Abstract
Vitamin D deficiency has recently been implicated as a possible risk factor in the etiology of numerous diseases, including nonskeletal conditions. In humans, skin synthesis following exposure to UVB is a potent source of vitamin D, but in regions with low UVB, individuals are at risk of vitamin D deficiency. Our objectives were to describe the prevalence of vitamin D deficiency and to investigate determinants of plasma 25-hydroxyvitamin D (25-OHD) concentrations in a high northern latitude country. Detailed dietary, lifestyle, and demographic data were collected for 2235 healthy adults (21–82 y) from Scotland. Plasma 25-OHD was measured by liquid chromatography-tandem MS. Among study participants, 34.5% were severely deficient (25-OHD <25 nmol/L) and 28.9% were at high risk of deficiency (25–40 nmol/L). Only 36.6% of participants were at low risk of vitamin D deficiency or had adequate levels (>40 nmol/L). Among participants who were taking supplements, 21.3% had a May-standardized 25-OHD concentration >50 nmol/L, 54.2% had 25–50 nmol/L, and 24.5% had <25 nmol/L, whereas this was 15.6, 43.3, and 41%, respectively, among those who did not take supplements (P < 0.0001). The most important sources of vitamin D were supplements and fish consumption. Vitamin D deficiency in Scotland is highly prevalent due to a combination of insufficient exposure to UVB and insufficient dietary intake. Higher dietary vitamin D intake modestly improved the plasma 25-OHD concentration (P = 0.02) and reduced the proportion of severely deficient individuals (P < 0.0001). In regions with low UVB exposure, dietary and supplement intake may be much more important than previously thought and consideration should be given to increasing the current recommended dietary allowance of 0–10 μg/d for adults in Scotland.
Introduction
In recent years, there has been an increasing awareness of the possible negative health impacts of vitamin D deficiency. Previously considered relevant only for bone and calcium metabolism, a diagnosis of vitamin D deficiency was rarely made in the absence of skeletal disease. However, vitamin D deficiency has recently been implicated as a possible risk factor in the etiology of cancer and metabolic, cardiovascular, infectious, and autoimmune diseases (1–6). Should the association prove to be causal, then vitamin D deficiency may have important adverse effects on population health.
UVB-induced synthesis in skin is a very potent source of vitamin D (7), but it is greatly affected by the UVB intensity and time of exposure (8). From April to October, to synthesize 10 μg, an individual with skin type III 12 would need to spend ~3–8 min in the sun at noon, with 25% of the body surface area exposed in Boston (42°N) or 3–6 min in Miami (26°N) (9). Alternatively, vitamin D can be obtained from dietary sources (1, 7). Due to the scarcity of vitamin D in food, the typical diet in most populations is rarely a sufficient source. Therefore, the lack of skin synthesis is a serious shortcoming in maintaining adequate vitamin D status.
The Scientific Advisory Committee on Nutrition in Scotland put forward a Reference Nutrient Intake13 for vitamin D of 10 μg/d for individuals over 65 y and zero for everyone else (excluding young children and pregnant and lactating women) (11). However, the Institute of Medicine 2011 report on dietary reference intakes proposed an RDA of vitamin D of 15 μg/d for ages 1–70 y and 20 μg/d for older persons and an Estimated Average Requirement14 of 10 μg/d. This recommendation is based on conditions of minimal sun exposure.
Due to the high northern latitude of Scotland (55° to 59° North) and the prevalent cloudy weather, conditions rarely facilitate vitamin D synthesis (12). In addition, an indoor-orientated lifestyle, obesity, high alcohol intake, and poor diet adversely affect vitamin D status (13, 14). As expected, in a nation-wide cohort of British adults, participants from Scotland were identified as the most likely to suffer from vitamin D deficiency (15).
In this large study, which included 2235 Scottish adults, we estimated the prevalence of vitamin D deficiency in Scotland. The aim of this study was to establish the main risk factors for vitamin D deficiency that are related to lifestyle patterns and dietary factors and to identify groups most at risk of vitamin D deficiency. This study attempted to recognize the major sources of vitamin D and clarify the role of dietary intake in general and supplements in particular on 25-hydroxyvitamin D (25-OHD) concentration at a time when vitamin D supplements are receiving increased attention as studies show the beneficial effect of vitamin D supplements on health and overall mortality (2, 16). To our knowledge, this is one of the largest studies to date to gather detailed demographic, lifestyle, and dietary data in a high latitude country.
Participants and Methods
The study population comprised 2235 healthy adults identified through the Community Health Index and invited by their general practitioner to take part as controls in a national case control study of colorectal cancer in Scotland. This study has been described in detail elsewhere (17). All participants gave written informed consent and were recruited in the period from 1999 to 2006. The participation rate among those invited to take part in the study was ~57%. More than 99% of all recruited individuals were white Caucasian. Approval for the study was obtained from the MultiCentre Research Ethics Committee for Scotland and Local Research Ethics committee.
Data collection and samples.
