Abstract
Objective
We examined the cross-sectional and longitudinal relationship between plasma carotenoids and depressive symptoms over a six-year follow-up in older persons.
Methods and Materials
This research is part of the InCHIANTI Study, a prospective population-based study of older persons in Tuscany, Italy. The sample for this analysis included 958 women and men aged 65 years and older. Plasma total carotenoids were assessed at baseline. Depressive symptoms were assessed at baseline and at the 3- and 6-year follow-up using the Center for Epidemiological Studies-Depression Scale (CES-D). Depressed mood was defined as CES-D≥20.
Results
At baseline, higher total carotenoids level were associated with lower probability of depressed mood (OR=0.82, 95%CI=0.68–0.99, p=0.04) after adjustment for sociodemographic, health and inflammation. After the exclusion of participants with baseline depressed mood and use of antidepressants, higher total carotenoids level were associated with lower risk of incident depressed mood (OR=0.72, 95%CI=0.52–0.99, p=0.04) at 6-year follow-up, after adjustment for confounders plus baseline CES-D. Inflammatory marker Interleukin-1 receptor antagonist partially mediated this association.
Discussion
Low plasma concentrations of carotenoids are associated with depressive symptoms and predict the development of new depressive symptoms in older persons. Understanding the mechanism of this association may reveal potential targets for prevention and treatment.
Keywords: Carotenoids, antioxidants, depression, inflammation, aging
INTRODUCTION
Depression is a major public health problem causing high disease burden for both communities and individuals worldwide. Chronic depressive syndromes are very common in older persons, especially in those affected by chronic medical illness, and strongly affect the risk of developing disability and death (Beekman et al. 2002, Alexopoulos 2005). According to the World Health Organization, depression is among the leading disorders causing disability and will be the second most important cause of disability worldwide in 2020 (Murray and Lopez 1997). Previous studies have shown that depression and stress are associated with upregulated inflammatory response, characterized by increased levels of pro-inflammatory cytokines and other acute phase proteins (Howren et al. 2009, Dowlati et al. 2010). Diet may also influence inflammation: a diet rich in fruit and vegetables is associated with lower levels of inflammatory markers, perhaps because of the anti-inflammatory properties of antioxidants (Giugliano et al. 2006, Semba et al. 2007a, Walston 2006). Few studies with mixed results investigated the associations of nutrient intake and biomarkers, such as folate, vitamin B12, vitamin D, and polyunsaturated fatty acids (PUFA) with depressive symptoms and depression diagnoses in older persons (Ng et al. 2009, Dimopoulos et al. 2007, Kim et al. 2008, Penninx et al. 2000, Skarupski et al. 2010, Kamphuis et al. 2008, Hoogendijk et al. 2008, Milaneschi et al. 2010a, Tiemeier et al. 2003, Féart et al. 2008, Kiecolt-Glaser et al. 2007, van de Rest et al. 2008). However, whether carotenoid levels are associated with depression in older persons has not yet been studied. According to the Food and Nutrition Board of the Institute of Medicine, blood concentrations of carotenoids are the best biological markers for consumption of fruits and vegetables (Institute of Medicine 2000).
In the present study we examined for the first time the cross-sectional and longitudinal relationship between plasma carotenoids and depressive symptoms over a six-year follow-up in a representative sample of older persons. We tested the hypothesis that participants with low carotenoid levels would be significantly more likely to have higher depressive symptoms and to develop clinically relevant depressed mood over time. Finally, we tested whether inflammatory markers mediated this relationship.
METHODS AND MATERIALS
Study Population
Participants were part of the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study, a prospective population-based study of older persons in Tuscany (Italy) designed to investigate factors contributing to decline of mobility in late life. A description of the study rationale, design and methods is given elsewhere (Ferruci et al. 2000). Briefly, in 1998–1999 the sample was randomly selected from two sites, Greve in Chianti and Bagno a Ripoli, using a multistage stratified sampling method: 1270 persons ≥65 years were randomly selected from the population registry of the two sites, another 29 subjects ≥90 years were oversampled. Thirty-nine participants were not eligible because they had already died or emigrated. Among those who were eligible, 1155 (91.6%) were enrolled. Data collection included: 1) a home interview 2) physical performance testing and medical examination at the study clinic; 3) a 24-h urine collection and a blood drawing. Participants were seen again for a three-year follow-up visit (2001–2003) and six-year follow-up visit (2004–2006). All respondents signed informed consent and the Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol.
Of the 1155 participants aged≥65 enrolled in the study, we excluded 175 because of missing data on plasma carotenoids or depressive symptoms at baseline. Subjects who did not participate in the blood drawing were generally older and had more comorbidity than those participating, as reported elsewhere (Schrager et al. 2007). Moreover, we additionally excluded 22 participants with dementia at baseline. In cross-sectional analyses we included all 958 remaining participants. In longitudinal analyses, consistent with a previous study based on the InCHIANTI cohort (Milaneschi et al. 2009), we considered participants with available data on depressive symptoms at 3- and 6-year of follow-up. At 3-year follow-up, 62 participants had already died, 90 were lost (77 refused, 10 emigrated, 3 not found) and 41 had missing depressive symptoms scores. This left 765 participants with available depression measures at the 3-year follow-up. At 6-year follow-up, 180 had died, 52 were lost (35 refused, 17 emigrated) and 65 had missing depressive symptoms scores. This left 661 participants for whom 6-year follow-up data on depression were available. As compared to participants with at least one available follow-up measure of depression, those lost at both follow-up were significantly older, more often sedentary and disabled, had poorer cognitive function, lower concentrations of plasma total carotenoids and higher depressive symptoms at baseline.
