Abstract
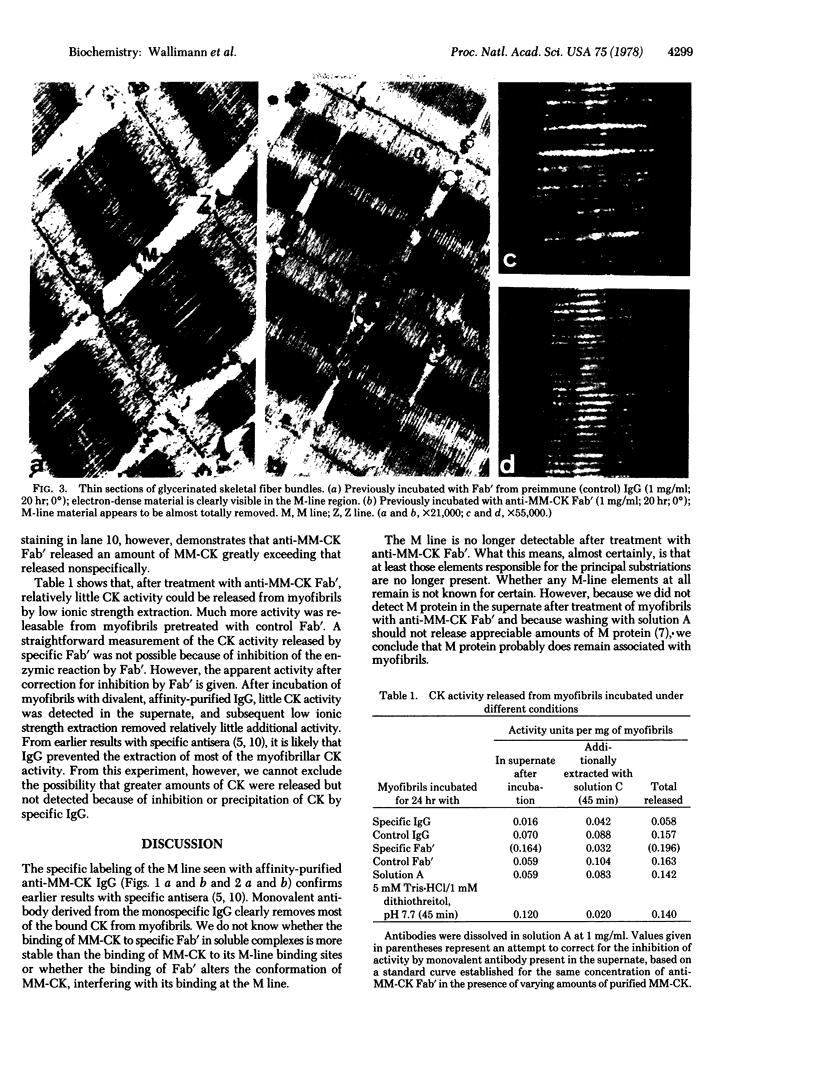
Column-purified antibodies against creatine kinase (EC 2.7.3.2) from chicken skeletal muscle (the homodimeric isoenzyme designated MM-CK) bind specifically to the M lines of chicken pectoral muscle myofibrils. Incubation of myofibrils with monovalent Fab' fragments of these antibodies solubilizes most of the myofibril-bound creatine kinase, concomitantly removing most of the electron-dense material from the M lines. This strongly indicates that MM-CK is an integral part of the M-line structure and is consistent with the suggestion that MM-CK molecules form the M bridges that are responsible for the principle M-line substriations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Heizmann C. W., Bläuenstein I. E., Eppenberger H. M. Comparison of the localization of several muscle proteins in relaxed and contracted myofibrils. Experientia. 1978 Jan 15;34(1):38–40. doi: 10.1007/BF01921887. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Eppenberger H. M. Isolation and characterization of glycogen phosphorylase b from chicken breast muscle: comparison with a protein extracted from the M. line. J Biol Chem. 1978 Jan 10;253(1):270–277. [PubMed] [Google Scholar]
- Knappeis G. G., Carlsen F. The ultrastructure of the M line in skeletal muscle. J Cell Biol. 1968 Jul;38(1):202–211. doi: 10.1083/jcb.38.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrat E., Pepe F. A. The M band. Studies with fluorescent antibody staining. J Cell Biol. 1971 Feb;48(2):340–347. doi: 10.1083/jcb.48.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landon M. F., Oriol C. Native conformation of M-protein. Biochem Biophys Res Commun. 1975 Jan 20;62(2):241–245. doi: 10.1016/s0006-291x(75)80129-x. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Isolation and physical chemical properties of an M-line protein from skeletal muscle. J Biol Chem. 1972 May 25;247(10):3052–3061. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977 Jan 5;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Trinick J., Lowey S. M-protein from chicken pectoralis muscle: isolation and characterization. J Mol Biol. 1977 Jun 25;113(2):343–368. doi: 10.1016/0022-2836(77)90146-2. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gmür R., Lebherz H. G., Siegrist M., Wallimann T., Eppenberger H. M. Differentiation in cultures derived from embryonic chicken muscle. II. Phosphorylase histochemistry and fluorescent antibody staining for creatin kinase and aldolase. Dev Biol. 1976 Feb;48(2):284–307. doi: 10.1016/0012-1606(76)90091-9. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]