Participants completed a lifestyle questionnaire, a semiquantitative FFQ (Scottish Collaborative Group FFQ, version 6.41), and a supplements intake questionnaire (18). The lifestyle questionnaire inquired about outdoor activities: walking; cycling; gardening; and jogging, swimming, fitness, or other aerobic exercise. These were recorded separately for winter and summer months and used as an estimate of the total number of hours spent outdoors per week as a proxy measure for sun exposure. The FFQ was used to calculate each participant’s dietary intake of food items and nutrients and to calculate the mean contribution of each food group to a participant’s dietary vitamin D intake (data available for 2056 participants). The main characteristics and validity of the FFQ were previously reported (19, 20).
Using an in-house calculation program (21), nutrients were calculated from the consumption frequencies of specified portion size for each food item from the FFQ and were standardized for total energy intake. Participants were also asked to give details about supplements taken and nutrient information was collected from the manufacturer’s product information.
Area deprivation scores were estimated from the postcodes of the participants (Carstairs deprivation index) based on 2001 Census data and took values from 1 (very low deprivation) to 7 (very high deprivation).
Blood was collected from all participants and transferred to the research center within 72 h of sampling. Plasma was prepared by gentle centrifugation of sodium EDTA tubes and 1.5 mL of each participant’s plasma was stored at −80°C.
Measurement of plasma 25-OHD.
The liquid chromatography-tandem MS (LC-MS/MS) method was used to measure 25-hydroxyergocalciferol and 25-hydroxycholecalciferol and the total of the 2 was used; however, most of our samples contained no 25-hydroxyergocalciferol. The lower limit of detection with the LC-MS/MS method was 10 nmol/L for 25-hydroxycholecalciferol (22). The LC-MS/MS method was performed following standard protocols and appropriate quality control procedures, including multiple measurements of the same sample from our cohort and standardization against standard reference material (SRM 972). Serum pools distributed by four Vitamin D External Quality Assessment Scheme distributions in 2010 (n = 20) were analyzed using the LC-MS/MS method with a mean negative bias of 10% compared with the method mean and 15% compared to the all laboratory trimmed mean. This method has been rated as the preferred 25-OHD measurement method for population studies by an international panel of experts (22, 23). More details can be found elsewhere (22, 24).
Vitamin D deficiency is defined in terms of circulating 25-OHD, which is a reliable marker of vitamin D status (25). Based on the evidence, the Institute of Medicine 2011 report judged that nearly all individuals (97.5%) meet their needs when their plasma 25-OHD concentration is >50 nmol/L and this corresponds to the RDA (the vitamin D intake that satisfies the needs of nearly everybody) (26). Participants with 25-OHD >50 nmol/L were considered to have an adequate 25-OHD concentration. On the other hand, we regarded individuals with 25-OHD < 25 nmol/L as severely deficient in concordance with other recent studies (7, 27).
The risk of deficiency increases gradually as 25-OHD concentration decreases from 50 nmol/L (adequate) to 25 nmol/L (severely deficient). The concentration of 40 nmol/L is associated with Estimated Average Requirement, a value that represents the median of the required intake in the population. Approximately one-half of individuals will have their needs met at this concentration; individuals below this cutoff (40 nmol/L) are more likely to be vitamin D deficient and those above are less likely to be deficient. Therefore, we consider individuals with an 25-OHD concentration between 25 and 40 nmol/L to be at high risk of deficiency and those with 40–50 nmol/L at low risk of deficiency.
Statistical analysis.
For the analysis of factors affecting vitamin D status, 25-OHD measurements were standardized to the month of May to remove the prominent effect of the sampling month on the 25-OHD concentration. May-standardized values are free of the sampling month effect and represent the expected values for the sample as if taken in May. Mean values in May (33.2 nmol/L) are close to the yearly mean (mean of monthly means is 36.7 nmol/L) and can therefore be taken as a proxy of the individual’s yearly mean of 25-OHD in plasma. Our dataset was imbalanced due to different numbers of participants sampled in each calendar month. Therefore, the dataset was balanced to calculate corrected percentages in each category of vitamin D status, which is equivalent to having sampled the same number of participants in each month.
Values in the text are presented as mean ± SD. Most of the statistical analysis was done using R software version 2.6.1 [Hornik (2009), The R FAQ, http://CRAN.R-project.org/doc/FAQ/R-FAQ.html, ISBN 3-900051-08-9] (28) and Microsoft Office Excel. The effects of dietary, demographic, and lifestyle factors on vitamin D status were assessed. May-standardized 25-OHD values were used in the analysis unless stated otherwise. The mean was calculated for each variable and participants were divided into 2 groups, one comprising 50% of participants below the mean for a given tested factor and a second comprising the remaining participants above the mean. For each variable, unpaired t test on log-transformed, May-standardized 25-OHD concentrations was performed. For categorical variables, we used the Pearson χ2 test.
To illustrate the differences in plasma 25-OHD in a low- and high-risk environment, participants were divided into quartiles according to the outdoor activities and subdivided further according to daily supplement intake: none (<1 μg/d), low intake (1–5 μg/d), medium intake (5–10 μg/d), and high intake (>10 μg/d). The same was repeated by subdividing the groups according to only food and total (from food and supplements) vitamin D intake quartiles.
Next, participants were grouped into quartiles according to the total intake of vitamin D and further subdivided according to age in 4 age groups (<50, 50–60, 60–70, and >70 y). The number in each group, mean vitamin D intake, median plasma 25-OHD concentration, and percent of severely deficient individuals was assessed for each subgroup, by total vitamin D intake, and by gender.