Carotenoids
The six major dietary carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene) comprise an important component of the antioxidant defense system in humans, and are considered a good indicator of fruit and vegetable intake (Institute of Medicine 2000). Measures for level of carotenoids were obtained from frozen plasma samples originally collected at baseline. Blood samples were collected in the morning after a 12-hour fast. Aliquots of serum and plasma were immediately obtained and stored at −80°C. Aliquots of plasma were shipped on dry ice to Dr. Semba’s laboratory for measurements of plasma carotenoids. Carotenoids were measured using high-performance liquid chromatography (HPLC). Total carotenoids were calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene in micromoles per liter (μmol/L). Within-run and between- run coefficients of variation, respectively, were 7.3% and 9.6% for α-carotene, 4.5% and 5.4% for β-carotene, 2.7% and 3.5% for β-cryptoxanthin, 2.6% and 7.1% for lutein, 6.2% and 6.8% for zeaxanthin, and 7.5% and 7.8% for lycopene.
Depressive symptoms
Depressive symptoms were assessed at baseline, at 3- and 6-year follow-up using the Center for Epidemiological Studies-Depression scale (CES-D) (Radloff 1977). The CES-D is a 20-item self report scale, ranging from 0 to 60. The CES-D has been shown to have good psychometric properties in assessing depressive symptoms in older population-based studies, also in an Italian sample (Fava 1983). In addition to a continuous CES-D score, a cut-off score ≥20 was used to additionally define clinically relevant “depressed mood”. While a cut-off of 16 is generally considered to represent relevant depression, we selected a cut-off of 20 that has been shown to avoid overestimation of depressed mood in older subjects (Beekman et al. 1997).
Other variables
The following covariates assessed at baseline were used in the analysis: age, gender, education (years), smoking habit (current/former versus non smoker), alcohol use (< 30 vs ≥ 30 g per day) and Mini Mental State Examination (MMSE) score. Use of antidepressants was coded according to Anatomical Therapeutic Chemical (ATC) classification system. Total number of chronic diseases (heart failure, coronary heart disease including angina and myocardial infarction, stroke, chronic obstructive lung disease, hypertension, diabetes, cancer, Parkinson’s disease and hip arthritis) was calculated as a global marker of poor physical health; diseases were ascertained according to standardized, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study (Guralnik et al. 1995) using information on self-reported history, pharmacological treatments, medical exam data and hospital discharge records. Presence of activities of daily livings (ADL) disabilities was defined as self-report of inability or needing personal help in performing any basic activities of daily living (Katz et al. 1976). Body mass index (BMI) was calculated as kg/m2 and categorized according to the World Health Organization definition (WHO Global Database on Body Mass Index): normal (BMI < 25), overweight (25–29.99) and obesity (BMI ≥ 30). Level of physical activity in the previous 12 months was classified as sedentary (completely inactive or light physical activity: ie, walking), light (light physical activity for 2 to 4 h/wk), and moderate to intense (light physical activity for more than 4 h/wk or moderate physical activity (ie, swimming etc)) (Ainsworth et al. 1993). Daily dietary energy intake was assessed by the food-frequency questionnaire created for the European Prospective Investigation on Cancer and Nutrition (EPIC) study, previously validated in the InCHIANTI population (Pisani et al. 1997).
Finally, measures of serum inflammatory markers were considered as potential mediators. Previously from the InCHIANTI Study, it has been reported (Milaneschi et al. 2009) that participants with high interleukin-1 receptor antagonist (IL-1ra) had a higher risk of developing depressive symptoms over time. Serum levels of interleukin 6 (IL-6) and IL-1ra were measured by enzyme linked immuno-absorbent assays (ELISA) (kits from BIOSOURCE International, Camarillo, California). Serum C-reactive protein (CRP) was measured in duplicate using the Dade Behring BNII nephelometer (Dade Behring Inc., Deerfield, IL, USA), utilizing a particle-enhanced immuno-nepholometric assay and monoclonal antibodies to CRP. The lowest detectable concentration was 0.1 pg/ml for IL-6, 4 pg/ml for IL1ra, and 0.03 mg/L for CRP. The inter-assay coefficient of variation was 4.5% for IL-1ra, 5% for CRP and 7% for IL-6.
