Abstract
C57BL/6 mice were maintained for up to 18-months on high-fat and low-fat diets with or without a multi-mineral-supplement derived from the skeletal remains of the red marine algae Lithothamnion calcareum. Numerous grossly observable liver masses were visible in animals on the “western-style” high-fat diet sacrificed at 12 and 18 months. The majority of the masses were in male mice (20 out of 100 males versus 3 out of 100 females; p=0.0002). There were more liver masses in animals on the high-fat diet than on the low-fat diet (15 out of 50 on high-fat versus 5 out of 50 on low-fat; p=0.0254). The multi-mineral supplement reduced the number of liver masses in mice on both diets (3 out of 25 male mice in the low-fat diet group without the supplement versus 1 out of 25 mice with supplement; 12 of 25 male mice in the high-fat diet group without the supplement versus 3 of 25 mice with supplement [p=0.0129]). Histological evaluation revealed a total of 17 neoplastic lesions (9 adenomas and 8 hepatocellular carcinomas), and 18 pre-neoplastic lesions. Out of 8 hepatocellular carcinomas, 7 were found in unsupplemented diet groups. Steatosis was widely observed in livers with and without grossly observable masses, but the multi-mineral supplement had no effect on the incidence of steatosis or its severity. Taken together, these findings suggest that a multi-mineral-rich natural product can protect mice against neoplastic and pre-neoplastic proliferative liver lesions that may develop in the face of steatosis.
Keywords: Calcium, hepatocellular carcinoma, liver disease, minerals, trace elements
INTRODUCTION
Overnutrition in the form of a high-fat or high caloric intake is highly associated with fat infiltration in the liver, or steatosis. While steatosis alone is typically well-tolerated, some individuals further develop inflammation (steatohepatitis) and fibrosis, culminating in cirrhosis. The spectrum of overnutrition-associated liver changes and metabolic alterations are clinically described as non-alcoholic fatty liver disease (NAFLD). NAFLD has recently been shown to increase the risk of hepatocellular carcinoma [1–3]. Mice on a long-term “Western-style” diet, with high caloric and dietary fat content, develop at least some of the features of NAFLD, specifically steatosis, and in some instances, perivenular fibrosis [4–8]. Additionally, long-term high fat diets have been demonstrated to increase neoplastic and pre-neoplastic liver lesions in mice [9–12].
Although the typical Western style diet has been epidemiologically and experimentally linked to liver disease, the specific dietary components that most contribute to these changes are still under investigation. In addition to a high content of saturated fat and sugar, the typical Western-style diet is deficient in calcium [13–15] and, possibly, other trace minerals that occur in conjunction with natural sources of calcium. We previously demonstrated that a calcium-rich, multi-mineral-rich marine algae-derived dietary supplement reduced the formation of adenomatous colonic polyps in mice on a high-fat Western diet (HFWD) [16]. The effects of mineral supplementation on hepatic disease have not been previously evaluated, to our knowledge. In the present study, we assessed the development of pre-neoplastic and neoplastic liver lesions in healthy mice, and determined whether the mineral supplement may have a protective effect against proliferative liver lesions.
MATERIALS AND METHODS
Mineral-rich natural product
The multi-mineral-rich material used in this study was obtained from the skeletal remains of the red marine algae, Lithothamnion calcareum (Pallas), also known as Phymatolithon calcareum (Pallas) [17]. The mineral-rich algae product is approximately 12% calcium by weight, and 1% magnesium, but also contains measurable levels of 72 other trace minerals. The mineral product is sold as a food supplement under the name Aquamin® (GRAS 000028) and is used in various products for human consumption in Europe, Asia, Australia, and North America [Marigot Ltd, Cork, IR]. The complete mineral composition of the batch used in the present study is shown in Supplement Table 1.