Plasma 25-OHD variance explained by total vitamin D intake and outdoor activity was independently calculated for winter (November to April) and summer months (May to October) and therefore, actual, nonstandardized measurements of plasma 25-OHD were used in this analysis.
Results
We investigated plasma 25-OHD concentrations in 2235 healthy individuals (988 females) from Scotland aged 21–82 y (61.3 ± 10.5 y). The plasma 25-OHD concentration measured in our samples was 35.9 ± 22.3 nmol/L (Supplemental Table 1), almost identical to the mean of the balanced dataset (35.0 ± 22.1 nmol/L).
The most striking finding was the very high prevalence of severely deficient individuals (34.5%) and of those at high risk of vitamin D deficiency (28.9%). Extremely low concentrations (<12.5 nmol/L) were measured in 264 participants (11.8%).
The distribution of vitamin D sufficiency categories for each month is presented in Figure 1 and Supplemental Table 1. Sample month was strongly associated with 25-OHD concentration (P < 0.0001) (Table 1). The proportion of individuals who were severely deficient or at high risk of vitamin D deficiency (25-OHD <40 nmol/L) was extremely high throughout the year. From December to May, 69–83% were deficient, but an improvement occurred from June to November, when the prevalence was 33–69% (P < 0.0001).
FIGURE 1.
Monthly 25-OHD concentration distribution in adults in Scotland (n = 2235). Nonstandardized 25-OHD measurements were used.
TABLE 1.
Plasma 25-OHD concentrations in adults in Scotland1
| Adequate |
Risk of deficiency |
Deficient |
||||||||
| ≥50 nmol/L | Low risk ≥40 and <50 nmol/L | High risk ≥25 and <40 nmol/L | <25 nmol/L | |||||||
| Month | n | Plasma 25-OHD, nmol/L | n | % | n | % | n | % | n | % |
| January2 | 139 | 26.9 ± 16.4 | 12 | 8.6 | 12 | 8.6 | 46 | 33.1 | 69 | 49.6 |
| August3 | 216 | 51.9 ± 26.1 | 109 | 50.5 | 35 | 16.2 | 41 | 19.0 | 31 | 14.4 |
| All | 2235 | 35.9 ± 22.3 | 503 | 22.5 | 314 | 14.0 | 646 | 28.9 | 772 | 34.5 |
Nonstandardized 25-OHD concentrations were used.
Months with the year's lowest and year's highest mean plasma 25-OHD concentration, respectively.
Plasma 25-OHD concentration was higher in younger (≤61.3 y) participants (P = 0.002) and in participants with lower BMI (≤26.8) (P < 0.0001) (Table 2), but there was no difference between males and females (P = 0.49). Plasma 25-OHD concentrations were affected by participants’ vitamin D intake from food (P = 0.005), supplements (P < 0.0001), and overall (P = 0.02). Participants who consumed >0.9 fish servings/d had a higher plasma 25-OHD concentration than those who consumed less (P = 0.006).
TABLE 2.
Descriptive analysis of plasma 25-OHD concentrations in adults in Scotland in relation to selected dietary and lifestyle factors1
| Plasma 25-OHD, nmol/L |
t test | |||
| Variable | ≤Mean2 | >Mean | P | |
| Age, y | 61.3 ± 10.7 | 35.7 ± 22.7 | 32.1 ± 17.3 | 0.002 |
| BMI, kg/m2 | 26.8 ± 4.7 | 35.6 ± 21.1 | 31.5 ± 18.8 | <0.0001 |
| Vitamin D from food, μg/d | 4.8 ± 2.9 | 32.9 ± 20.9 | 35.3 ± 19 | 0.005 |
| Vitamin D from supplements, μg/d | 3.9 ± 4.0 | 34.7 ± 20.7 | 40.2 ± 19.7 | <0.0001 |
| Total vitamin D, μg/d | 8.7 ± 5.2 | 35.4 ± 20.1 | 40 ± 20.2 | 0.02 |
| Food calcium, g/d | 1.2 ± 0.3 | 33.3 ± 20.3 | 34.5 ± 20.2 | 0.21 |
| Supplement calcium, mg/d | 48 ± 167 | 37.3 ± 20.3 | 38.3 ± 21 | 0.41 |
| Energy intake, MJ/d | 10.7 ± 3.9 | 33.4 ± 19.4 | 34.3 ± 21.4 | 0.36 |
| Food fiber, g/d | 22.9 ± 6.1 | 33.1 ± 20.2 | 34.7 ± 20.3 | 0.02 |
| Alcohol, g/d | 13.2 ± 15.2 | 33.2 ± 19.4 | 34.9 ± 21.6 | 0.03 |
| White fish,3servings/d | 0.4 ± 0.4 | 33.7 ± 19.6 | 34.1 ± 21.4 | 0.67 |
| Oily fish,3servings/d | 0.2 ± 0.4 | 33.3 ± 20.8 | 34.8 ± 19.1 | 0.096 |
| All fish and seafood,3servings/d | 0.9 ± 0.9 | 32.9 ± 19.6 | 35.4 ± 21.2 | 0.006 |
| All red meat,4servings/d | 1.4 ± 1 | 34.5 ± 21.4 | 32.9 ± 18.4 | 0.27 |
| Liver products,5servings/d | 0 ± 0.1 | 33.9 ± 20.6 | 33.7 ± 19.4 | 0.88 |
| All outdoor activities, h/wk | 16.7 ± 15.4 | 32.6 ± 19.4 | 36.4 ± 22 | <0.001 |
| Cycling and gardening, h/wk | 4.1 ± 6.7 | 32.9 ± 20.6 | 36.1 ± 19.7 | <0.001 |
| Summer months: all outdoor activities, h/wk | 19.1 ± 15.3 | 32.4 ± 19.4 | 36 ± 21.4 | <0.0001 |
| Summer months: cycling and gardening, h/wk | 5.8 ± 7 | 32.8 ± 20.6 | 35.5 ± 19.5 | <0.001 |
| Deprivation score | 3.3 ± 1.4 | 33.8 ± 20.7 | 34.0 ± 19.6 | 0.56 |
Values are mean ± SD. Plasma 25-OHD measurement, age, and deprivation score data were available for 2235 participants, FFQ data for 2056, supplement intake data for 2089, BMI data for 2067, and outdoor activity data for 2036 participants.