Statistical Analyses
Variables were reported as percentage, or means ± standard deviation (SD) for categorical and continuous variables as appropriate. Continuous variables with a skewed distribution are shown as median and interquartile range (IQR) and log-transformed values were used in the analyses. Partial correlations between baseline characteristics and total carotenoids were examined using Pearson and Spearman coefficients controlling for age and sex. Multivariate linear regression models were used to analyze the association between total carotenoids (per SD increase) and CES-D score at baseline. Multivariate logistic regressions were used to compare the odds of prevalent depressed mood at baseline per SD increase in total plasma carotenoids and across carotenoid quartiles. Then, we excluded participants with depressed mood and/or use of antidepressants at baseline and logistic regression was used to test whether plasma carotenoids predicted incident depressed mood over the follow-up period. Consistent with our previous study on inflammatory markers and depression (Milaneschi et al. 2009), separate analyses were performed for incident depressed mood at 3- and at 6-year follow-ups, because the mean increase of CES-D score was small after 3 years, while the 6-year interval allowed for characterization a greater mean increase in depressive symptoms. This could influence the findings in terms of risk of developing incident depressed mood at each time point. Finally, to address reverse causation, analyses were repeated to examine the association between plasma carotenoids and 6-year follow-up incident depressed mood after the additional exclusion of participants who became depressed after 3 years. Ancillary analyses were also performed to study the association between single carotenoid compounds and depression. All multivariate analyses were adjusted for age, sex, and for covariates that showed a significant correlation with total carotenoids. All cross-sectional analyses were also adjusted for use of antidepressants. All longitudinal analyses were additionally adjusted for baseline CES-D score in order to correct for “regression to the mean”. Finally, we tested whether inflammatorymarkers could be considered mediators in the relationship between carotenoid concentrations and incident depressed mood at 6-year follow-up. We applied meditational analyses that use bootstrapping techniques, a nonparametric resampling procedure (Preacher and Hayes 2008). Cases were randomly selected, with replacement, from the original sample of N. For each bootstrap sample, the model was estimated and the parameter estimates saved and their distribution examined. The indirect effect was deemed significant if the confidence interval around that effect did not include zero. We set the number of bootstrap samples to 1000. We utilized the SPSS macro developed by Preacher and Hayes (Preacher and Hayes 2008) which allows to estimate models with binary outcome. All other analyses were performed using SAS (v. 9.1, SAS Institute, Inc., Cary, NC) with a statistical significance level set at P <0.05.
RESULTS
Baseline characteristics and their correlation with total plasma carotenoids are shown in Table 1. The mean (±SD) age of the study sample was 74.3 (±6.8) years and 55.7% were women. The mean plasma level of total carotenoids was 1.8 (±0.7) μmol/L. At baseline, higher plasma concentrations of total carotenoids were associated with female gender, being non-disabled, higher level of physical activity, lower number of chronic diseases, lower BMI and lower serum inflammatory markers. The adjusted Pearson’s correlation coefficient between total carotenoids and depressive symptoms assessed with CES-D was −0.09 (p<.01). Table 2 shows the relations between total carotenoids and other covariates with depressive symptoms at baseline. Higher plasma level of total carotenoids (per SD increase) was significantly associated with lower CES-D score after adjustment for age, sex and antidepressants use (β = −0.76, SE= 0.26, p = 0.004). Additional adjustment for BMI, physical activity, number of chronic diseases and disability reduced by 24% the strength of the association, which nevertheless remained significant (β = −0.57, SE= 0.27, p = 0.03). Analyses were additionally adjusted for inflammatory markers. Collinearity between inflammatory markers was examined using correlation and variance inflation factors in regression models. Correlation coefficient were low (from 0.32 to 0.50) and all variance inflation factors were below 10, indicating non-significant multicollinearity. Additional simultaneous adjustment for all inflammatory markers marginallyreduced the association between carotenoids and depressive symptoms (β = −0.55, SE= 0.27, p = 0.047). When we considered the single compounds separately, only higher plasma levels (per SD increase) of lycopene (β = −0.84, SE= 0.27, p = 0.002) and β-cryptoxanthin (β = −0.52, SE= 0.27, p = 0.05) were significantly associated with lower baseline CES-D score after full adjustment for confounders. Non-significant negative associations were also found for lutein and zeaxanthin.
Table 1.
Characteristics of the study population at baseline and partial correlations with total carotenoids.
|
|
|
|
|---|---|---|
| Total sample (n=958)
|
Correlation with Total carotenoids
|
|
| Characteristics | ||
| Age (years) | 74.3±6.8 | − 0.05 |
| Sex (F) | 55.7 | 0.15** |
| Education (years) | 5.5±3.3 | 0.01 |
| Alcohol use (≥3 drinks/day) | 15.3 | − 0.04 |
| Smoking habit | − 0.06 | |
| non smoker | 58.5 | |
| former smoker | 27.4 | |
| current smoker | 14.0 | |
| MMSE scores | 25.4±3.1 | 0.03 |
| BMI (Kg/m2) | − 0.08* | |
| normal | 28.1 | |
| overweight | 46.4 | |
| obesity | 25.5 | |
| Physical activity | 0.14** | |
| Low | 18.6 | |
| Medium | 76.0 | |
| High | 5.4 | |
| CES-D score | 12.8±8.8 | − 0.09** |
| Antidepressants use | 4.4 | 0.02 |
| Vit supplementation | 3.3 | 0.05 |
| Total carotenoids (μmol/L) | 1.8±0.7 | 1 |
| ADL disabilities | 4.7 | − 0.09** |
| No. of chronic diseases | 1.2±1.0 | − 0.09** |
| Energy intake (Kcal/day) | 1931.5±564.8 | 0.06 |
| CRP (μg/mL) | 2.7 (4.3) | − 0.11** |
| IL-6 (pg/mL) | 1.4 (1.3) | − 0.13** |
| IL-1ra (pg/mL) | 132.0 (87.4) | − 0.10** |
Values are shown as means ± SD for continuous variable or percentage for categorical variable; continuous variables with a skewed distribution are shown as median (IQR) and were log-transformed for the analysis.