Diet groups
Four diet groups were included in this study, the AIN 76A (low-fat) rodent diet, a high-fat, western-style diet (HFWD), which is a variant of AIN 76A, and the same two diets supplemented with the mineral-rich algae product. AIN 76A contains 5% fat from corn oil. The HFWD was prepared according to the formulation of Newmark et al [18] and designed to mimic the diet consumed by many individuals in Western society. It contains 20% fat from corn oil. On a per weight basis, the percentage of calories from fat in this diet is 37.8% as compared to 11.5% in the AIN 76A diet. Although sucrose is reduced in the HFWD relative to AIN 76A, the overall calories provided in the HFWD is 4767 kcal% versus 3902 kcal% in AIN 76A. In the HFWD, the calcium level is reduced from 5.25 gm/kg to 0.41 gm/kg. Both diets contain a mix of essential trace elements including potassium, magnesium, manganese, chromium, copper, iron, selenium and zinc. For half of the animals in the present study, diets were supplemented with the mineral-rich natural product at 62 gm%, providing comparable dietary calcium to what is in the AIN 76A diet. Diets were formulated and provided by Research Diets Incorporated (New Brunswick, NJ). The compositions of the four diets are presented in Supplement Table 2.
Treatment protocol and necropy
Separate cohorts of male and female C57BL/6 mice were maintained for 5, 12 or 18 months on their respective diet. Food was provided ad libitum. Animals were monitored at 2-day intervals throughout the maintenance phase and were weighed every two weeks. Animals were euthanized at each time-point and subjected to necropsy. After the abdominal cavity was opened and the liver exposed, it was photographed in situ, if there was any gross lesion. Then the liver was removed, weighed, and fixed in 10% buffered formalin for histology. Tissue sections from selected normal-appearing areas and from all grossly-visible lesions (35 lesions in all) were stained with hematoxylin and eosin and examined at the light microscopic level by a board-certified veterinary pathologist (I.B).
Liver lesions were classified according to recently revised, standardized guidelines established by the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND) project [19]. This project represents consensus criteria for histopathological lesions in rodents as established by the North American, European, British, and Japanese Societies of Toxicologic Pathology. Proliferative lesions were classified as foci of cellular alteration (FCA), non-regenerative hyperplasia (NRH), regenerative hyperplasia (RH), hepatic adenoma (HA), or hepatocellular carcinoma (HCC). These can be considered as a spectrum of pre-neoplastic and neoplastic lesions. The pre-neoplastic lesions (foci of cellular alteration, non-regenerative and regenerative hyperplastic nodules) have been used as early markers of hepatic carcinogenesis in the mouse in short and medium-term carcinogenicity studies [20].
Blood was obtained at the time of necropsy. A clinical chemistry profile including serum levels of albumin, globulin, total protein, alkaline phosphatase (ALKP), alanine aminotransaminase (ALT), cholesterol and glucose was obtained for a subset of the animals (i.e., male mice at month-18). Serum calcium levels were assessed in the same samples. Values were determined using the Vet-Test dry chemistry analyzer (Idexx Laboratories, Westbrook, ME). Calcium levels in long bones were determined in parallel. Long bones (one femur and one tibia from each animal) were carefully separated from the surrounding connective tissue. Bones from all animals were “pooled” in order to obtain a sufficient quantity of bone, and the bone tissue converted to ash. Calcium levels were then determined by atomic adsorption spectroscopy (Advanced Laboratories, Inc., Salt lake City, UT). C-reactive protein was assessed in serum using an enzmye-linked immunosorbent assay (ELISA) kit (Life Diagnostics Inc., West Chester, PA). Finally, a panel of cytokines was measured in serum samples using the Bio-Plex bead-based cytokine assay from Bio-Rad Laboratories (Hercules, CA).
All of the procedures involving animals were reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan.
Statistical evaluation
Differences in the incidence of liver lesions among diet groups were assessed for statistical significance using the Fisher Exact Test (2-tailed). Biochemical data were evaluated statistically by ANOVA followed by paired group comparisons. Differences were considered significant at the p<0.05 level.