For each tested variable, the sample was split into 2 groups, above and below the mean.
One fish serving is one small fish fillet (50–150 g).
One meat serving is 2 tablespoons (50–110 g) of mince or casserole, or one burger, sausage, or steak.
The number of liver, liver sausage, and liver pate servings was assessed in the FFQ.
The estimated total time spent on outdoor activities (h/wk) was strongly associated with plasma 25-OHD concentrations (P < 0.001). The strong association remained for each activity (walking, cycling, physical exercise, and gardening) when independently assessed both in summer and winter months (P < 0.03), with the exception of gardening in winter months (P = 0.14).
The majority of participants (66%) had an estimated intake of vitamin D from diet alone of <5 μg/d (Fig. 2). The majority of participants (74%) did not take any form of vitamin D supplements, 7% reported taking <5 μg/d, and 18% took ≥5 μg/d of vitamin D in the form of supplements. The total vitamin D intake from food and supplements was calculated; only 48% consumed >5 μg/d and 14% consumed >10 μg/d.
FIGURE 2.
Total daily vitamin D intake by adults in Scotland who take vitamin D supplements (n = 535) and do not (n = 1554).
The mean intake of vitamin D from food by the participants who were not taking vitamin D supplements was 4.7 ± 2.6 μg/d (Fig. 2). The plasma 25-OHD concentration of this group was 32.3 ± 20.4 nmol/L. For participants who did take vitamin D supplements, the mean total daily intake of 10.7 ± 4.9 μg/d was higher (P < 0.0001) and the plasma 25-OHD concentration of 38.3 ± 18.9 nmol/L was also higher (P < 0.0001). More information on the relationship between supplementary and dietary intakes of vitamin D to plasma 25-OHD concentration is given in Table 3. Among participants who took supplements, 21.3% had a May-standardized 25-OHD concentration >50 nmol/L, 54.2% had 25–50 nmol/L, and 24.5% had <25 nmol/L, whereas this was 15.6, 43.3, and 41%, respectively, among those who did not take supplements (P < 0.0001).
TABLE 3.
Distribution of individuals in different plasma 25-OHD categories, in respect to total vitamin D and supplements intake, in adults in Scotland1
| Vitamin D supplements |
Total (from food and supplements) vitamin D intake >5 μg/d |
|||||||
| No |
Yes |
No |
Yes |
|||||
| 25-OHD | n | % | n | % | n | % | n | % |
| >50 nmol/L | 243 | 15.6 | 114 | 21.3 | 167 | 15.8 | 183 | 18.5 |
| >25 and ≤50 nmol/L | 673 | 43.3 | 290 | 54.2 | 430 | 40.6 | 511 | 510.8 |
| >12.5 and ≤25 nmol/L | 471 | 30.3 | 100 | 18.7 | 329 | 31.1 | 230 | 23.3 |
| ≤12.5 nmol/L | 167 | 10.7 | 31 | 5.8 | 132 | 12.5 | 63 | 6.4 |
| Total | 1554 | 74.4 | 535 | 25.6 | 1058 | 51.7 | 987 | 48.3 |
The total number of participants with 25-OHD and supplement intake data available was 2089. For 44 participants, information on vitamin D intake from food was not available.
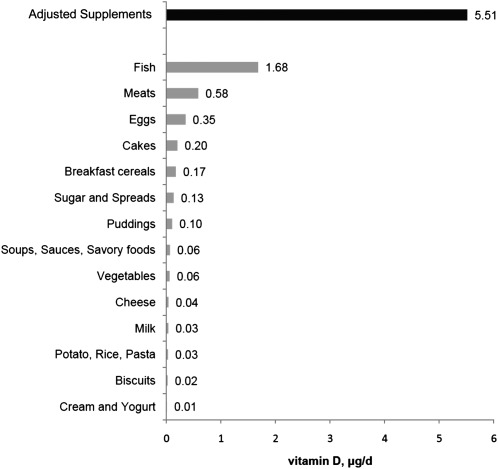
The assessment of the mean contribution of different food groups to an individual’s vitamin D intake is presented in Figure 3. As expected, fish intake was the single most important dietary source of vitamin D.
