Abstract
Compelling evidence has accumulated over the last several years from our laboratory, as well as others, indicating that central hyperactive states resulting from neuronal plastic changes within the spinal cord play a critical role in hyperalgesia associated with nerve injury and inflammation. In our laboratory, chronic constriction injury of the common sciatic nerve, a rat model of neuropathic pain, has been shown to result in activation of central nervous system excitatory amino acid receptors and subsequent intracellular cascades including protein kinase C translocation and activation, nitric oxide production, and nitric oxide-activated poly(ADP ribose) synthetase activation. Similar cellular mechanisms also have been implicated in the development of tolerance to the analgesic effects of morphine. A recently observed phenomenon, the development of “dark neurons,” is associated with both chronic constriction injury and morphine tolerance. A site of action involved in both hyperalgesia and morphine tolerance is in the superficial laminae of the spinal cord dorsal horn. These observations suggest that hyperalgesia and morphine tolerance may be interrelated at the level of the superficial laminae of the dorsal horn by common neural substrates that interact at the level of excitatory amino acid receptor activation and subsequent intracellular events. The demonstration of interrelationships between neural mechanisms underlying hyperalgesia and morphine tolerance may lead to a better understanding of the neurobiology of these two phenomena in particular and pain in general. This knowledge may also provide a scientific basis for improved pain management with opiate analgesics.
Keywords: hyperalgesia, protein kinase C, excitatory amino acid, analgesia, nociception
A number of studies, both from our laboratory as well as others, indicate that central hyperactive states resulting from neuronal plastic changes within the spinal cord play a critical role in hyperalgesia associated with nerve injury and inflammation. We have recently shown in a rat model of neuropathic pain that chronic constrictive injury (CCI) of the common sciatic nerve can result in activation of central nervous system excitatory amino acid receptors and subsequent intracellular cascades including protein kinase C translocation and activation, nitric oxide (NO) production, and NO-activated poly(ADP ribose) synthetase (PARS) activation. Similar cellular mechanisms also have been implicated in the development of tolerance to the analgesic effects of morphine. Of particular interest is that morphological changes in the spinal cord dorsal horn, the development of so called “dark neurons,” are associated with both CCI and morphine tolerance. A site of action involved in both hyperalgesia and morphine tolerance has also been shown to be in the superficial laminae of the spinal cord dorsal horn. We will first summarize recent evidence indicating central mechanisms of hyperalgesia and morphine tolerance. The main focus of this article will be on the recent development in our understanding of the involvement of PARS activation in central mechanisms of morphine tolerance. A working hypothesis with regard to interactions between hyperalgesia and morphine tolerance within the spinal cord dorsal horn will be discussed. Finally, clinical implications of such interactions will be addressed.
Central Mechanisms Subserving Hyperalgesia.
Recent insights into neural mechanisms of hyperalgesia are based on knowledge of the involvement of the N-methyl-d-aspartate (NMDA) receptor and associated intracellular cascades. These insights result from studies of central nervous system (CNS) neuronal plasticity in general and from studies of spinal cord mechanisms of neurogenic and inflammatory hyperalgesia in particular (1–8). Hyperalgesia after tissue injury and inflammation may reflect central sensitization resulting from prolonged and excessive activation of spinal cord excitatory amino acid receptors and subsequent intracellular cascades. Tonic activation of NMDA receptors activates second-messenger systems, an ultimate result of which is phosphorylation and hence sensitization of ion channel complexes, including that of the NMDA receptor (9). These central changes are initiated by abnormal and often tonic input to the spinal cord. This tonic input, in turn, may result from peripheral nerve injury or tissue inflammation. Potential peripheral generators of this input include nerve injury-induced impulse discharges (3), generation of ectopic nerve action potentials (10), aberrant sympathetic influences (11), and/or sensitization of peripheral nociceptors (2, 11). Of the several central changes initiated by tonic nociceptive afferent input, protein kinase C (PKC) translocation/activation and/or NO production are pivotal intracellular events within spinal cord neurons. Translocation/activation of PKC enhances postsynaptic neuronal excitability by means of increasing the efficacy of receptor–ion channel complexes (12–15). Similar changes may also occur at presynaptic sites via activation of presynaptic NMDA receptors localized on primary afferent fibers (16) and/or via the effects of extracellular NO (17). Central hyperactive states reflect the combined effects of these pre- and postsynaptic mechanisms. Direct support of the involvement of PKC in these mechanisms is provided by an experiment in which a phorbol ester (a PKC activator) increased spontaneous and stimulus-evoked activity of dorsal horn spinothalamic tract neurons (18) and by an experiment in which a PKC activator produced a long-lasting increase in the amplitude and duration of excitatory postsynaptic potentials evoked in dorsal horn neurons by orthodromic dorsal root stimulation (19). Both experiments show that activation of PKC can indeed modulate activity of spinal cord nociceptive neurons. These experiments are complemented by studies that demonstrate elevated dorsal horn levels of translocated PKC or increased biosynthesis of PKC in neuropathic rats (20, 21).
