior to entering the scan; subjects also completed a 4-item craving questionnaire (Questionnaire of Smoking Urges; QSU-4) just before and after the scan, with the average taken to represent overall craving for each scanning session.
Details of our fMRI task are available through several earlier reports (Forbes et al., 2009; Hariri et al., 2006; Hariri et al., 2009). Briefly, subjects were instructed that they would be guessing whether a hidden number was higher or lower than five, indicated by pressing their middle or index finger, respectively. For each trial, subjects were presented with a question mark indicating that they should make their guess. Following each trial, the actual number was shown, followed by a green up arrow indicating they won money if they got it right (positive feedback) or a red down arrow indicating they lost money if they got it wrong (negative feedback). The blocked design consisted of pseudorandom presentation of trials organized into blocks of mostly positive feedback (4 out of 5 trials) or mostly negative feedback (4 out of 5 trials), interleaved with control blocks in which subjects were presented with comparable visual stimuli and were required to press a button with either their middle or index finger to control for motor activity. Subjects were unaware of the fixed outcome probabilities associated with each block and were led to believe that their performance would determine their net monetary gain, although all subjects received $10 upon completion of the task.
Subjects were scanned using a Siemens 3T MAGNETOM scanner (Siemens AG, Medical Solutions, Erlangen, Germany). Whole-brain image analysis was completed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Following preprocessing, data sets were analyzed using second-level random effects models. For each subject and scan, predetermined condition effects at each voxel within a predefined VS region of interest were calculated using a t-statistic, producing a statistical image for each contrast: (1) positive feedback > control (2) negative feedback > control and (3) positive feedback > negative feedback2.
3.2 Results
Analysis of behavioral measures revealed that abstinence was associated with significantly lower CO levels and greater craving relative to non-abstinence. Although the increase in withdrawal symptoms during abstinence was in the predicted direction, this difference did not reach significance (see Table 1).
Table 1.
Mean scores (and standard deviations) on smoking measures for 10 smokers assessed during abstinence and non-abstinence, and t score for the test of significant difference between conditions.