FIGURE 3.
Mean contributions of different food products and supplements to daily vitamin D intake in adults in Scotland (n = 2056). The contribution of supplements was calculated for the 527 individuals who take >1 μg/d of vitamin D from supplements.
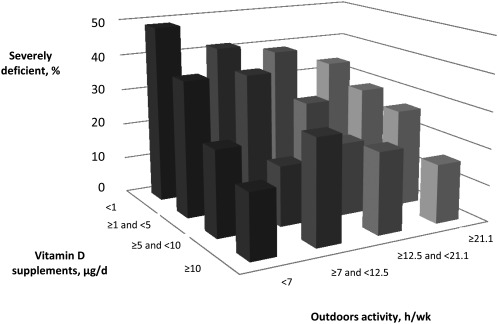
The analysis of joint and independent effects of sun exposure and supplement intake on plasma 25-OHD revealed an increase in the median plasma 25-OHD concentration with increased outdoor activity and with greater supplementation for a given activity quartile (P = 0.02) (Fig. 4;Table 4). Results were similar when vitamin D intake from food and total intake were analyzed (Supplemental Tables 2 and 3). Notably, in the group of participants in the lowest outdoor activity quartile (<7 h/wk), the percent of severely deficient individuals decreased with increasing supplementation level. Among those who took no supplements, 49.6% were severely deficient, followed by 34.4, 23.4, and 14.3% among those taking <5, 5–10, and >10 μg/d, respectively.
FIGURE 4.
Proportion of severely deficient individuals with plasma 25-OHD <25 nmol/L according to the outdoor activity and levels of supplement intake.
TABLE 4.
Plasma 25-OHD concentrations in adults in Scotland by level of supplemental vitamin D intake and quartile of outdoor activity12
| Supplemental vitamin D intake | Quartiles of outdoor activity, h/wk |
|||
| <7 | ≥7.0 and <12.5 | ≥12.5 and <21.1 | ≥21.1 | |
| <1 μg/d | ||||
| n | 385 | 381 | 371 | 361 |
| 25-OHD median, nmol/L | 25.1 | 28.5 | 28.9 | 30.8 |
| 25-OHD < 25 nmol/L, % | 49.6 | 41.2 | 38.5 | 33.5 |
| ≥1 and <5 μg/d | ||||
| n | 32 | 35 | 40 | 31 |
| 25-OHD median, nmol/L | 29.5 | 32.4 | 35.9 | 34.6 |
| 25-OHD < 25 nmol/L, % | 34.4 | 37.1 | 25.0 | 29.0 |
| ≥5 and <10 μg/d | ||||
| n | 64 | 73 | 78 | 79 |
| 25-OHD median, nmol/L | 34.1 | 38.4 | 37.7 | 38.2 |
| 25-OHD < 25 nmol/L, % | 23.4 | 16.4 | 19.2 | 26.6 |
| ≥10 μg/d | ||||
| n | 21 | 7 | 18 | 32 |
| 25-OHD median, nmol/L | 38.0 | 39.9 | 41.5 | 45.9 |
| 25-OHD < 25 nmol/L, % | 14.3 | 28.6 | 22.2 | 15.6 |
May-standardized values of 25-OHD were used.
In total, 245 participants were excluded from this analysis due to missing data regarding outdoor activity and supplements intake.
An analysis of total vitamin D intake by gender and age showed that men (9.8 ± 5.3 μg/d) had a higher intake than women (8.1 ± 4.8 μg/d) (P < 0.0001) (Table 5). Plasma 25-OHD concentrations were higher and the percent of severely deficient participants was lower in younger adult age groups of both genders. The percent that were severely deficient and the median plasma concentration of 25-OHD by vitamin D intake quartile and age are shown in Table 5.
TABLE 5.