Contributions of Central Excitation to Hyperalgesia.
It has been proposed that central sensitization may be manifested as increases in spontaneous and stimulus-evoked neuronal activity within the spinal cord, which, in turn, contribute to the development and maintenance of neurogenic and inflammatory pain syndromes. There may be an increase in spontaneous neural activity of pain transmission pathways and hence spontaneous pain. This increased spontaneous action potential activity includes that of spinal cord dorsal horn neurons (22, 23), and that of pain-related thalamic neurons (24) of CCI rats with neuropathic hyperalgesia (25). These extensive elevations in neural activity, which have also been mapped by using the 2-deoxyglucose metabolic technique (26, 27), occur within the spinal cord and a variety of pain-related brain regions even in the absence of overt somatic stimulation. Furthermore, responses of central pain-related neurons of CCI rats show exaggerated responses to innocuous and noxious peripheral stimulation. This is indicated by increased responses of spinothalamic neurons to mechanical or thermal stimulation (23). An exaggerated response to innocuous stimulation may contribute to allodynia, whereas the enhanced response to noxious stimulation is likely related to hyperalgesia. Peripheral receptive fields also expand after tissue inflammation (28). Given the role of receptive field size in neuronal recruitment, expanded receptive fields would mean that a nociceptive stimulus would activate more central neurons than would normally occur, leading to exaggerated pain and exaggerated spatial radiation of the painful sensation. Taken together, elevated spontaneous discharges, increased stimulus-evoked impulse frequencies, and expanded receptive fields are likely to operate in concert to cause persistent hyperalgesia, spontaneous pain, allodynia, and radiation of pain.
Contributions of Central Disinhibition to Hyperalgesia.
Excitatory amino acid-induced PKC translocation/activation and NO production may also result in disinhibitory processes associated with excitotoxic consequences including neuronal death within the CNS. The involvement of such excitotoxic processes in mechanisms of injury-induced pain syndromes has been suggested by several experimental observations. There is histological evidence showing that peripheral nerve injury induces excitotoxic transsynaptic morphological changes of superficial dorsal horn (laminae I–II) neurons (dark neurons), which have been proposed to be inhibitory interneurons (29). Importantly, a PARS has been shown to be activated in CCI rats, which is likely to contribute to the development of dark neurons (30). The activation of PARS occurs in the presence of DNA fragmentation, a process that may be initiated by the production of NO and that may eventually lead to programmed cell death. These morphological changes may reflect a pathological process in which injury-induced central responses result in a persistent imbalance of the excitatory–inhibitory circuitry within the spinal cord dorsal horn. This possibility is supported by the demonstration of Aβ-mediated mechanical allodynia in neuropathic pain patients (31) and the demonstration that pharmacological blockade of the spinal cord γ-aminobutyric acid and glycine inhibitory system augments thermal hyperalgesia in rats with sciatic nerve injury (32). Thus, in addition to direct central sensitzation described above, the possible disinhibition resulting from the loss of function of spinal cord inhibitory interneurons may also contribute to central hyperexcitability after peripheral nerve injury and inflammation.
Central Mechanisms Subserving Morphine Tolerance.
Similar mechanisms, including the activation of NMDA receptors, translocation and activation of PKC, and the production of NO have been implicated in the development and maintenance of morphine tolerance. Detailed discussion of these mechanisms of morphine tolerance is presented elsewhere (9). The following sections will emphasize new evidence indicating the involvement of PARS in mechanisms of morphine tolerance.
As discussed above for the development of hyperalgesia, a consequence of excessive NMDA receptor activation is the initiation of a cascade of intracellular events leading to PARS activation (33). In view of the evidence implicating NMDA receptor activation and the subsequent intracellular events resulting from this, we have conducted a series of experiments to examine the possible role of PARS in the development of morphine tolerance. In addition, because a consequence of PARS activation may include alterations in cell morphology, we examined the development of dark neurons as a marker of altered cellular morphology.
For these experiments, adult male Sprague–Dawley rats were prepared for intrathecal (i.t.) injection by implanting a polyethylene (PE-10) tube into the lumbar spinal cord. The vehicle or drugs were delivered slowly (1–3 minutes) through the i.t. catheter followed by 10 μl of saline, which flushed the catheter. To examine the antinociceptive effects of morphine, the tail-flick test was used. The light bulb intensity of the tail-flick device was set to produce baseline tail-flick latencies between 3.5 and 4.5 sec. To ensure that no tissue damage occurred, the light bulb was automatically turned off after 8 sec, even if no tail-flick occurred. An average of three tail-flick trials each separated by a 1-min intertrial interval constituted the mean baseline latency (BL). The antinociceptive effects of morphine and the development of tolerance were then determined by measuring test (tail-flick) latencies (TL) after drug administration. Thus, data were expressed as percent maximal possible antinociceptive effect (%MPAE) using the equation %MPAE = [(TL − BL)/(8 − BL)] × 100.