| Measure | Abstinent | Non-Abstinent | Paired T Test |
|---|---|---|---|
| Exhaled carbon monoxide (CO) | 6.3 (2.7) | 22.4 (5.9) | 9.02** |
| Questionnaire of smoking urges (QSU-4) | 68.0 (27.7) | 32.7 (21.2) | 5.11* |
| Minnesota withdrawal scale (MNWS) | 31.7 (23.2) | 17.1 (25.6) | 1.94+ |
p < 0.001
p < 0.01
p < 0.10
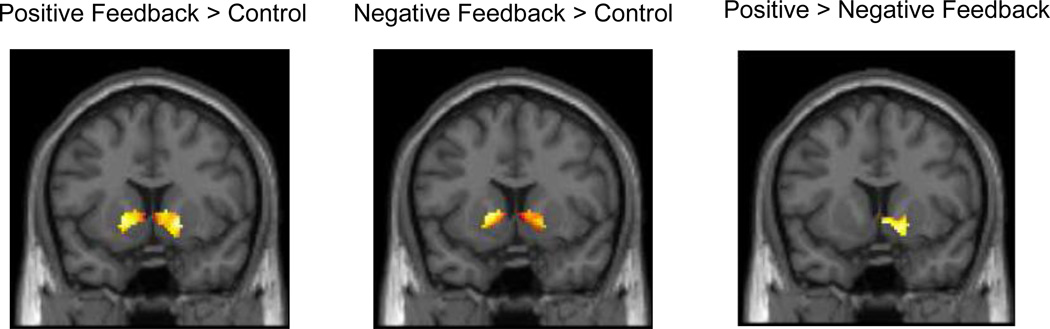
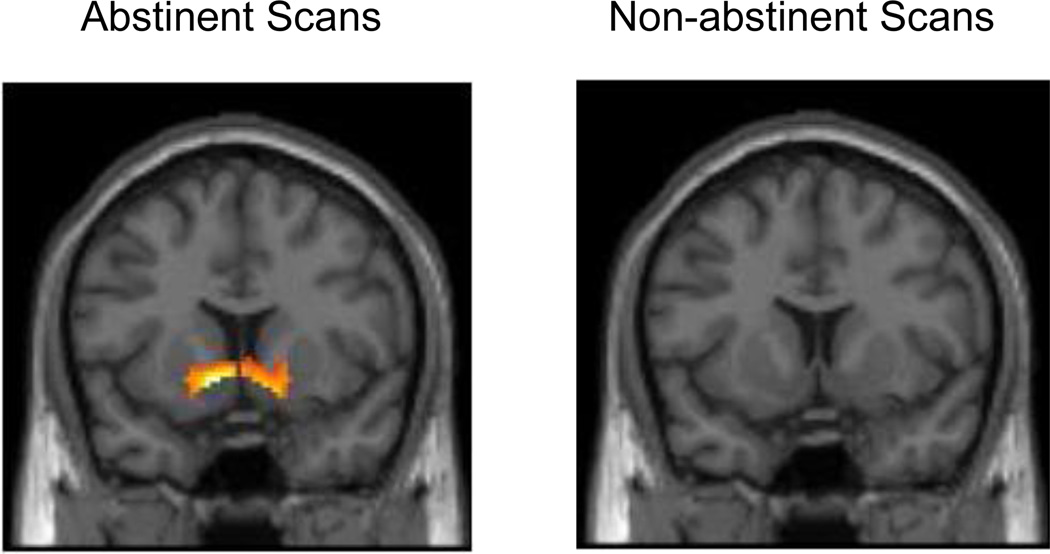
Consistent with previous studies, we observed strong bilateral VS activity associated with both positive and negative feedback blocks, relative to control blocks, collapsed across both abstinent and non-abstinent conditions (Figure 2a,b). We also observed relatively greater right VS activation in response to positive compared with negative feedback blocks (Figure 2c). When analyzed separately by condition, bilateral VS activation was observed for both positive and negative feedback blocks relative to control blocks during both abstinence and non-abstinence. However, the differential effect of positive > negative feedback was significantly associated with VS activation only during abstinence, while no effect was observed during non-abstinence (Figure 3a,b). Despite these apparent differences between conditions when analyzed separately, direct comparisons of VS reward-related activation during abstinence compared with non-abstinence were not statistically significant.
Figure 2.
Average ventral striatal (VS) activity associated with general feedback as well as the differential effect of reward (p < 0.05, 125 cluster extent threshold for all contrasts), collapsed across both conditions. All slices are presented at Y = 10. a. VS activation associated with the contrast of positive feedback > control (right cluster: 18, 10, −4; t = 5.95; left cluster: −16, 10, −2; t = 5.35). b. VS activation associated with the contrast of negative feedback > control (right cluster: 18, 6, −2; t = 5.08; left cluster: −10, 6, 2; t = 7.38). c. VS activation associated with the contrast of positive feedback > negative feedback (right cluster: 8, 6, −6; t = 3.68).
Figure 3.
Average ventral striatal (VS) activity associated with the contrast of positive feedback > negative feedback (p < 0.05, 125 cluster extent threshold for all contrasts) for each condition. Slices presented at Y=10. a. Differential effect of reward during abstinence (right cluster: 14, 10, −10; t = 3.57; left cluster: −10, 10, −6; t = 5.85). b. No significant differential effect of reward observed during non-abstinence.