Mean total vitamin D intake, median plasma concentration of 25-OHD and percent of study participants that were severely deficient, by vitamin D intake quartile and age12
| Total vitamin D intake quartile | All Age group, y |
Men Age group, y |
Women Age group, y |
||||||||||||
| <50 | ≥50 and <60 | ≥60 and <70 | ≥70 | All | <50 | ≥50 and <60 | ≥60 and <70 | ≥70 | All | <50 | ≥50 and <60 | ≥60 and <70 | ≥70 | All | |
| Q1 | |||||||||||||||
| Intake, μg/d | 2.3 | 2.5 | 2.4 | 2.6 | 2.5 | 2.8 | 2.9 | 2.4 | 2.8 | 2.7 | 2.1 | 2.2 | 2.2 | 2.3 | 2.2 |
| 25-OHD, nmol/L | 35.0 | 28.5 | 26.4 | 22.1 | 26.4 | 38.4 | 32.8 | 27.4 | 21.4 | 26.5 | 31.0 | 27.8 | 23.4 | 22.6 | 25.9 |
| <25 nmol/L, % | 35.6 | 41.3 | 43.9 | 57.0 | 45.3 | 31.4 | 42.1 | 39.1 | 61.9 | 45.6 | 40.5 | 41.0 | 51.9 | 51.0 | 45.9 |
| n | 72 | 166 | 141 | 135 | 514 | 35 | 88 | 87 | 84 | 294 | 37 | 78 | 54 | 51 | 220 |
| Q2 | |||||||||||||||
| Intake, μg/d | 4.0 | 4.0 | 4.1 | 4.0 | 4.1 | 3.8 | 4.1 | 4.4 | 4.2 | 4.2 | 4.0 | 3.8 | 3.7 | 3.8 | 3.8 |
| 25-OHD, nmol/L | 29.5 | 28.1 | 28.1 | 26.0 | 27.8 | 26.6 | 27.0 | 29.6 | 30.1 | 29.6 | 33.5 | 28.0 | 28.8 | 21.9 | 26.6 |
| <25 nmol/L, % | 37.8 | 43.3 | 40.4 | 44.3 | 42.0 | 41.9 | 41.7 | 41.9 | 31.4 | 38.8 | 33.3 | 45.7 | 33.3 | 56.5 | 44.6 |
| n | 76 | 142 | 141 | 155 | 514 | 43 | 72 | 93 | 86 | 294 | 33 | 70 | 48 | 69 | 220 |
| Q3 | |||||||||||||||
| Intake, μg/d | 6.5 | 6.7 | 6.5 | 6.7 | 6.6 | 6.4 | 6.8 | 6.6 | 7.0 | 6.8 | 6.3 | 6.4 | 6.3 | 6.3 | 6.3 |
| 25-OHD, nmol/L | 30.2 | 32.0 | 31.3 | 30.5 | 31.4 | 32.6 | 30.8 | 30.6 | 33.6 | 31.4 | 27.9 | 34.2 | 30.7 | 27.4 | 29.6 |
| <25 nmol/L, % | 30.9 | 29.3 | 33.9 | 35.8 | 32.7 | 28.1 | 28.1 | 35.6 | 32.9 | 32.0 | 32.4 | 32.3 | 37.1 | 43.6 | 36.8 |
| n | 66 | 144 | 163 | 141 | 514 | 32 | 82 | 101 | 79 | 294 | 34 | 62 | 62 | 62 | 220 |
| Q4 | |||||||||||||||
| Intake, μg/d | 12.2 | 12.3 | 12.3 | 13.0 | 12.5 | 13.8 | 12.5 | 12.6 | 13.5 | 12.9 | 11.4 | 11.9 | 11.8 | 12.1 | 11.8 |
| 25-OHD, nmol/L L | 34.9 | 39.3 | 34.1 | 33.8 | 35.2 | 24.0 | 37.3 | 37.3 | 34.5 | 36.2 | 38.8 | 44.4 | 31.4 | 31.6 | 37.2 |
| <25 nmol/L, % | 36.4 | 23.0 | 25.9 | 27.4 | 26.5 | 50.0 | 23.0 | 19.2 | 24.5 | 23.9 | 25.9 | 20.6 | 32.8 | 29.8 | 27.4 |
| n | 45 | 142 | 166 | 161 | 514 | 18 | 74 | 99 | 103 | 294 | 27 | 68 | 67 | 58 | 220 |
| All | |||||||||||||||
| Intake, μg/d | 7.7 | 8.8 | 9.2 | 9.3 | 9.0 | 8.0 | 9.5 | 9.9 | 10.1 | 9.8 | 7.6 | 8.1 | 8.3 | 8.2 | 8.1 |
| 25-OHD, nmol/L | 30.9 | 31.8 | 30.6 | 28.4 | 30.1 | 30.4 | 31.5 | 31.1 | 29.9 | 30.8 | 32.0 | 33.0 | 29.9 | 25.8 | 29.1 |
| <25 nmol/L, % | 35.1 | 34.5 | 35.6 | 40.6 | 36.7 | 36.7 | 33.9 | 33.9 | 37.0 | 35.1 | 33.6 | 35.3 | 38.5 | 45.8 | 38.8 |
| n | 259 | 594 | 612 | 591 | 2056 | 128 | 316 | 381 | 351 | 1176 | 131 | 278 | 231 | 240 | 880 |
Quartiles of vitamin D intake were calculated independently for male, female, and all participants.
Only participants with available information on total vitamin D intake are presented ( = 2056).
For plasma 25-OHD, the variance explained by total vitamin D intake was 5.05% and by outdoor activity was 0.24% in the winter months and 1.15 and 0.56% in the summer months, respectively.
Discussion
A number of different 25-OHD thresholds to define categories of vitamin D deficiency have been published, but a final consensus on what plasma 25-OHD concentration is required for optimal health, skeletal and extra-skeletal, has not yet been reached (12, 15, 29–31). The Institute of Medicine 2011 report, based on available evidence, judged that 97.5% of all individuals meet their needs when their 25-OHD concentration is >50 nmol/L, whereas a concentration of 40 nmol/L is associated with the median requirement in the population (26). In a national sample, 63.4% of individuals had a 25-OHD concentration < 40nmol/L and were at high risk of mild or severe vitamin D deficiency. Only 22.5% of the participants could be classified as having an adequate vitamin D status (25-OHD >50 nmol/L) based upon IOM guidelines.
Bone mineral density decreases with decreasing concentrations of 25-OHD in plasma (31) and osteomalacia and rickets can arise from low concentrations of plasma 25-OHD (22). A concentration of 25 nmol/L is commonly used as the threshold and individuals with lower 25-OHD are at risk of skeletal problems and are regarded as severely deficient; for those, treatment with high doses of vitamin D should be considered (7, 27). As many as one-third of adults in our sample were severely vitamin D deficient.