Partial Correlations based on Pearson or Spearman coefficient as appropriate and adjusted for age and sex.
MMSE, Mini Mental State Examination; BMI, Body Mass Index; CES-D, Center for Epidemiological Studies-Depression Scale; ADL, Activities of Daily Living; CRP, C-Reactive Protein; IL-6, Interleukin 6; IL-1ra, Interleukin 1 receptor antagonist.
p < .05
p < .01
Table 2.
Relationship between total carotenoids and other covariates with baseline depressive symptoms.
| Baseline CES-D scores
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
Model 2
|
|||||||
| β | SE | P | β | SE | P | β | SE | P | |
|
|
|
|
|||||||
| Total Carotenoids * | − 0.75 | 0.26 | 0.005 | − 0.57 | 0.27 | 0.03 | − 0.55 | 0.27 | 0.047 |
| Age | 0.21 | 0.04 | <.0001 | 0.15 | 0.04 | 0.0003 | 0.16 | 0.04 | 0.0002 |
| Sex | 5.46 | 0.53 | <.0001 | 5.14 | 0.55 | <.0001 | 5.13 | 0.60 | <.0001 |
| Antidepressants use | 5:05 | 1:28 | <.0001 | 4:09 | 1:34 | 0.002 | 3.90 | 1:35 | 0.004 |
| No. chronic diseases | 0.39 | 0.29 | 0.18 | 0.29 | 0.30 | 0.32 | |||
| Physical activity | − 2.26 | 0.63 | 0.0004 | − 2.27 | 0.62 | 0.0004 | |||
| ADL disabilities | 3.17 | 1.52 | 0.04 | 3.05 | 1.52 | 0.05 | |||
| BMI | − 0.26 | 0.37 | 0.48 | − 0.43 | 0.38 | 0.26 | |||
| (log) CRP | −0.41 | 0.38 | 0.27 | ||||||
| (log) IL-6 | 0.04 | 0.30 | 0.89 | ||||||
| (log) IL-1ra | 1.41 | 0.50 | 0.005 | ||||||
Per SD increase; Total Carotenoids SD = 0.68 μmol/L
CES-D, Center for Epidemiological Studies-Depression Scale; SE, Standard Error; MMSE, Mini Mental State Examination; BMI, Body Mass Index; ADL, Activities of Daily Living; CRP, C-Reactive Protein; IL-6, Interleukin 6; IL-1ra, Interleukin 1 receptor antagonist.
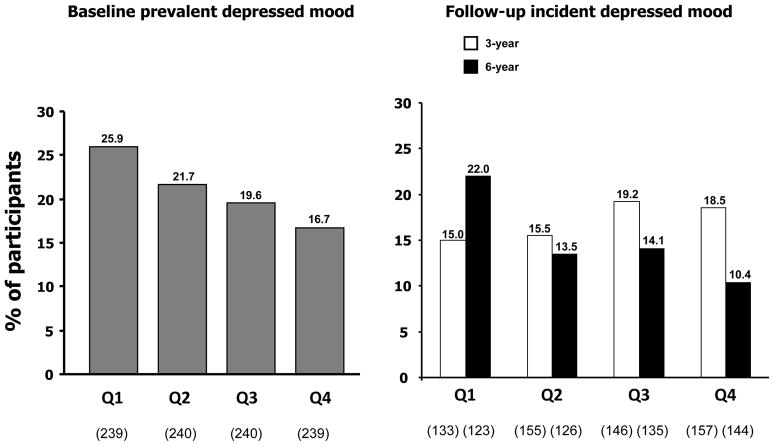
Figure 1 shows the percentages of participants with prevalent depressed mood at baseline and incident depressed mood over follow-up across quartiles of carotenoids. At baseline, 21% of participants had depressed mood. Table 3 reports the odds ratios (ORs) for prevalent depressed mood at baseline and incident depressed mood at 3- and 6-year follow-up per SD increase in total plasma carotenoids and across carotenoids quartiles. Higher total plasma carotenoids (per SD increase) concentrations were associated with a lower probability (OR=0.82, 95%CI=0.68–0.99, p=0.04) of depressed mood at baseline after adjustment for age, sex, antidepressants use, BMI, physical activity, number of chronic diseases, disability and inflammatory markers. Estimated ORs for participants in the lowest carotenoid quartile, compared to those in the highest quartile, were 1.72 (95%CI:1.05–2.83, p=0.03) after full adjustment for confounders.
Figure 1.
Proportions of participants with prevalent depressed mood at baseline and incident depressed mood at 3- and 6-year follow-ups across quartiles of total carotenoid concentrations.