The procedure for the histological examination of dark neurons was similar to that described previously (29, 30). It has been reported by Sugimoto et al. (29) that there are three principal characteristics of dark neurons: (i) irregular cellular outlines; (ii) increased amounts of chromatin throughout the nucleoplasm and cytoplasm, which is why they are called dark neurons; and (iii) intensely and homogeneously stained nucleoplasm with almost indiscernible heterochromatin. Dorsal horn neurons that are normal sometimes show increased cytoplasmic staining also, but they do not exhibit enhanced nucleoplasmic staining. Some glial cells, especially oligodendrocytes, also exhibit chromophilia that is as dark as that seen in dark neurons. Although this is true, oligodendrocytes are distinguishable from dark neurons, because glial cells have aggregates of heterochromatin that are seen under high-power magnification, whereas dark neurons do not exhibit this characteristic. Neurons that exhibited all three of the above-mentioned characteristics were counted as dark neurons, whereas other cells were excluded.
The spinal cord sections were divided into three zones for examination by microscope. They were divided, by using Rexed’s laminar system, into laminae I–II, III–IV, and V–VI. Sections were examined with the experimenter blind to the treatment regimen. Dark neurons were counted in each zone mentioned above under medium-power magnification. If there was a dark neuron that was in question, it was then viewed under high-power magnification to discern if it was indeed a dark neuron. At least three 1-μm spinal cord sections were viewed for each rat. This yielded an average number of dark neurons for each subdivision.
The tail-flick data were analyzed by using two-way ANOVA to discern differences among treatment groups. When main effects were seen, a Waller–Duncan D ratio t test was performed to determine the source of variations between the groups. Dark neurons were counted for the left and right sides of the dorsal horn in laminae I–VI. The numbers were then averaged and analyzed by using a two-way ANOVA. The total number of dark neurons from a sampled region was analyzed to determine (i) differences in the number of dark neurons between the left and right side of the dorsal horn; (ii) differences in numbers of dark neurons among sampled dorsal horn regions (i.e., laminae I–II); and (iii) differences in the number of dark neurons among treatment groups.
An initial experiment was performed to determine the effect of the chronic administration of morphine on the development of dark neurons. Three groups of rats (n = 5–9 per group) were used: (i) rats treated with saline i.t. once a day; (ii) rats treated with 10 μg of morphine i.t. once daily; and (iii) rats treated with 20 μg of morphine i.t. once daily. The doses of morphine given have previously been shown to induce the development of tolerance to the antinociceptive effects of morphine (34). All of the agents were given once daily for 8 days.
On day 8, those rats receiving morphine and saline showed the development of tolerance to the analgesic effects of morphine. Rats that were made tolerant to morphine exhibited a reliable increase in the number of dark neurons in the dorsal horn of the lumbar spinal cord (P < 0.01). Several features characterized this increase in dark neurons. Dark neurons were primarily located in laminae I–II and to a much lesser degree to laminae III–IV. Also, there was no statistical difference in the number of dark neurons observed on the left and right sides of the spinal cord (P > 0.05).
Because chronic administration of morphine induced tolerance and the development of dark neurons, we examined the effect of the selective PARS inhibitor benzamide, which has been shown to prevent the development of hyperalgesia in the CCI model (30), on the development of morphine tolerance and dark neurons resulting from chronic morphine administration. For this experiment, seven groups of rats were used. They included rats receiving 100, 200, or 400 nmol benzamide and 20 μg of morphine on days 1–8, rats receiving 400 nmol benzamide and saline on days 1–8, rats receiving 20 μg morphine and saline on days 1–8, rats receiving only saline on days 1–8, and rats receiving saline on days 1–7 and 20 μg of morphine on day 8.
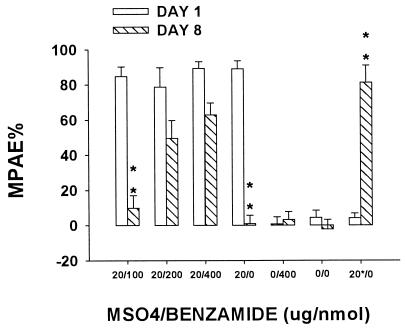
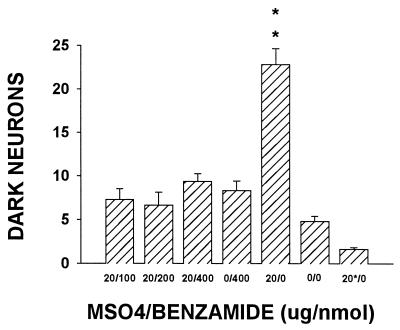
As shown in Fig. 1, coadministration of 20 μg of morphine with 200 or 400 nmol (not 100 nmol) benzamide for 7 days reliably attenuated the development of tolerance (P < 0.01). Neither baseline tail-flick latency nor the response to a single injection of 20 μg of morphine changed after repeated saline treatment for 7 days. Coadministration of 20 μg of morphine with benzamide (100–400 nmol) for 7 days also reliably prevented the increase in dark neurons (P < 0.01; Fig. 2). Neither repeated benzamide (400 nmol) treatment alone nor a single injection of 20 μg of morphine on day 8 (the 20*/0 group) affected the occurrence of dark neurons as compared with the saline group.