RESULTS
Suppression of liver injury with the mineral-rich natural product: Gross findings
Male and female mice were maintained on their respective diets for 5, 12 and 18 months. At all three time-points, mice on the HFWD diet were heavier than animals on the low-fat diet. However, the presence or absence of the mineral-rich supplement in the diet had no effect on weight gain in either sex. Average weights for each group at the end of the 18-month time-point are shown in Supplement Table 3.
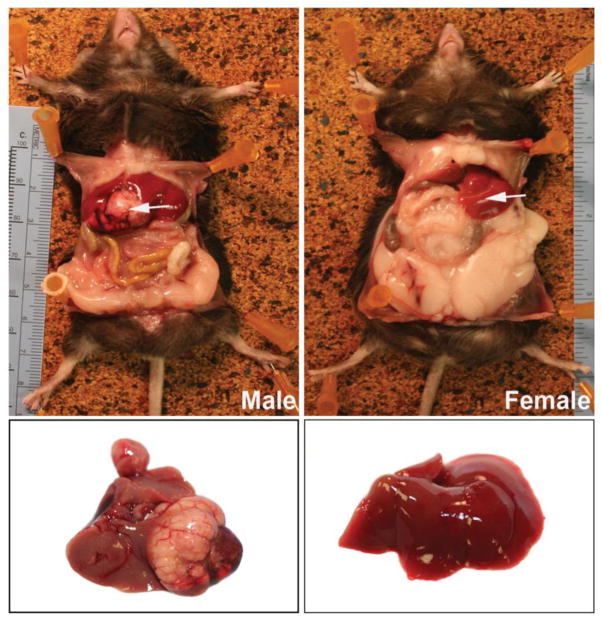
Livers obtained at necropsy were examined in situ and after removal. While all livers appeared normal at the 5-month time-point, distinct liver masses were observed in 20 male mice (out of 100 animals) at the 12 and 18-month time-points (combined) as compared to only 3 lesions in 100 female mice (p= 0.0002). Figure 1 demonstrates a large tumor in the liver of a male mouse while the female mouse has a normal-appearing liver.
Figure 1. In situ appearance of livers in a male and female mouse on the HFWD for 18 months.
In the male mouse (left), a large liver mass is visible (arrow). In the female mouse, the liver is normal in appearance (arrow). A large amount of fat in the peritoneal wall of the female mouse can be seen, but this is not seen in the male mouse.
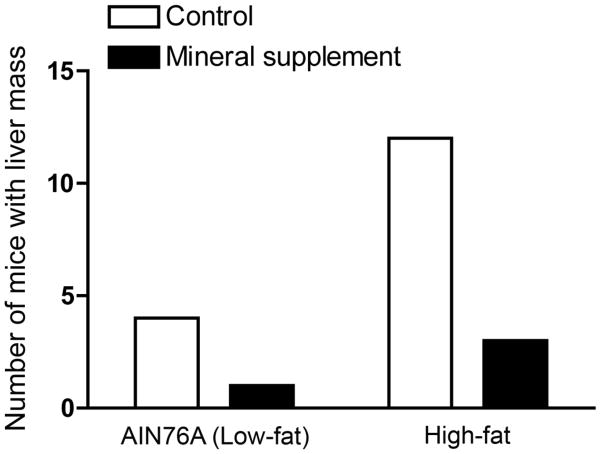
Among male mice, there was a higher incidence of liver mass formation in the high-fat diet group than in the low-fat diet group (15 out of 50 males on high-fat versus 5 out of 50 on low fat; p= 0.0254, Figure 2). Inclusion of the mineral-rich supplement in the diet was associated with a reduction in liver mass formation among male mice. In the high-fat diet group, 12 of 25 mice without the supplement had liver masses versus 3 of 25 mice with the supplement (p= 0.0129) (Figure 2). There was also a lower incidence of liver mass formation with the mineral supplement in the low-fat diet. However, with such a small number of lesions to begin with, there was no statistical difference (Figure 2). With the high-fat and low-fat diet groups combined, the difference was significant (16 of 50 without the supplement versus 4 of 50 with the supplement (p= 0.0054).