Table 3.
Relationship between total carotenoids with prevalent depressed mood at baseline and with incident depressed mood at 3- and 6-year follow-up.
| Baseline Prevalent Depressed Mood (201/958)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 a
|
Model 2 a
|
Model 3 a
|
|||||||
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
|
|
|
|
|||||||
| Total Carotenoids * | 0.78 | (0.65 – 0.92) | 0.004 | 0.82 | (0.68 – 0.99) | 0.03 | 0.82 | (0.68 – 0.99) | 0.04 |
| Quartile 4 | Ref | Ref | Ref | ||||||
| Quartile 3 | 1.27 | (0.78 – 2.06) | 0.35 | 1.20 | (0.73 – 1.99) | 0.47 | 1.27 | (0.77 – 2.12) | 0.35 |
| Quartile 2 | 1.71 | (1.06 – 2.77) | 0.03 | 1.58 | (0.96 – 2.61) | 0.07 | 1.58 | (0.95 – 2.63) | 0.08 |
| Quartile 1 | 2.14 | (1.33 – 3.45) | 0.002 | 1.84 | (1.11 – 3.07) | 0.02 | 1.87 | (1.11 – 3.14) | 0.02 |
| 3-year Incident Depressed Mood (101/591)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 b
|
Model 2 b
|
Model 3 b
|
|||||||
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
|
|
|
|
|||||||
| Total Carotenoids * | 0.97 | (0.76 – 1.24) | 0.81 | 1.01 | (0.78 – 1.30) | 0.94 | 1.02 | (0.79 – 1.32) | 0.88 |
| Quartile 4 | Ref | Ref | Ref | ||||||
| Quartile 3 | 1.18 | (0.64 – 2.18 | 0.59 | 1.14 | (0.61 – 2.12) | 0.67 | 1.12 | (0.60 – 2.10) | 0.73 |
| Quartile 2 | 0.90 | (0.48 – 1.70) | 0.75 | 0.81 | (0.42 – 1.55) | 0.52 | 0.81 | (0.42 – 1.55) | 0.52 |
| Quartile 1 | 0.97 | (0.49 – 1.89) | 0.92 | 0.90 | (0.45 – 1.82) | 0.77 | 0.86 | (0.42 – 1.75) | 0.68 |
| 6-year Incident Depressed Mood (78/528)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 b
|
Model 2 b
|
Model 3 b
|
|||||||
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
|
|
|
|
|||||||
| Total Carotenoids * | 0.62 | (0.45 – 0.84) | 0.002 | 0.71 | (0.52 – 0.98) | 0.04 | 0.72 | (0.52 – 0.99) | 0.04 |
| Quartile 4 | Ref | Ref | Ref | ||||||
| Quartile 3 | 1.61 | (0.75 – 3.43) | 0.22 | 1.42 | (0.64 – 3.13) | 0.39 | 1.44 | (0.65 – 3.22) | 0.37 |
| Quartile 2 | 1.73 | (0.79 – 3.77) | 0.17 | 1.42 | (0.63 – 3.20) | 0.39 | 1.50 | (0.66 – 3.45) | 0.33 |
| Quartile 1 | 3.77 | (1.77 – 8.03) | 0.001 | 2.70 | (1.20 – 6.04) | 0.02 | 2.63 | (1.16 – 6.00) | 0.02 |
Model 1: Adjustment for age and sex.
Model 2: Model 1 + additional adjustment for BMI, physical activity, number of chronic diseases and disability.
Model 3: Model 2 + additional adjustment for (log)CRP, (log)IL-6 and (log)IL-1ra.
Additionally adjusted for antidepressants use.
Additionally adjusted for baseline CES-D
Per SD increase; Total Carotenoids SD = 0.68 μmol/L.
O.R. Odds Ratio; C.I., Confidence Interval.
Depressed Mood: CES-D ≥20.
For the incidence analyses, we excluded 220 participants with depressed mood and use of antidepressants at baseline. Incident depressed mood was developed by 17.1% of the participants available at 3-year follow-up and by 14.8% of those available at 6-year follow-up. In multivariate analyses, plasma levels of total carotenoids significantly predicted incident depressed mood status after 6 years. However, this relationship between carotenoids and depressive symptoms was not detectable after the first 3 years. At 6-year follow-up, higher total carotenoid concentrations (per SD increase) were associated with a lower risk (OR=0.72, 95%CI=0.52–0.99, p=0.04) of depressed mood after adjustment for age, sex, baseline CES-D, BMI, physical activity, number of chronic diseases, disability and inflammatory markers. Participants in the lowest quartile of plasma carotenoids, as compared to those in the highest quartile, had a higher risk of developing depressed mood (OR=2.63, 95%CI=1.16–6.00, p=0.02) after 6 years. To test whether this association was consistent across gender we included a “carotenoid-by-sex” interaction term in the previous Cox regression models. The interaction term was not statistically significant (all p >0.15) suggesting that the nature of association between serum carotenoids and depression is substantially similar in the two sexes. For all individual carotenoids, higher plasma concentrations (per SD increase) of all compounds were associated with lower probability of incident depressed mood, although these associations were non-significant after full adjustment. To verify that the longitudinal findings were not biased by reverse causation (that is, participants who were subclinically depressed at baseline and could have had lower levels of carotenoids as a consequence of depression), the 6-year follow-up analyses were repeated after the additional exclusion of 79 participants, among those available after 6 years, who became depressed after 3 years. Again, risk of incident depressed mood decreased by 37% for each SD increase in plasma total carotenoids concentrations (full adjusted OR =0.63, 95%CI=0.43–0.94, p=0.02).