Figure 1.
Effect of benzamide on morphine tolerance. Tolerance to the antinociceptive effect of morphine developed in rats treated with 20 μg of morphine for 7 days. Coadministration of 20 μg of morphine with 200 or 400 nmol (not 100 nmol) benzamide for 7 days reliably attenuated the development of tolerance. Neither baseline tail-flick latency nor the response to a single injection of 20 μg of morphine changed after repeated saline treatment for seven days. ∗∗, P < 0.01, as compared with that of day 1 in each corresponding group. Neither repeated benzamide (400 nmol) treatment alone nor a single injection of 20 of μg morphine on day 8 (the 20*/0 group) affected the degree of tolerance as compared with the saline group. MPAE%, percent of maximal possible antinociceptive effect.
Figure 2.
Effect of benzamide on incidence of dark neurons. Coadministration of 20 μg of morphine with benzamide (100–400 nmol) for 7 days reliably prevented the increase in dark neurons. Neither repeated benzamide (400 nmol) treatment alone nor a single injection of 20 μg of morphine on day 8 (the 20*/0 group) affected the occurrence of dark neuron as compared with the saline group. ∗∗, P < 0.01, as compared with each of the rest groups.
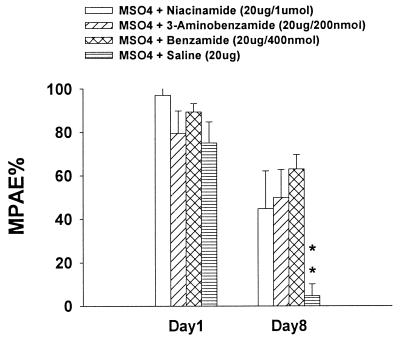
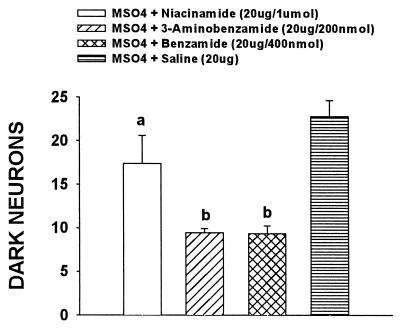
The data from the previous experiment showed that benzamide was effective in inhibiting the development of morphine tolerance and dark neurons. The specificity of this effect to PARS inhibition was examined by utilizing other PARS inhibitors. For this experiment, four groups of rats were used; they included rats receiving 400 nmol benzamide and 20 μg of morphine on days 1–8, rats receiving 200 nmol 3-aminobenzamide and 20 μg of morphine on days 1–8, rats receiving 1 μmol niacinamide (nicotinamide) and 20 μg of morphine on days 1–8, and rats receiving 20 μg of morphine and saline on days 1–8. Coadministration of 20 μg of morphine with either 200 nmol 3-aminobenzamide or 1 μmol niacinamide (nicotinamide) for 7 days reliably (P < 0.01) attenuated the development of tolerance as compared with that of day 1 in the same group (Fig. 3). As shown in Fig. 4, coadministration of 20 μg of morphine with either 200 nmol 3-aminobenzamide or 1 μmol niacinamide for 7 days also reliably (P < 0.05 and P < 0.01 for the drugs respectively) prevented the increase in dark neurons as compared with the morphine + saline group.
Figure 3.
Effect of nicotinamide and 3-aminobenzamide on morphine tolerance. Coadministration of 20 μg of morphine with either 200 nmol 3-aminobenzamide or 1 μmol niacinamide (nicotinamide) for 7 days reliably attenuated the development of tolerance. ∗∗, P < 0.01, as compared with that of day 1 in the same group.
Figure 4.
Effect of nicotinamide and 3-aminobenzamide on incidence of dark neurons. Coadministration of 20 μg of morphine with either 200 nmol 3-aminobenzamide or 1 μmol niacinamide (nicotinamide) for 7 days also reliably prevented the increase in dark neurons. a, P < 0.05; b, P < 0.01, as compared with the MSO4 + saline group.
To confirm that the development of tolerance and dark neurons in morphine-treated rats is associated with the activation of opioid receptors, we examined the effect of the opioid receptor antagonist, naltrexone, on the ability of morphine to produce tolerance and dark neurons. For this experiment, two groups of rats were used; they included rats receiving 10 mg/kg naltrexone intraperitoneally 5 min before 20 μg of morphine i.t. on days 1–8 and rats receiving 20 μg of morphine and saline on days 1–8. Coadministration of 20 μg of morphine with 10 mg/kg naltrexone for 7 days reliably prevented both the development of the antinociceptive tolerance (P < 0.01) and the increase in dark neurons (P < 0.01) as compared with the morphine + saline group.