Figure 2. Distribution of liver masses in male mice by diet group.
Values shown represent the number of male mice at 12 and 18 months (combined) with overt liver masses at necropsy. Differences in the incidence of liver mass formation was assessed for statistical significance using the Fisher Exact Test (two tailed). Differences between high-fat and AIN 76A (low-fat) were significant at p=0.0254; differences between unsupplemented (high-fat alone) and mineral-supplemented (high-fat) were significant at p=0.0129; differences between unsupplemented and mineral-supplemented (high-fat and low-fat combined) were significant at p=0.0054.
Histological findings
Liver masses identified grossly were histologically classified as pre-neoplastic or neoplastic proliferative lesions according to the guidelines established by the INHAND project [19]. Table 1 summarizes lesion histology among male mice as a function of diet group and Figure 3 presents representative histology. Seventeen lesions in male mice were classified as neoplasms, with 9 lesions identified as adenomas (benign tumors) and 8 as hepatocellular carcinoma (malignant tumors). Thirteen of these 17 lesions occurred in animals without the dietary mineral supplement. Six lesions were identified as FCA, all in animals without the mineral supplement (Table 1). The remaining lesions were identified as RH or NRH. Among female mice, a total of three lesions were characterized histologically. All were non-malignant in nature (regenerative or non-regenerative hyperplasia). With so few lesions, not much can be said about diet influence.
Table 1.
Histological characterization of liver masses in male mice
| Group | Histological description | ||||
|---|---|---|---|---|---|
| FCA | NRH | RH | HA | HCC | |
| AIN 76A (Low-fat) | 3 | 1 | 0 | 2 | 3 |
| AIN 76A (Low fat) + mineral supplement | 0 | 1 | 0 | 2 | 0 |
| HFWD | 3 | 6 | 1 | 4 | 4 |
| HFWD + mineral supplement | 0 | 2 | 1 | 1 | 1 |
A total of 35 distinct liver masses were observed in 20 male mice. Each lesion was characterized by light microscopy of hematoxylin and eosin-stained sections, and classified according to the INHAND project (19).
FCA = focus of cellular alteration, NRH = non-regenerative hyperplasia, RH = regenerative hyperplasia, HA = hepatic adenoma, HCC = hepatocellular carcinoma
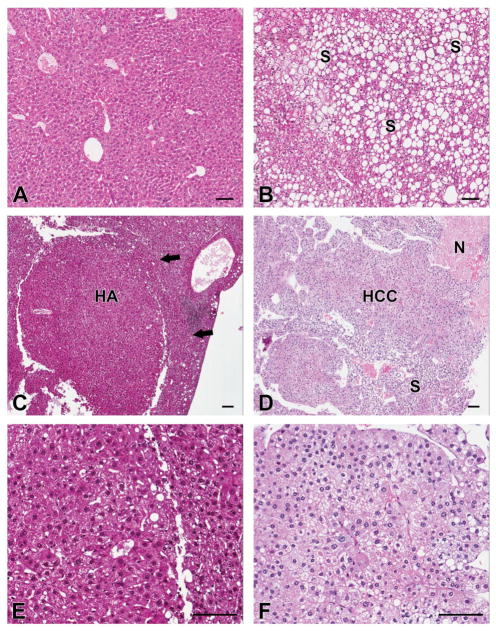
Figure 3. Representative histological sections of liver in male mice.
A: Normal liver appearance from a male mouse on the mineral-supplemented high-fat diet at 18 months; B: Steatotic liver from a male mouse on the mineral-supplemented high-fat diet at 18 months. Mineral supplementation did not affect the number of mice with steatosis. C and E: Hepatic adenoma (HA) from a male mouse on the high-fat diet without supplementation at 18 months. Arrow indicates compression of adjacent parenchyma. D and F: Hepatocellular carcinoma (HCC) in a male mouse on the high-fat diet without supplementation at 18 months. Hepatocytes form sheets and thick trabeculae without normal architectural features. Fat accumulation (steatosis, S) and necrosis (N) are evident in some areas. Neoplastic (and pre-neoplastic) lesions were more frequent in non-supplemented male mice. All sections are hematoxylin and eosin. (Bars=100 μm).