Finally, we tested the role of inflammatory markers as potential mediators of the relationship between blood total carotenoid concentrations and incident depressed mood at 6-year follow-up. Among the inflammatory markers, bootstrapping analyses identified IL-1ra as a significant mediators between carotenoids levels and subsequent depressed mood (point estimate = −0.04, bias corrected 95%CI=−0.09 - −0.01). The findings were essentially unchanged when age, sex and baseline depressive symptoms were added to the model (IL-1ra point estimate = −0.04, bias corrected 95%CI=−0.1 - −0.004).
DISCUSSION
Using data from a population-based study in older persons, we found evidence of a strong cross-sectional and prospective independent association between plasma carotenoid concentrations and depressive symptoms. Participants with lower total carotenoids level had higher depressive symptoms at baseline and were more likely to develop incident depressed mood after six years of follow up. To our knowledge, this is the first study examining this relationship. Previous studies examining the relationship of nutrient intake and biomarkers with depression in older persons obtained mixed results. Lower levels of folate and vitamin B12 have been shown to be associated with depression in two cross sectional-studies of Chinese (Ng et al. 2009) and Greek (Dimopoulos et al. 2007) older adults, and to be a risk for incident depression over a period of 2–3 years in a prospective studies of Korean older persons (Kim et al. 2008). Moreover, in a sample of older disabled women serum levels of vitamin B12 were associated with higher risk of severe depression (Penninx et al. 2000). High total intakes of vitamins B6 and B12 have been recently shown to be protective of depressive symptoms over time in community-dwelling older adults (Skarupski et al. 2010). However, in a sample of healthy elderly men in the Netherlands, intake of folate and vitamins B6 and B12 were not related to depressive symptoms (Kamphuis et al. 2008). Decreased serum vitamin D levels have been shown to be associated with depression status in the Longitudinal Aging Study Amsterdam (Hoogendijk et al. 2008) and with higher risk of developing depressed mood over time in the InCHIANTI Study (Milaneschi et al. 2010a). Depressive symptomatology in older persons has also been related to plasma PUFAs concentrations and fatty acid composition (Tiemeier et al. 2003, Féart et al. 2008, Kiecolt-Glaser et al. 2007). However, a recent randomized controlled trials on 302 Dutch elderly showed no significant effect of eicosapentaenoic acid and docosahexaenoic acid on depressive symptoms (van de Rest et al. 2008). Further studies are required of sufficient methodological quality, duration, and sample size to confirm these findings.
Interestingly, in our study baseline plasma levels of carotenoids were not predictive of depressed mood at 3-years follow-up. We could hypothesize that the influence of carotenoids levels on the development of depressive symptoms is a slow process that takes several years. As shown in Figure 1, after the exclusion of participants already depressed at enrolment, proportions of participants with incident depressed mood across carotenoid quartiles after 3 years did not follow the same clear trend as for prevalent depressed mood at baseline. The same trend (proportions of participants with depressed mood decreased from the lowest quartile to the highest) was instead detected again after 6 years. However, the reasons for the lack of association after 3 years remain unknown, and further research is needed. Moreover, although all the single carotenoids compounds were associated with a lower risk of incident depressed mood over time, effects appeared especially strong and significant only for the combined indicator of total carotenoids. Several explanations for this finding are possible. First of all, individual compounds may have small effects that emerge only when they are integrated. Moreover, there may be biologic interactions between the different compounds that may be difficult to detect unless very large samples are used. Indeed, previous study on the impact of plasma carotenoids on the health of older adults (Milaneschi et al. 2010b) commonly considered the measure of total plasma carotenoids.