The major findings of this series of studies are (i) the incidence of dark neurons increased significantly within the spinal cord dorsal horn, particularly the superficial laminae I–II, of rats injected daily for 8 days with i.t. morphine; (ii) benzamide and other PARS inhibitors reduced or prevented the development of analgesic tolerance and dark neurons; and (iii) the development of dark neurons after repeated morphine injection is opioid receptor-mediated, because concurrent administration of naltrexone with morphine prevents the development of dark neurons. These studies demonstrate that in vivo administration of morphine for 8 days produces dark neurons similar in morphology and location to transsynaptic alterations resulting from chronic constrictive injury of the sciatic nerve. The morphological characteristics of these cells are consistent with those of cells undergoing programmed cell death.
These results, along with others, indicate that the cascade of events leading to the formation of dark neurons in the spinal cord after morphine administration involves activation of the NMDA receptor, PKC, NO, and PARS. Thus, morphine tolerance may be reduced by intervening at any one of these steps. These findings suggest that neurotoxicity contributes to neural mechanisms underlying opioid tolerance and that PARS inhibitors are protective from such neurotoxicity. The results from these studies, combined with our results in similar studies using the CCI model (30), suggest that opioids may exacerbate the excitotoxicity underlying at least certain types of neuropathic pain. A corollary of this is that the excitotoxicity from neuropathic pain may, under some circumstances, reduce the response to opioids.
Several perplexing observations about neuropathic pain, opioid tolerance, and the interactions between them led us to propose an early model of the events involved (34) and now lead us to propose a revised version of this model here (Fig. 5). These observations include the following. (i) Neuropathic pain syndromes often present with symptoms indicative of both hyperexitability (e.g., hyperalgesia) and disinhibition (e.g., spontaneous pain, allodynia). Some of these symptoms, such as hyperalgesia, can be at least partially reversed in animal models by NMDA receptor antagonists (35), whereas others, such as allodynia, cannot (36). (ii) NMDA receptor antagonists prevent, but do not acutely reverse, tolerance to opioids (37). (iii) Our model assumes, for numerous reasons (34), that postsynaptic opioid and NMDA receptors are on the same neurons, at least in the spinal cord. In fact, we have reported direct immunohistochemical evidence of this (38). Opioids are known to produce hyperpolarization via an inwardly rectifying K+ channel. Despite the hyperpolarized state in these cells, voltage/ligand-gated NMDA receptors must be activated during the process of tolerance development, because NMDA receptor antagonists block the development of tolerance. (iv) At the spinal cord level, it is likely, except in the presence of nociceptive input, that presynaptic elements release only small amounts of glutamate onto postsynaptic elements in the superficial laminae of the dorsal horn that contain NMDA receptors related to opioid tolerance, yet tolerance to opioids occurs in the absence of nociceptive input. (v) PKC translocation to the cell membrane is greatly increased by chronic as compared with acute morphine treatment (34). (vi) Chronic opioid administration produces hyperalgesia, which can be reversed by NMDA receptor antagonists (27). (vii) CCI, which produces hyperalgesia causes a rightward shift in the dose–response curve to morphine (39). (viii) NO is involved in opioid tolerance (40). (ix) CCI-induced hyperalgesia (30) and opioid tolerance (39) are associated with the development of dark neurons (30). As reviewed in this article, the development of hyperalgesia, morphine tolerance, and dark neurons can be prevented by inhibiting the nuclear repair enzyme PARS.
Figure 5.
A proposed model for the excitotoxic formation of dark neurons in the dorsal horn of the spinal cord from peripheral nerve injury or repeated morphine administration. Excessive excitation of the NMDA receptor and subsequent influx of Ca2+ occurs either directly by glutamate release from primary afferent input (CCI model) or indirectly by activation of μ-opioid receptors (repeated opiate administration). Activation of the μ-opioid receptor results in indirect NMDA receptor activation by initiating a second-messenger PKC translocation to the membrane(16) This PKC translocation activates the NMDA receptor by removal of the Mg2+ blockade(17). The removal of the Mg2+ blockade from the NMDA receptor allows for an increased influx of Ca2+. The influx of Ca2+, via direct or indirect activation of the NMDA receptor, has several effects (5). It activates either a separate pool of PKC (PKC2) or much greater amounts of the original pool of PKC1. This second pool of PKC may be translocated directly to the membrane, modifying various excitatory amino acid or other receptors. It also may function as a transcription factor, resulting in the production of more PKC (PKC3), which can result in uncoupling of the μ-opioid receptor from its associated G protein. Another effect of the influx of Ca2+ is that it activates NO synthase, which increases the production of NO. In addition, the influx of Ca2+ results in the production of superoxide from mitochondria. The simultaneous generation of these two molecules favors the production of peroxynitrite (ONOO−), a very potent initiator of DNA strand breakage, which, in turn, initiates the production of the nuclear repair enzyme, PARS. Pronounced activation of PARS can result in cell dysfunction and eventually cell death because of inhibition of mitochondrial respiration and depletion of cellular energy stores, which in turn may lead to the formation of dark neurons, perhaps by way of programmed cell death. PKCx, various pools of protein kinase C; GPro, heterotrimeric guanine nucleotide binding protein; NOS, nitric oxide synthase.