In addition to replicative abnormalities, we also assessed livers for fatty infiltration (steatosis) and for inflammation. Virtually every animal (both males and females) at the 12 and 18 month time-points demonstrated some degree of fatty infiltration. Even in livers with no grossly visible anomalies, steatosis was observed. Steatosis was more extensive in males than in females. The incidence was higher in mice on the high-fat diet than on the low-fat diet, but there was little difference between mice with the mineral-rich supplement and controls (not shown).
None of the animals euthanized at 5 months or 12 months (male or female on any diet) showed significant inflammatory changes. At the 18 month time point, wide-spread liver inflammation was observed in three of the male mice on high fat diet and some degree of inflammatory changes was also seen in four others.
Metabolic parameters, cytokines and calcium levels in male mice
A number of metabolic markers reflective of liver function were assessed in serum of male mice at the 18 month time-point. For several parameters, no diet-specific differences were detected (not shown). Not surprisingly, when animals with liver masses were compared to those without overt disease, differences were observed. Specifically, alkaline phosphatase and alanine aminotransaminase levels were increased in tumor-bearing mice (Table 2). Serum cytokine levels showed similar trends. That is, there were no diet-dependent differences, but there were differences between animals with liver tumors versus those without (Table 2).
Table 2.
Blood chemistry and Cytokines Analysis in mice with and without liver tumors.
| No Liver Tumor | With Liver Tumor | ||
|---|---|---|---|
|
Blood Chemistry |
|||
| ALBUMIN | (g/dL) | 2.8±0.4 | 2.7±0.8 |
| GLOBULIN | (g/dL) | 3.2±0.3 | 3.5±0.4 |
| T PROTEIN | (g/dL) | 6.0±0.5 | 6.2±1.0 |
| CHOLESTEROL | (mg/dL) | 157±38 | 150±103 |
| GLUCOSE | (mg/dL) | 263±49 | 233±66 |
| ALKP | (U/L) | 77±25 | 136±87* |
| ALT | (U/L) | 67±48 | 263±305* |
| C - Reactive Protein | (ng/mL) | 238±167 | 242±374 |
|
Cytokines |
|||
| IL-1α | (pg/mL) | 0.09±0.19 | 0.66±0.82* |
| IL-6 | (pg/mL) | 6.8±14.3 | 16.5±25.9 |
| TNF-α | (pg/mL) | 0.7±1.9 | 2.3±3.6 |
| KC (IL-8) | (pg/mL) | 30.7±21.5 | 41.4±23.6 |
| MCP-1 | (pg/mL) | 146±102 | 172±94 |
| RANTES | (pg/mL) | 14.2±7.8 | 12.1±5.7 |
| Calcium | (mg/dL) | 10.2±1.1 | 9.5±1.5 |
Values shown are means and standard deviations, based on n= 8 mice with liver masses and n=41 mice without masses. Differences were evaluated using the Student T-test. Asterisks indicate significance at p<0.05.
ALKP = alkaline phosphatase
ALT = alanine aminotransaminase
IL-1 α = interleukin 1 alpha
IL-6 = interleukin 6
TNF-α = tumor necrosis factor alpha
KC = keratinocyte chemoattractant
MCP-1 = monocyte chemoattractant protein 1
RANTES = Regulated upon Activation, Normal T-cell Expressed, and Secreted
Calcium levels were assessed in male mice at 18-months. Serum calcium levels were not statistically different between mice in any diet group, irrespective of mineral supplementation (Supplement Table 4). Additionally, no difference in serum calcium levels between male mice with tumors and those without were seen (Table 2). Finally, bone reserves of calcium were also compared. Differences were slight (Supplement Table 4). Since bones from all mice in a group were pooled to obtain sufficient sample for analysis, it was not possible to compare animals with tumor versus those without.