In the current study, we found evidence that inflammatory markers, in particular blood levels of IL-1ra, partially mediated the relationship between carotenoid concentrations and development of depressed mood after 6 years. In our previous study (Milaneschi et al. 2009), we found that older persons with high plasma levels of IL-1ra had a higher risk of developing relevant depressive symptoms over time. Moreover, the association between IL-1ra and depression has been confirmed also in a recent meta-analysis (Howren et al. 2009). IL-1ra, which is considered an acute phase protein (Gabay et al. 1997), is a reliable marker of IL-1 signaling network activation. IL-1ra production increases under the same inflammatory conditions that stimulate IL-1α and IL-1β, but while these molecules are produced locally, rapidly metabolized and their serum concentrations is often below the detectable, IL-1ra is produced by the liver in larger quantities and remains in the circulation for long time (Dibbs et al. 1999, Biasucci et al. 1999, Granowitz et al. 1991). The fact that inflammation mediated the relationship between plasma carotenoids and depression suggests the hypothesis of a shared biological pathway. Antioxidants, by reducing free radical concentrations, may modulate redox balance and activation of transcription nuclear factor κB (NF-kB) (Semba et al 2007a), a major transcriptional factor involved in the expression of proinflammatory cytokines, which have been shown to be associated with psychosocial stress, sickness behavior and depression (Miller et al. 2009, Bierhaus et al. 2003) In animal model of depression, it has been shown that NF-kB is the key mediator linking stress-induced increases in IL-1β with impaired hippocampal neurogenesis and depressive-like behaviors (Koo et al. 2010). Different biological and behavioral pathways should also be considered through which level of carotenoids may potentially influence depressive symptoms. Carotenoids, as antioxidants, play an important role in counterbalancing the age-dependent increase in oxidative stress. As people age, the central nervous system may became more vulnerable to the effect of free radicals in terms of damage and mutation of proteins, lipids and mitochondrial DNA, which in turn leads to impaired mitochondrial function and further generation of free radicals, with increased lipid peroxidation and impaired oxidative DNA repair in the nucleus (Joseph et al. 1996, Balaban et al. 2005). It has hypothesized that the inability to buffer the effects of this oxidative stress may be responsible for age-related neuronal decrements and neurodegenerative disease (Beal 2005, Patten 2010). Brain imaging studies have shown that depression is associated with structural and functional alterations of limbic and cortical structures, particularly in the hippocampus (Lorenzetti et al. 2009, Videbech and Ravnkilde 2004).Antioxidants may play a preventive role in neuron damage by reducing oxidative injury through the quenching of hydroxyl radicals and reduction in lipid peroxidation (Mayne 2003). Furthermore, a lower level of carotenoids, considered a good index of fruit and vegetable intake, could reflect an unhealthy dietary pattern associated with overweight and obesity, which have been shown to increase the risk of developing depression through inflammation or hypothalamic-pituitary-adrenal axis dysregulation (Luppino et al. 2010, Vogelzangs et al. 2008, Miller et al. 2003). Finally, an unhealthy dietary pattern may be associated with other lifestyle factors, such as lower level of physical activity, alcohol consumption and smoking habit, considered risk factor for depression (van Gool et al. 2007, van Gool et al. 2003). While the causal pathway from carotenoids blood levels and changes in mood has not been elucidated, it is interesting to note that the same micronutrients associated with depression have also been associated with some of the phenotypes that characterize age-related frailty, such as sarcopenia and mobility disability (Semba et al. 2007a, Milaneschi et al. 2010b, Semba et al. 2003, Alipanah et al. 2009, Semba et al. 2007b, Lauretani et al. 2008a, Lauretani et al. 2008b).
A limitation of the present study is the loss of participants to follow-up. Participants lost to follow-up were significantly older, more disabled and had poorer cognitive function as compared to those available for longitudinal analysis; this could limit the generalization of the findings. However, those lost at follow up had also higher depressive symptoms and lower concentrations of plasma total carotenoids at baseline. Therefore, censoring of these participants probably led to an underestimation of the relationship between carotenoids and depression. Another limitation is that depressive symptoms were evaluated by the CES-D questionnaire and the diagnosis of depression was not confirmed by a clinical psychiatric diagnosis. However, the CES-D is a commonly used scale to measure depressive symptoms and has been widely used in older population-based studies (Beekman et al. 1997). Moreover DSM affective disorders are not highly prevalent among elderly persons in the community, while subsyndromal depression is more common (Beekman et al. 2002, Alexopoulos 2005). Another limitation is that the study design has not allowed us to detect depressive episodes that started and remitted between subsequent follow-up visits. Finally, reverse causation should be considered. In fact, depression and stress may promote unhealthy dietary preference (Wardle et al. 2000) which in turn could result in lower intake and blood concentration of carotenoids. However, when we subsequently excluded from longitudinal analyses participants who became depressed after 3 years, the association between carotenoids and incident depression after 6 years was still present.
Despite these limitations, we believe that our findings suggest that low plasma concentrations of dietary carotenoids may be considered a potential risk factor for the onset of depressive symptoms in older persons. However, further longitudinal studies are needed in order to confirm this conclusion. Moreover, since we measures dietary carotenoids, any interpretation of the findings of the present study as evidence sustaining the need for supplementation should be avoided. Attempts to translate the results from observational studies to dietary intervention trials may result in disappointing outcomes. Results from the Beta-Carotene and Retinol Efficacy Trial and the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study showed that β-carotene supplementation on participants with high risk of lung cancer was associated with an excess in risk of mortality (Omenn et al. 1996, The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group 1994). Moreover, along with nutrition other physiologic and behavioral mechanisms that affect the absorption, storage, and utilization of carotenoids may influence their blood concentrations (Brady et al. 1996).
Evidence emerging from the study of the nutritional determinants of late-life depression may instead provide the rationale for intervention studies aimed to test whether improving the quality of diet may be especially effective in improving depression in older persons. Dietary patterns rich in antioxidants, such as a Mediterranenan-style diet (characterized by a high intake of fruit, vegetables and olive oil), have already been shown to be associated with longer survival, reduced cardiovascular and cancer mortality, lower risk of chronic degenerative disease and cognitive decline (Trichopoulou et al. 2003, Sofi et al. 2008). Moreover, two trials showed that nutritional interventions based on Mediterranean diet significantly reduced the levels of inflammatory markers in participants with cardiovascular risk factors (Esposito et al. 2004, Estruch et al. 2006).