A model of our current working hypothesis concerning the development of neuropathic pain, opioid tolerance, and their interactions that is consistent with these observations is presented in Fig. 5. In this model, excessive release of glutamate resulting from peripheral events, such as those occurring in the CCI model, initiates a series of intracellular events, which, via different messenger systems, leads to (i) at least partially NMDA antagonist reversible hyperexcitability that results from a PKC-mediated alteration of NMDA receptors (pathway 1, Fig. 5); (ii) events such as allodynia, which are not reversible with NMDA antagonists (36) but are prevented by inhibition of the NO/PARS pathway (30) and thus may be mediated by cellular dysfunction resulting from depletion of cellular energy stores (pathway 2, Fig. 5). This cellular dysfunction may be morphologically manifested by the appearance of dark neurons, which can also be prevented by inhibition of the NO/PARS pathway (30); and (iii) the rightward shift of the morphine dose–response curve resulting from CCI (39), which has been hypothesized to result from relatively long duration, PKC-mediated alterations in gene expression (pathway 3, Fig. 5), which may result in PKC-mediated opioid receptor/K+ channel uncoupling (41).
With regard to opioid tolerance, in this model, activation of the μ-opioid receptor may initiate PKC translocation to the membrane (42). This PKC translocation allows the NMDA receptor to function as a ligand-gated channel by removal of the voltage-dependent Mg2+ blockade (15). The removal of the Mg2+ blockade from the NMDA receptor allows for an increased influx of Ca2+ despite membrane hyperpolarization by μ-opioids and low levels of presynaptic glutamate release. This influx of Ca2+ has two effects. It activates either a separate pool of PKC (PKC2) or much greater amounts of the original pool of PKC1. The other pool of PKC may be translocated directly to the membrane, modifying various excitatory amino acid and/or other receptors (pathway 1), and/or it may modify nuclear transcription (pathway 3), the products of which result in delayed and persistent changes in cellular function such as opioid receptor/K+ channel uncoupling (41). This consequence cannot be reversed by acute administration of NMDA antagonists. A second effect of the influx of Ca2+ is that it activates NO synthase, which increases the production of NO and superoxide (43) (pathway 2). The simultaneous generation of these two molecules favors the production of peroxynitrite (ONOO−) (44). ONOO− is a very potent initiator of DNA strand breakage (45). Thus, ONOO− initiates the production of the nuclear repair enzyme, PARS. Pronounced activation of PARS can result in cell dysfunction and eventually cell death because of inhibition of mitochondrial respiration and depletion of cellular energy stores (33). This then leads to the formation of dark neurons perhaps by way of programmed cell death. Such an excitotoxic cascade may underlie at least some aspects of opioid tolerance. A third series of events initiated by influx of Ca2+ is the activation of either a separate pool of PKC (PKC2, Fig. 5) or much greater amounts of the original pool of PKC1. This other pool of PKC may function as a transcription factor resulting in the production of more PKC (PKC3, Fig. 5), which can modulate μ-opioid receptor responsiveness (46), resulting in desensitization of the μ-opioid receptor coupling through its associated G protein to an inwardly rectifying K+ channel, a mechanism know to exist in vitro (40).
Although we have focused here on NMDA receptor activation as a primary initiator of neuropathic pain, opioid tolerance, and their interactions, these events, as we have pointed out previously (34), are likely to involve additional factors. It is likely that Ca2+/calmodulin, cAMP, and other second and third messengers, non-NMDA receptors, and cholecystokinin (47) and other non-glutamate receptors also participate. Nevertheless, it is clear from the data reviewed here that the complex events resulting from NMDA receptor activation are of critical importance in neuropathic pain, opioid tolerance, and their interactions.
Clinical Implications.
Recent progress in investigating neural mechanisms subserving neuropathic and inflammatory pain as well as opioid tolerance has significantly advanced our knowledge about pain and pain modulation. These studies represent two important frontiers in pain research. First, these studies have led to the concept that pathological pain may reflect a disease process with both dynamic and progressive changes during its course (1, 2, 9). A key feature of this process is that neuronal plastic changes occur within the CNS in association with the progress of pathological pain states. This concept provides, at least in part, a basis for explaining pathological pain that often persists long after the initial insults. Second, mechanisms of opioid tolerance may involve neuroplastic changes within the CNS as well (9, 39). Neuroplastic changes in relation to opioid tolerance have much in common with those of pathological pain, both of which begin with the activation of NMDA receptors (9). Evidence also exists indicating that interactions do indeed occur between cellular and intracellular mechanisms of pathological pain and opioid tolerance, and such interactions are likely to be a contributing factor to a generally weak analgesic effect of opioids in pathological pain states (39).