DISCUSSION
A high-fat diet is known to produce fatty changes in the liver. Fatty changes are common and not considered serious, but more destructive liver disease including steatohepatitis, cirrhosis/fibrosis can follow. In addition, proliferative changes, leading, eventually, to hepatocellular carcinoma occur in a subset of individuals with pre-existing fatty changes [7,21–23]. While dietary fat is clearly linked with liver disease, other components of the typical Western-style diet including high carbohydrate content, and a deficiency of methyl group donors (i.e., certain b-vitamins, methionine and choline) also appear to contribute to deleterious changes in the liver [24].
In addition to a high content of saturated fat and sugar, and a lack of methyl group donors, the typical Western-style diet also has a deficiency in calcium [13–15]. Possibly, other trace minerals that occur in conjunction with calcium are deficient in the same diet, as well. What role a deficiency in calcium and / or other inorganic minerals play in liver disease is not known. What is known is that another consequence of a Western style diet (i.e., adenomatous colon polyps), is clearly linked to an inadequate level of calcium. This has been demonstrated in humans in both epidemiological [25–30] and interventional [31,32] studies. Experimental animal studies have shown that calcium alone reduces polyp formation in healthy rodents maintained on a high-fat diet [18,33,34] and our own recent studies have shown that a calcium-rich, multi-mineral supplement provides almost complete protection [16]. The current study demonstrates that the same multi-mineral-rich natural product used for polyp prevention also reduces liver injury. Specifically pre-neoplastic and neoplastic (adenoma and hepatocellular carcinoma) lesions are decreased. In our study with C57BL/6 mice, male mice maintained for up to 18 months on a high-fat diet had significantly more liver lesions than animals fed a low-fat diet. However, even on the low-fat diet, some animals developed liver lesions. In both groups of animals, the mineral supplement reduced both the incidence and severity of disease.
How supplementation of the diet with a mineral-rich product protects against liver injury is not understood. Previous short-term (6 week) studies demonstrated that including high levels of chromium (to provide up to 80 μg/kg of body weight) in the diet reduced liver injury in chronic cholestasis [35] as well as liver injury occurring in diabetic mice [36]. In another short-term study, sodium selenite (4 mg/kg of diet) reduced carcinogen-induced liver tumors in rats [37]. Mechanisms of action were not clearly delineated in these studies as multiple histo-pathological and biochemical changes were seen. Two other trace elements – zinc and copper – have also been shown to partially reverse liver injury in experimental animals [38]. An anti-oxidant effect was postulated to underlie protective activity. While all of these minerals are present in the multi-mineral-rich natural product used here, we do not believe that our results can be attributed to the activities of these important minerals alone. First of all, the amounts used in the previous, short-term studies were much higher than the levels found in the natural product (see Supplement Table 1). Second, both the low-fat diet (AIN 76A) and the high-fat diet – which is a variant of AIN 76A – contain significant amounts of essential minerals (including chromium, selenium, copper and zinc) (see Supplement Table 2).
One possibility is that calcium in the multi-mineral-rich supplement is primarily responsible for protection from liver injury. In other tissues that have been studied in detail, including the colon, calcium promotes differentiation and, concomitantly, reduces epithelial cell proliferation [39–41]. Colon polyp chemopreventive activity is thought to reflect this property. The colon, of course, can be exposed to high levels of calcium and other minerals via the colonic fluid. The liver, on the other hand, relies on plasma calcium levels, which are tightly regulated. In our study, serum calcium levels differed by approximately 5% between male mice receiving the mineral-rich supplement in the high-fat diet and those on the high-fat diet without supplement. Whether this difference is sufficient to account for liver protection against tumor formation is not clear. Additional studies will be needed to address this issue.