Interestingly, two recent studies have also demonstrated that adherence to a Mediterranean-style diet could lower the risk of incident depression in a cohort of adults (Sánchez-Villegas et al. 2009) and could buffer the inflammatory process boosted by depression among community-dwelling older persons (Milaneschi et al. 2010c). In the future dietary interventions may become a cost-effective strategy to promote healthy aging and reduce the burden of age-related depression in the population.
Acknowledgments
“The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health. This work was supported also by NIA Grant R01 AG027012.
Role of the Sponsor: None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported herein.
Footnotes
Author Contributions: All the authors took part in every aspect of this paper including the design, preparation of the manuscript and writing of this paper.
Financial Disclosure: None reported.
Potential Conflicts of Interest: The authors do not have any conflict of interest in the publication of the manuscript.
References
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–70. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Alipanah N, Varadhan R, Sun K, Ferrucci L, Fried LP, Semba RD. Low serum carotenoids are associated with a decline in walking speed in older women. J Nutr Health Aging. 2009;13:170–5. doi: 10.1007/s12603-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–5. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, Braam AW, Penninx BW, van Tilburg W. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59:605–11. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–37. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–42. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Gazi F, Papadimitriou A, Lea RW, Kalofoutis A. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem. 2007;40:604–8. doi: 10.1016/j.clinbiochem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. Clin Psychol. 1983;39:249–51. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Féart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, Barberger-Gateau P. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr. 2008;87:1156–62. doi: 10.1093/ajcn/87.5.1156. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM for the InCHIANTI Group. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–40. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, Dinarello CA. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991;338:1423–4. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: health and social characteristics of older women with disability. National Institute on Aging; Bethesda: 1995. NIH Publication No.95–4009. [Google Scholar]
- Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–12. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Villalobos-Molina R, Denisova N, Erat S, Cutler R, Strain J. Age differences in sensitivity to H2O2- or NO-induced reductions in K(+)-evoked dopamine release from superfused striatal slices: reversals by PBN or Trolox. Free Radic Biol Med. 1996;20:821–30. doi: 10.1016/0891-5849(95)02225-2. [DOI] [PubMed] [Google Scholar]
- Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–45. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–24. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–74. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008a;63:376–83. doi: 10.1093/gerona/63.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Lauretani F, Corsi AM, Guralnik JM, Ferrucci L. Carotenoids as protection against disability in older persons. Rejuvenation Res. 2008b;11:557–63. doi: 10.1089/rej.2007.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133:933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65:973–8. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, Ferrucci L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010a;95:3225–33. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Tanaka T, Ferrucci L. Nutritional determinants of mobility. Curr Opin Clin Nutr Metab Care. 2010b;13:625–9. doi: 10.1097/MCO.0b013e32833e337d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Bandinelli S, Penninx BW, Vogelzangs N, Corsi AM, Lauretani F, Kisialiou A, Vazzana R, Terracciano A, Guralnik JM, Ferrucci L. Depressive symptoms and inflammation increase in a prospective study of older adults: a protective effect of a healthy (Mediterranean-style) diet. Mol Psychiatry. 2010c doi: 10.1038/mp.2010.113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–85. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Niti M, Kua EH, Yap KB. Folate, vitamin B12, homocysteine, and depressive symptoms in a population sample of older Chinese adults. J Am Geriatr Soc. 2009;57:871–6. doi: 10.1111/j.1532-5415.2009.02229.x. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996 Nov;88:1550–9. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; 2000. Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of DRIs, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. [Google Scholar]
- Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–67. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B(12) deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. Am J Psychiatry. 2000;157:715–21. doi: 10.1176/appi.ajp.157.5.715. [DOI] [PubMed] [Google Scholar]
- Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, Martínez-González MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66:1090–8. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- Semba RD, Blaum C, Guralnik JM, Totin D, Ricks MO, Fried LP. Low carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15:482–487. doi: 10.1007/BF03327377. [DOI] [PubMed] [Google Scholar]
- Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007a;458:141–5. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Varadhan R, Bartali B, Ferrucci L, Ricks MO, Blaum C, Fried LP. Low serum carotenoids and development of severe walking disability among older women living in the community: the women’s health and aging study I. Age Ageing. 2007b;36:62–7. doi: 10.1093/ageing/afl122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92:330–5. doi: 10.3945/ajcn.2010.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–6. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;14(330):1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32:81–7. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GI, Bosma H, van Boxtel MP, Jolles J, van Eijk JT. Associations between lifestyle and depressed mood: longitudinal results from the Maastricht Aging Study. Am J Public Health. 2007;97:887–94. doi: 10.2105/AJPH.2004.053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Hoefnagels WH, Beekman AT, de Groot LC. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88:706–13. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BW. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65:1386–9. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Antioxidants, inflammation, and mortality among women living in the community. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- [Accessed June 16, 2010];WHO Global Database on Body Mass Index Web site. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.