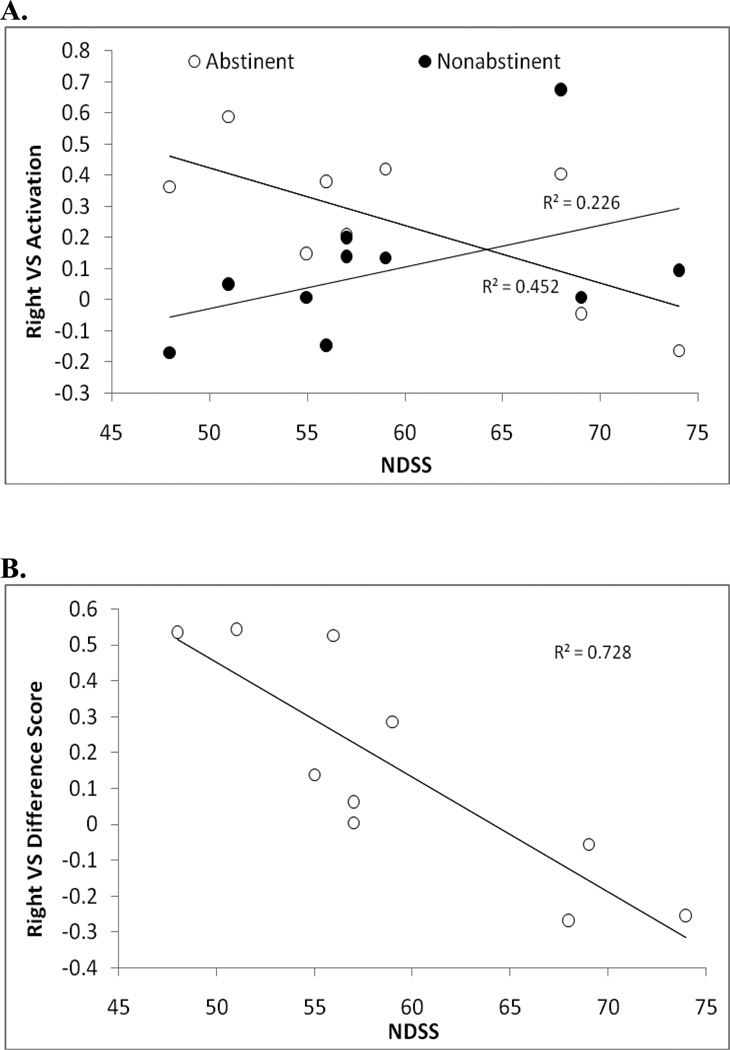
Given the theoretical and behavioral evidence suggesting that abstinence from smoking may result in an attenuated response to reward, the lack of an overall effect of abstinence compared with non-abstinence was surprising. However, we hypothesized that substantial inter-individual variability in reward-related VS activation induced by abstinence could be masking any group-level differences. Consequently, we sought to examine whether variation could be observed across individuals, and whether this variability was related to severity of nicotine dependence. To do this, we extracted right VS activation values from the contrast of positive > negative feedback, including scans from both conditions (Figure 2c). Extracted values for both abstinent and non-abstinent scans for each subject are illustrated in Figure 4a, presented as a function of nicotine dependence as measured by the NDSS. As can be seen, individuals low in dependence appeared to show increases in VS reactivity during abstinence compared with non-abstinence, while individuals high in dependence showed the opposite pattern. This effect was further apparent when examining difference scores calculated by subtracting non-abstinent from abstinent scan VS activation values for each subject. Variability in difference scores was significantly related to NDSS scores (R2=.728, p=.002; Figure 4b)—an effect which remained significant when controlling for age, race, session order, and change in CO between abstinence and non-abstinence (Partial r = −.900, p= .015). To assess the extent to which these findings generalized to left VS activation, we also extracted activation values from a left VS cluster associated with differential response to reward identified using a more liberal threshold3. Although relaxing the significance threshold means it is less clear that the extracted values truly reflect reward-related activation, analyses based on this cluster revealed a pattern identical to that observed with the right VS (Partial r = −.907, p= .013). Furthermore, the same pattern was observed with the FTND predicting right VS change in activation (Partial r = −.828, p= .042), although this did not reach significance in the left VS (Partial r = −.735, p =.096).