In Scotland, skin synthesis, normally a potent source of vitamin D, is greatly impaired due to the very low yearly quota of solar radiation (32) and a large additional loss of UVB in the atmosphere given the high latitude (33, 34). Indeed, not even summer sunlight can produce optimal plasma 25-OHD concentrations at UK latitudes (12). These adverse environmental factors coupled with typical indoor lifestyle patterns contribute to the epidemic proportions of vitamin D deficiency in Scotland.
Epidemic hypovitaminosis D in Scotland has been previously reported. However, our study revealed an even higher proportion of vitamin D-deficient individuals. From May to November, Hypponen et al. (15) reported 8.3, 27.5, and 74.9% of individuals with 25-OHD <25, 40, and 75 nmol/L, respectively, whereas we observed 25.2, 53.7, and 92.4%, respectively. The disparity is likely due to the age difference; their cohort included exclusively 45-y-old individuals, whereas the median age in our cohort was 62 y. It is apparent that levels in the Scottish population are very low relative to other populations, such as in Italy or France (35–37).
There are increasing reports of associations between low vitamin D levels and a variety of diseases of public health importance such as cancer (5, 6), cardiovascular disease (2, 3), and diabetes (4), as well as with increased total mortality rates (2, 16). A recent study in the Scottish population (75 y and older) showed that vitamin D status at baseline was inversely related to mortality (38). Although it has not been proven that these associations are causal in nature, it is clear that this merits further research attention. If these associations are causal, then preventing vitamin D deficiency could have the potential to reduce the disease and health care burden from these conditions and this would also have to be considered when defining “optimal” vitamin D status (39).
Factors associated with 25-OHD.
We observed a significant association between total dietary vitamin D intake and vitamin D status. The number of individuals who had higher levels of vitamin D intake was too low to attempt to define a daily intake that could maintain a sufficient vitamin D status in Scotland. In our study, supplement intake was significantly associated with an individual’s plasma 25-OHD and contributed most to the total dietary vitamin D intake. Fish intake was the single most important food source of vitamin D, in contrast to the US and Canada, where fortified dairy products contribute the most.
When assessed together, the absolute level of increase in median plasma 25-OHD and the decrease in the proportion of severely deficient individuals were greater across observed levels of supplementation than across quartiles of outdoor activity. The effect of supplements was greatest in the lowest outdoor activity quartile, emphasizing the value of supplementation for individuals deprived of skin synthesis. With increasing total vitamin D intake, the largest reduction in severe deficiency occurred in the older age groups.
Unlike in the US or Canada, vitamin D food fortification in Scotland is currently optional, except fortification of margarine, which is required (7.5–10 μg vitamin D/100 g end product). In recent years, a selection of vitamin D-fortified products, primarily milk, yogurt, orange juice, and breakfast cereals, became available on the market, but the amount added is highly variable. Current measures of fortification and dietary recommendations of 10 μg/d of vitamin D for individuals over 65 y and zero for everyone else (excluding children and pregnant or lactating women) do not seem to prevent hypovitaminosis D in Scotland, because very few individuals achieve the recommended 25-OHD concentration >40 nmol/L.
A growing body of evidence suggests that even higher supplement doses of vitamin D (20–30 μg/d) are safe and well tolerated (40). In the IOM 2011 report, the upper limit15 for vitamin D intake was set at 100 μg/d for individuals over the age of 14 y. Even doses of 250 μg/d given to breast cancer patients for 4 mo were reported to be safe (41). Our study found a high prevalence of deficiency even among participants in the highest quartile of total vitamin D intake and therefore supports calls for a higher RDA for vitamin D and for the consideration of food fortification. The RDA of 600–800 IU/d (15–20 μg/d) is required to achieve a plasma 25-OHD concentration of 50 nmol/L and is based on the assumption that there is no contribution from skin synthesis (26). This may also be applicable to Scotland given the complete lack of UVB during many months and low synthesis even in the summer months due to the weather. In addition, because food fortification with vitamin D in Scotland is not mandatory, individuals living in Scotland might be getting less vitamin D from food than those living in the US or Canada.
Strengths and limitations of the study.
Plasma 25-OHD concentrations are very stable and unlikely to be affected by time in transport to research centers or low temperature storage (42). Analysis of multiple measurements of the same sample from our cohort was in agreement with laboratory internal quality control data. Because it is very difficult to measure diet, some measurement errors in FFQ-derived vitamin D intakes are expected. The correlation coefficients between FFQ-derived measures of vitamin D intake and weighted dietary record measurements for vitamin D were 0.51 for men and 0.39 for women (19). When using outdoor activity as a proxy for sun exposure, measurement error could arise from the fact that vitamin D synthesis depends strongly on numerous factors, such as atmospheric conditions and time of day, and also some of these activities may be performed indoors. Persons who agreed to participate might have had a healthier lifestyle (participation bias). A relatively large sample size was studied and all samples were treated in the same manner and analyzed in one laboratory only, thereby increasing the reliability of the results.