Thus far, little information exists with regard to interactions between hyperalgesia and analgesic tolerance in man. It is conceivable that reduced morphine analgesia after repeated administration to patients with chronic pain could result from the development of both pharmacological tolerance to morphine analgesia and tolerance-associated hyperalgesia. Because of the coincidental development of morphine tolerance and tolerance-associated hyperalgesia, progressively higher morphine doses may be needed to overcome both conditions. In turn, a vicious cycle may be initiated involving higher opiate doses, more tolerance, and greater hyperalgesia. Thus, the need for higher opiate doses in a clinical setting of opiate treatment could be partly due to the development of hyperalgesia that may result from repeated opiate administration. Conceivably, the development of tolerance-associated hyperalgesic states may also contribute to withdrawal signs of opioid dependence. This possibility also suggests that an inappropriate opiate treatment schedule in pain management may precipitate unexpected hyperalgesic responses to preexisting pain conditions and could be a source of the clinical complexity with respect to the responsiveness to opiate treatment.
On the other hand, evidence indicating hyperalgesia-associated reduction of morphine antinociception (27) has bearing on the controversy concerning opiate effects or lack of effects on neuropathic pain states in man. It is conceivable that the diversity of clinical response patterns to opiate treatment in neuropathic pain patients may result from varying degrees of CNS neuronal plastic changes initiated by nerve injury or injury to other tissues. Such neuronal plastic changes can underlie the development of neuropathic pain syndromes and result in reduced morphine analgesia even before opiate treatment starts (9). To complicate matters further, neuropathic pain syndromes as well as other chronic pain states (such as cancer pain) often present a dynamic and progressive course that demands increased opiate doses for adequate pain relief.
The complexity of opioid tolerance, hyperalgesia, and their interactions calls for a new look into some clinical issues of pain management. Because the development of pathological pain states often involves the activation of NMDA receptors and because there exists an intimate relationship between the NMDA and opioid receptor systems that may lead to changes in responsiveness to opioid analgesics, early recognition of clinical conditions that may lead to the development of pathological pain states would be of utmost importance. Such clinical conditions may include, but may not be limited to, nerve injury, tissue inflammation, and prolonged and ongoing peripheral nociceptive input (such as those seen in persistent postoperative pain). Some of these conditions (e.g., postoperative pain) may be treated preemptively to produce a favorable outcome. However, nerve injury and inflammatory tissue diseases often occur in an unpredictable manner, thereby obviating the possibility of preemptive treatment. Thus, effective early interruption of ongoing nociceptive input from the injured site (e.g., using nerve block or field block), thereby reducing CNS activation of NMDA receptors, could be the key to preventing or minimizing changes in NMDA and opioid systems that may eventually lead to persistent pain and reduced opioid effectiveness for treating such pain states.
As discussed above, interactions between NMDA and opioid receptors could occur in both directions (9). Thus, any condition that results in activation of NMDA receptors within the CNS could modulate opioid receptors, causing reduced efficacy of opioid analgesia; conversely, repeated treatment with opioids could set up a condition mimicking ongoing nociceptive input through interactions between opioid and NMDA receptors (9, 39). This concept is the basis for recommending a combined use of opioids and clinically available NMDA receptor antagonists (9). Importantly, such a strategy should be integrated into the treatment regimen not only for chronic pain management (treating an existing pain condition) but also for preventing an evolving pain condition, such as that after nerve injury. By the same token, effective nerve block or field block should be part of an integrated therapeutic regimen for treating clinical conditions that may later lead to the development of intractable chronic pain states. It should be noted that, although early treatment of pain after tissue injury and inflammation with opioids often provides satisfactory clinical pain relief, opioids alone offer little help for stopping the process of an evolving pathological pain state, because evidence presented in this article and elsewhere suggests that opioids alone could actually contribute to the development of neuronal plastic changes via interactions with NMDA receptors. It can be anticipated that our further understanding of neural mechanisms subserving hyperalgesia, opioid tolerance, and their interactions would advance and improve clinical management of debilitating and intractable pain syndromes.
Acknowledgments
Portions of this work were supported by Public Health Service Grants DA08835 and NS24009.
ABBREVIATIONS
- PARS
poly(ADP ribose) synthetase
- CCI
chronic constrictive injury
- NMDA
N-methyl-d-aspartate
- CNS
central nervous system
- PKC
protein kinase C
- i.t.