Figure 4.
Correlations between Nicotine Dependence Syndrome Scale (NDSS) scores and ventral striatal (VS) differential reward-related activity (right hemisphere cluster, maximal voxel coordinates: x = 8, y = 6, z = −6) from the contrast of positive > negative feedback. a. Relationship between NDSS and right VS reward-related activation, plotted separately for abstinent (partial r = −.772, p = 0.072, controlling for age, race, session order, and pre-session CO) and non-abstinent sessions (partial r = .198, ns). b. Relation between NDSS scores and the difference in right VS reward-related activation during abstinence compared with non-abstinence (partial r = −.900, p = 0.015).
It should be noted that the high correlations observed within this small sample likely represent an overestimation of the true effect size for the association between nicotine dependence and withdrawal induced changes in VS activation (see Yarkoni et al., 2009 for a discussion of this issue), and replication in a larger sample is needed. However, these preliminary findings suggest that, although abstinence from smoking was not associated with a statistically significant generalized decrease in reward-related VS activation, substantial individual variability was observed in the degree of change in activation induced by abstinence from smoking compared with non-abstinence. Importantly, this variability was significantly related to nicotine dependence. Only the three most highly dependent individuals appeared to show decrements in VS response to reward as a function of abstinence, while those low in dependence appear to show the opposite pattern. While replication is clearly needed, these preliminary findings suggest that a relative decrease in neural sensitivity to reward during abstinence may be associated with high levels of dependence. These findings are consistent with a recent study demonstrating a negative association between reward-related BOLD activation among detoxified alcoholics and number of subsequent drinking days (Heinz et al., 2007), suggesting a heightened vulnerability to relapse among those exhibiting the greatest reward decrements during abstinence. Furthermore, studies with heroin and cocaine dependent subjects have demonstrated similar blunting of BOLD activation to positive affective stimuli relative to healthy controls (Garavan et al., 2000; Zijlstra et al., 2009), suggesting a potential pathway of common liability to addiction.
As described above, multiple neural pathways and environmental factors are likely to contribute to complex behaviors such as difficulty quitting smoking. Although the findings described above require replication in a larger sample, they suggest one potential pathway which may be related to inter-individual variability in more distal processes. Characterization of this neural phenotype among chronic smokers paves the way for further analysis of the mechanisms underlying this variability. Given the influence of common genetic variants (e.g., DAT1, DRD2 −141 Ins/Del) on dopamine signaling associated with reward circuitry and impulsivity, it is possible that these or related genes may also be related to the severity of nicotine dependence by way of their impact on the susceptibility to neuroadaptations induced by chronic smoking. Extending research to behavioral and neural processes unique to addicted populations also opens the door for testing the impact of genetic variation in other pathways such as nicotinic cholinergic or opioid signaling with clear prior relevance to drug abuse and addiction.
4. Summary and conclusions
We have presented a framework for understanding the neurobiological basis for inter-individual variability in complex traits. With the advent of human genome sequencing and advances in neuroimaging technologies in recent years, we now have the capacity to trace complex human behaviors such as addiction to the level of their basic genetic building blocks by identifying the intermediate behavioral phenotypes, associated neural circuitry, and underlying molecular signaling pathways. Such ongoing efforts to understand the detailed mechanisms that mediate individual differences in addictive disorders and closely related behavioral traits may provide guiding principles to develop more effective prevention strategies and individually tailored treatment regimes. Where genetic association studies predicting treatment outcomes may yield little benefit in terms of significant or informative findings, an integrated, multilevel approach that traces the neural pathways linking candidate genes to core behavioral phenotypes can help to identify markers of risk for addiction. Researchers, armed with a detailed conceptualization of the neurobiological and behavioral sequelae of candidate functional genetic polymorphisms, have the potential to identify underlying mechanisms which may contribute to initiation, relapse, or recovery; mechanisms which likely vary across individuals and which could be specifically targeted for tailored behavioral and/or pharmacological interventions.