Several of the minor trace minerals, especially members of the lanthanoid family of “rare earth” elements, are known to enhance calcium’s epithelial cell growth-controlling activity. Lanthanoid elements bind to calcium-binding sites on regulatory molecules, including the extracellular calcium-sensing receptor (CaSR); in some cases, the affinity is higher than that of calcium itself [42–45]. Our own studies have shown that proliferation of epithelial cells can be suppressed by the multi-mineral-rich natural product under conditions in which equivalent amounts of calcium alone are not growth-inhibitory [46]. Individual lanthanoid elements duplicate growth-inhibiting activity [47]. The capacity of the lanthanoid elements to suppress epithelial growth may be particularly important in tissues such as the liver, where exposure to calcium is limited by plasma concentrations. This may be especially important under conditions of relative (dietary) calcium deficiency.
Whether the multi-mineral-rich marine algae extract can be used under conditions needed to protect the liver will require clinical studies in humans. Given that in this animal study no measurable adverse effects were attributed to the supplement, a clinical study evaluating biomarkers of hepatic function may be informative.
In summary, mice on a high fat diet with and without a multi-mineral supplement had similar degrees of steatosis, but mice without the supplement developed the majority of the pre-neoplastic and neoplastic lesions. This suggests that mineral supplementation, while it may not reverse fat accumulation in the face of a high fat content diet, may provide protection against the more deleterious effects of such a diet. Given the clinical difficulties in limiting liver steatosis associated with human dietary habits and contributing metabolic conditions (diabetes mellitus), such a therapy may be very useful. Ongoing clinical studies related to polyp prevention could, perhaps, be modified to incorporate liver function biomarkers.
Supplementary Material
Acknowledgments
This study was supported in part by grant CA140760 from the National Institutes of Health, Bethesda, MD, and by grant 11-0577 from the Association for International Cancer Research, St. Andrews, Fife, Scotland.
The authors would like to acknowledge Ron Craig (Histomorphometry Core) for his ScanScope services. The core laboratory is supported by the Department of Pathology at the University of Michigan. The authors would also like to thank Marigot, Ltd. (Cork, Ireland) for providing the multi-mineral supplement (Aquamin®) as a gift.
References
- 1.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatology Research. 2011 doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 2.Ertle J, Dechêne A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–43. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 3.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng QG, She H, Cheng JH, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–14. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 6.Charlton M, Krishnan A, Viker K, et al. The Fast Food Diet Mouse - A Novel Small Animal Model of NASH with High Histologic and Physiologic Fidelity to the Human Condition. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Suzuki J, Tsujioka S, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50–7. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara M, Hirose A, Ono M, et al. A novel and comprehensive mouse model of human non-alcoholic steatohepatitis with the full range of dysmetabolic and histological abnormalities induced by gold thioglucose and a high-fat diet. Liver Int. 2011;31:542–51. doi: 10.1111/j.1478-3231.2010.02443.x. [DOI] [PubMed] [Google Scholar]
- 9.Stauffer JK, Scarzello AJ, Andersen JB, et al. Coactivation of AKT and β-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–27. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soga M, Hashimoto S, Kishimoto Y, Hirasawa T, Makino S, Inagaki S. Insulin resistance, steatohepatitis, and hepatocellular carcinoma in a new congenic strain of Fatty Liver Shionogi (FLS) mice with the Lepob gene. Exp Anim. 2010;59:407–19. doi: 10.1538/expanim.59.407. [DOI] [PubMed] [Google Scholar]
- 12.Hill-Baskin AE, Markiewski MM, Buchner DA, et al. Diet-induced Hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–88. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. Eating patterns and risk of colon cancer. Am J Epid. 1998;148:4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 15.Potter JD. Risk factors for colon neoplasia – epidemiology and biology. Eur J Cancer. 1995;31:1033–1038. doi: 10.1016/0959-8049(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 16.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Res. 2010;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adey WH, McKibbin DL. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Botanical Marina. 1970;13:100–106. [Google Scholar]