intrathecal
References
- 1.Woolf C J, Thompson S W N. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 2.Dubner R. In: Proceedings of 5th World Congress on Pain. Bond M, Charlton E, Woolf C J, editors. Vol. 5. Amsterdam: Elsevier; 1991. pp. 263–276. [Google Scholar]
- 3.Seltzer Z, Cohn S, Ginzgurg R, Berlin B. Pain. 1991;45:69–75. doi: 10.1016/0304-3959(91)90166-U. [DOI] [PubMed] [Google Scholar]
- 4.Davar G, Hama A, Deykin A, Vos B, Maciewicz R. Brain Res. 1991;553:327–330. doi: 10.1016/0006-8993(91)90844-l. [DOI] [PubMed] [Google Scholar]
- 5.Mao J, Mayer D J, Hayes R L, Lu J, Price D D. Brain Res. 1992;598:271–278. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Price D D, Mayer D J, Lu J, Hayes R L. Brain Res. 1992;576:254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- 7.Mao J, Price D D, Hayes R L, Lu J, Mayer D J, Frenk H. Brain Res. 1993;605:164–168. doi: 10.1016/0006-8993(93)91368-3. [DOI] [PubMed] [Google Scholar]
- 8.Tal M, Bennett G J. Neurosci Lett. 1993;151:107–110. doi: 10.1016/0304-3940(93)90058-s. [DOI] [PubMed] [Google Scholar]
- 9.Mao J, Price D D, Mayer D J. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 10.Kajander K C, Wakisaka S, Bennett G J. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 11.Sato J, Perl E R. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarek L K. Trends Neurosci. 1987;10:30–34. [Google Scholar]
- 13.Numann R, Catterall W A, Scheuer T. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- 14.West J W, Numann R, Murphy B J, Scheuer T, Catterall W A. Science. 1991;254:866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Huang L Y M. Nature (London) 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Wang H, Sheng L Y, Jan Y N, Basbaum A I. Soc Neurosci Abstr. 1994;20:482. [Google Scholar]
- 17.Kitto K F, Haley J E, Wilcox G L. Neurosci Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- 18.Palecek J, Paleckova V, Dougherty P M, Willis W D. J Neurophysiol. 1994;71:529–537. doi: 10.1152/jn.1994.71.2.529. [DOI] [PubMed] [Google Scholar]
- 19.Gerber G, Kangraga I, Ryu P D, Larew J S A, Randic M. J Neurosci. 1989;9:3606–3617. doi: 10.1523/JNEUROSCI.09-10-03606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao J, Mayer D J, Hayes R L, Price D D. J Neurophysiol. 1993;70:470–481. doi: 10.1152/jn.1993.70.2.470. [DOI] [PubMed] [Google Scholar]
- 21.Mao J, Price D D, Phillips L L, Lu J, Mayer D J. Brain Res. 1995;677:257–267. doi: 10.1016/0006-8993(95)00161-i. [DOI] [PubMed] [Google Scholar]
- 22.Laird J M A, Bennett G J. J Neurophysiol. 1993;69:2071–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- 23.Palecek J, Paleckova V, Dougherty P M, Carlton S M, Willis W D. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- 24.Guilbaud G, Benoist J M, Jazat F, Gautron M. J Neurophysiol. 1990;64:1537–1554. doi: 10.1152/jn.1990.64.5.1537. [DOI] [PubMed] [Google Scholar]
- 25.Bennett G J, Xie Y K. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 26.Mao J, Coghill R C, Mayer D J, Hayes R L. Pain. 1992;50:89–100. doi: 10.1016/0304-3959(92)90116-S. [DOI] [PubMed] [Google Scholar]
- 27.Mao J, Mayer D J, Price D D. J Neurosci. 1993;13:2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hylden J L K, Nahin R L, Traub R J, Dubner R. Pain. 1989;37:329–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto T, Bennett G J, Kajander K C. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- 30.Mao J, Price D D, Zhu J, Lu J, Mayer D J. Pain. 1997;72:355–366. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 31.Price D D, Long S, Huitt C. Pain. 1992;49:163–174. doi: 10.1016/0304-3959(92)90139-3. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Yaksh T L. Pain. 1992;49:121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Dawson V L, Dawson T M, Snyder S H. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 34.Mayer D J, Mao J, Price D D. Pain. 1995;61:365–374. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- 35.Mao J, Price D D, Mayer D J, Lu J, Hayes R L. Brain Res. 1992;576:254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- 36.Tal M, Bennett G J. NeuroReport. 1994;5:1438–1440. doi: 10.1097/00001756-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Trujillo K A, Akil H. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 38.Keniston L, Mao J, Price D D, Lu J, Mayer D J. Soc Neurosci Abstr. 1998;24:390. [Google Scholar]
- 39.Mao J, Price D D, Mayer D J. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 40.Kolesnikov Y A, Pick C G, Pasternak G W. Eur J Pharmacol. 1992;221:399–400. doi: 10.1016/0014-2999(92)90732-j. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Yu L. J Biol Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- 42.Chen L, Huang L Y. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 43.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 44.Pryor W A, Squadrito G L. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 45.Szabo C. Free Radical Biol Med. 1996;21:855–869. doi: 10.1016/0891-5849(96)00170-0. [DOI] [PubMed] [Google Scholar]
- 46.Harada H, Ueda H, Katada T, Ui M, Satoh M. Neurosci Lett. 1990;113:47–49. doi: 10.1016/0304-3940(90)90492-r. [DOI] [PubMed] [Google Scholar]
- 47.Watkins L R, Kinscheck I B, Mayer D J. Science. 1984;224:395–396. doi: 10.1126/science.6546809. [DOI] [PubMed] [Google Scholar]