One implication of the focus on the neural and genetic pathways underlying core features of addiction is that many of the features are not specific to any single drug of abuse, or even to drug addiction per se. That is, genetic variation related to fundamental psychological processes (e.g., dopamine signaling, reward circuitry and impulsivity) may shape risk for many psychological disorders depending on the influence of other genetic and/or environmental factors. Of course, some sources of variation may also be drug-specific. For example, variation in nicotinic receptors is likely to be more closely linked to the pharmacological actions of nicotine and symptoms of nicotine dependence (e.g., tolerance to nicotine) than other drugs of abuse. Indeed, by tracing the neural and behavioral pathways linking candidate genes to disease state, we are likely to reveal drug-specific as well as more general mechanisms.
The examples described above focused on the effects of a single signaling pathway on behaviorally relevant brain circuitry. However, it is likely that most genetic contributors to addictive disorders do not operate in an isolated, linear manner. It is clear that there are numerous complex interactions between pathways, and more than one signaling pathway contributes to the regulation of brain circuitry. Similarly, alterations within signaling pathways are likely to impact multiple brain regions and more than one behavioral phenotype. Thus, future work must elucidate the complex interactions between multiple signaling pathways and integrate across multiple behavioral phenotypes to provide a comprehensive understanding of the neurobiological basis of vulnerability to addiction. Furthermore, gene-environment interactions and epigenetic effects are also likely to modulate the influence of genotype on brain circuitry and behavior, further underscoring the complexity of potential pathways to behavior.
In addition, because addiction involves neuroadaptations resulting from long-term drug exposure, prospective studies are needed to ultimately distinguish between those neural phenotypes which confer risk for addiction and those which are the result of years of drug abuse. Furthermore, given that substance use is often initiated during adolescence, prospective studies are needed to identify developmental shifts in neurogenetic pathways mediating individual variability in behavior (Viding et al., 2006). Finally, consideration of ethnic background is critical to imaging genetics, given that specific polymorphisms may differ in allelic frequencies across populations, and may even exhibit altered functional effects dependent on genetic ancestry (Kim et al., 2000; Lee and Ham, 2008; Munafo et al., 2008; Yoshida et al., 2002). Ultimately, these strategies have the potential to dramatically improve our understanding of the neurobiological pathways leading to vulnerability to addiction, and may contribute to the discovery of novel therapeutic strategies targeting underlying disease processes.
Supplementary Material
Acknowledgements
Ms. Sweitzer was supported by NIH training grant (T32GM081760) and the Center for the Neural Basis of Cognition. The authors would like to thank Adam Gorka, Patrick Fisher, and Karen Munoz for their assistance with imaging analyses.
Role of Funding Source: This work was funded by Western Psychiatric Institute and Clinic and the following grants from the National Institutes of Health: MH072837 (ARH), DA023459 (ECD), and DA027441 (ECD). Dr. Donny also received a Global Research Award on Nicotine Dependence from Pfizer, Inc. WPIC, NIH and Pfizer had no further role in study design, collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Author Disclosures
Contributors: MMS and ARH managed the literature searches and summaries of previous related work. For new data, MMS, ECD, and ARH designed the study and wrote the protocol. MMS and ARH undertook statistical analyses, and MMS wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of Interest: The authors declare no conflicts of interest.