- 18.Newmark HL, Yang K, Lipkin M, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57BI/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 19.Thoolen B, Maronpot RR, Harada T, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 20.Kushida M, Kamendulis LM, Peat TJ, Klaunig JE. Dose-related induction of hepatic preneoplastic lesions by diethylnitrosamine in C57Bl/6 mice. Toxicol Pathol. 2011;39:776–786. doi: 10.1177/0192623311409596. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Shimada M, Hashimoto E, Taniai E, et al. Hepatocellula carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–160. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 23.Demigne C, Bloch-Faure M, Picard N, et al. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr. 2006;45:298–306. doi: 10.1007/s00394-006-0599-6. [DOI] [PubMed] [Google Scholar]
- 24.Denda A, Kitayama W, Kishida H, et al. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, l-amino acid-defined diet. Jpn J Cancer Res. 2002;93:125–132. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough ML, Robertson AS, Rodriguez C, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States) Cancer Causes Control. 2003;14:1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- 26.Flood A, Peters U, Chatterjee N, Lacey JV, Jr, Schairer C, Schatzkin A. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev. 2005;14:126–132. [PubMed] [Google Scholar]
- 27.Wakai K, Hirose K, Matsuo K, et al. Dietary risk factors for colon and rectal cancers: a comparative case-control study. J Epidemiol. 2006;16:125–135. doi: 10.2188/jea.16.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 29.Kampman E, Giovannucci E, van ‘t Veer P, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol. 1994;139:16–29. doi: 10.1093/oxfordjournals.aje.a116931. [DOI] [PubMed] [Google Scholar]
- 30.Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States) Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 31.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 32.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 33.Yang K, Kurihara N, Fan K, et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 34.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57BI/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W-Y, Chen C-J, Liao J-W, Mao FC. Chromium attenuates hepatic damage in a rat model of chronic cholestasis. Life Sci. 2009;84:606–614. [PubMed] [Google Scholar]
- 36.Chen W-Y, Chen C-J, Liu C-H, Mao FC. Chromium attenuates high-fat diet-induced nonalcoholic fatty liver disease in KK/HIJ mice. Biochem Biophys Res Commun. 2010;397:459–464. doi: 10.1016/j.bbrc.2010.05.129. [DOI] [PubMed] [Google Scholar]
- 37.Alwahaibi N, Mohamed J, Alhamadani A. Supplementation of selenium reduces chemical hepatocarcinogenesis in male Sprague-Dawley rats. J Trace Elements Biol Med. 2010;24:119–123. doi: 10.1016/j.jtemb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Kang X, Zhong W, Liu J, et al. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: Promotion of E-cadherin expression and suppression of -catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 40.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: Interaction with Ca2+ and 1,25-Dihydroxyvitamin D3. Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]
- 41.Bhagavathula N, Hanosh AW, Nerusu KC, et al. Regulation of E-cadherin and β-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. Int J Cancer. 2007;121:1455–1462. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Zhou Y, Castiblanco A, et al. Multiple Ca(2+) -binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273(23):14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 44.McLarnon SJ, Riccardi D. Physiological and pharmacological agonists of the extracellular Ca2+ -sensing receptor. Eur J Pharmacol. 2002;447:271–278. doi: 10.1016/s0014-2999(02)01849-6. [DOI] [PubMed] [Google Scholar]
- 45.Riccardi D, Maldonado-Perez D. The calcium-sensing receptor as a nutrient sensor. Biochem Soc Trans. 2005;33:316–320. doi: 10.1042/BST0330316. [DOI] [PubMed] [Google Scholar]
- 46.Aslam MN, Bhagavathula N, Chakrabarty S, Varani J. Growth-inhibitory effects of Aquamin, a mineralized extract from the red algae, Lithothamnion calcerum, on Ca2+ - sensitive and Ca2+ - resistant human colon carcinoma cells. Cancer Letters. 2009;283(2):186–192. doi: 10.1016/j.canlet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins W, Perone P, Walker K, et al. Fibroblast response to lanthanoid metal ion stimulation: Potential contribution to fibrotic tissue injury. Biol Trace Element Res. 2011 doi: 10.1007/s12011-011-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.