For a complete description of methods, see supplementary materials by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
See supplementary materials for more information on image acquisition, processing, and analyses by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
See supplementary materials by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav. Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Moeller FG, Rhoades HM, Cherek DR. Impulsivity and history of drug dependence. Drug Alcohol Depend. 1998;50:137–145. doi: 10.1016/s0376-8716(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol. Biochem. Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum. Mol. Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur. Neuropsychopharmacol. 2001;11:449–455. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol. Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch. Gen. Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl.) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur. Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch. Gen. Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol. Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology (Berl.) 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- de Wit H, Flory JD, Acheson A, McLoskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Pers. Individ. Dif. 2007;42:111–121. [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol. Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Bogdan R, Fagerness J, Holmes AJ, Perlis RH, Pizzagalli DA. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum. Brain Mapp. 2010;31:210–221. doi: 10.1002/hbm.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl.) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug Alcohol Depend. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. USA. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat. Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol. Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Kullmann PH, Horn JP, Levitan ES. D2 autoreceptors chronically enhance dopamine neuron pacemaker activity. J. Neurosci. 2006;26:5240–5247. doi: 10.1523/JNEUROSCI.4976-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu. Rev. Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J. Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am. J. Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol. Clin. Exp. Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Jomphe C, Lemelin PL, Okano H, Kobayashi K, Trudeau LE. Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur. J. Neurosci. 2006;24:2789–2800. doi: 10.1111/j.1460-9568.2006.05151.x. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, Carroll BJ. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000;11:215–219. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict. Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat. Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BT, Ham BJ. Serotonergic genes and amygdala activity in response to negative affective facial stimuli in Korean women. Genes Brain Behav. 2008;7:899–905. doi: 10.1111/j.1601-183X.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- Logue AW. Self-control: Waiting Until Tomorrow for What You Want Today. Englewood Cliffs, NJ: Prentice-Hall; 1995. [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp. Clin. Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am. J. Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Martin-Soelch C, Missimer J, Leenders KL, Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. Eur. J. Neurosci. 2003;18:680–688. doi: 10.1046/j.1460-9568.2003.02791.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, Laruelle M. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, Kapur S. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biol. Psychiatry. 2007;62:765–772. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J. Neurochem. 2001;79:1033–1038. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am. J. Med. Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Craig I, D'Souza UM. Transient expression analysis of allelic variants of a VNTR in the dopamine transporter gene (DAT1) BMC Genet. 2005;6:3. doi: 10.1186/1471-2156-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl.) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav. Processes. 2011;87:10–17. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am. J. Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol. Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl.) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl.) 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J. Stud. Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J. Neurosci. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol. Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict. Behav. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav. Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv. Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ, Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. Int. Rev. Neurobiol. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, Smolka MN, Buchholz HG, Schreckenberger M, Rosch F, Cumming P, Mann K, Bartenstein P, Heinz A. Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur. J. Neurosci. 2006;24:305–313. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- Stulz N, Gallop R, Lutz W, Wrenn GL, Crits-Christoph P. Examining differential effects of psychosocial treatments for cocaine dependence: an application of latent trajectory analyses. Drug Alcohol Depend. 2009;106:164–172. doi: 10.1016/j.drugalcdep.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob. Res. 2008;10:1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, Weinberger DR, Mattay VS. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J. Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J. Nucl. Med. 2005;46:745–751. [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Williamson DE, Hariri AR. Developmental imaging genetics: challenges and promises for translational research. Dev. Psychopathol. 2006;18:877–892. doi: 10.1017/s0954579406060433. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl. 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. Am. J. Psychiatry. 1992a;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992b;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch. Gen. Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp. Clin. Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Weaver MT, Sweitzer M, Coddington S, Sheppard J, Verdecchia N, Caggiula AR, Sved AF, Donny EC. Precipitated withdrawal from nicotine reduces reinforcing effects of a visual stimulus for rats. Nicotine Tob. Res. 2012 doi: 10.1093/ntr/ntr293. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, Braus DF, Buchel C. Gene-gene interaction associated with neural reward sensitivity. Proc. Natl. Acad. Sci. USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies: Inflated fMRI correlations reflect low statistical power--commentary on Vul et al. (2009) Perspect. Psychol. Sci. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Ito K, Sato K, Takahashi H, Kamata M, Higuchi H, Shimizu T, Itoh K, Inoue K, Tezuka T, Suzuki T, Ohkubo T, Sugawara K, Otani K. Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:383–386. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.