In view of the limited opportunity for the production of vitamin D in skin and restricted dietary vitamin D intake, most Scottish adults appear to be unable to attain an adequate vitamin D status. Although increasing vitamin D intake from food and supplements significantly but modestly increased the plasma 25-OHD concentration, the decrease in the proportion of severely deficient individuals is notable and important.
Given the epidemic proportions of vitamin D deficiency, the current RDA for vitamin D seems to be insufficient for Scotland and this is reflected in the high proportion of deficient individuals, even among those whose dietary intake is more than the current RDA. Because these results might reflect the situation in other regions with low UVB radiation, such as other high latitude countries (43), our results suggest that the recommended daily vitamin D intake in countries with low UVB exposure should be reviewed.
Supplementary Material
Acknowledgments
L.Z. and E.T. analyzed the data and wrote the manuscript; F.A., K.J., and G.M. analyzed the data; H.C. designed the study and wrote the manuscript; M.D. designed the study; S.K., S.F., R.C., M.W., H.C., M.D., and M.P. collected the data; and G.M., K.J., S.K., and A.M.W. provided consultation in their areas of expertise. All authors read and approved the final manuscript.
Footnotes
Supported by grants from Cancer Research UK (C348/A3758, C348/A8896), the Scottish Government Chief Scientist Office (K/OPR/2/2/D333, CZB/4/94); Medical Research Council (G0000657-53203), and a Centre Grant from CORE as part of the Digestive Cancer Campaign (http://www.corecharity.org.uk). E.T. is funded by Cancer Research UK Fellowship C31250/A10107.
Supplemental Tables 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
On the Fitzpatrick Scale, skin type III is beige in color, sometimes mildly burns, and tans gradually.
RDA is the amount that will cover the needs of 97.5% of the population. It is defined as the level of nutrient required, which is two standard deviations above the Estimated Average Requirement: assuming a normal population distribution, this covers the needs of at least 97.5% of the population and is therefore more than most people will actually need. This is equivalent to UK Reference Nutrient Intake (10).
Estimated Average Requirement (EAR) is the average requirement for a nutrient by a particular group of people. Individual requirements will vary from this mean, some people requiring more and others less than EAR (10).
Upper limit indicates a level above which there is risk of adverse events.
Literature Cited
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 2.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9 [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9 [DOI] [PubMed] [Google Scholar]
- 6.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91 [DOI] [PubMed] [Google Scholar]
- 7.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med. 1989;114:301–5 [PubMed] [Google Scholar]
- 9.Terushkin V, Bender A, Psaty E, Engelsen O, Wang S, Halpern A. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62:929.e1–9 [DOI] [PubMed] [Google Scholar]
- 10.Thomas B, Bishop J. Manual of dietetic practice. Oxford: Blackwell Publishing; 2007 [Google Scholar]
- 11.Department of Health Dietary Reference values for food energy and nutrients for the United Kingdom. London: Stationary Office Books, 1991 [Google Scholar]
- 12.Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, O'Brien SJ, Vail A, Berry JL. Recommended summer sunlight exposure levels can produce sufficient (>/=20 ng ml(-1)) but not the proposed optimal (>/=32 ng ml(-1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130:1411–8 [DOI] [PubMed] [Google Scholar]
- 13.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 15.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8 [DOI] [PubMed] [Google Scholar]
- 16.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–7 [DOI] [PubMed] [Google Scholar]
- 17.Tenesa A, Campbell H, Theodoratou E, Dunlop L, Cetnarskyj R, Farrington SM, Dunlop MG. Common genetic variants at the MC4R locus are associated with obesity, but not with dietary energy intake or colorectal cancer in the Scottish population. Int J Obes (Lond). 2009;33:284–8 [DOI] [PubMed] [Google Scholar]
- 18.The Scottish Collaborative Group Food frequency questionnaire. Scottish Collaborative Group. Internet: http://www.foodfrequency.org (accessed 31 March 2011)
- 19.Masson LF, McNeill G, Tomany JO, Simpson JA, Peace HS, Wei L, Grubb DA, Bolton-Smith C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003;6:313–21 [DOI] [PubMed] [Google Scholar]
- 20.Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684–93 [DOI] [PubMed] [Google Scholar]
- 21.Holland B, Welcha AA, Unwin ID, Buss DH, Paul AA, Southgate DAT. McCance and Widdowson's the composition of foods, 5th editionCambridge, UK: Royal Society of Chemistry, 1991 [Google Scholar]
- 22.Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75:477–88 [DOI] [PubMed] [Google Scholar]
- 23.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:S2030–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox S, Harris J, Calton L, Wallace AM. A simple automated solid-phase extraction procedure for measurement of 25-hydroxyvitamin D3 and D2 by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem. 2009;46:226–30 [DOI] [PubMed] [Google Scholar]
- 25.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009;89:S1997–2008 [DOI] [PubMed] [Google Scholar]
- 26.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, et al. The 2011 Report on Dietary Reference Intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–6 [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010 [Google Scholar]
- 29.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 30.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22 [DOI] [PubMed] [Google Scholar]
- 32.Gies P, Roy C, Javorniczky J, Henderson S, Lemus-Deschamps L, Driscoll C. Global solar UV index: Australian measurements, forecasts and comparison with the UK. Photochem Photobiol. 2004;79:32–9 [PubMed] [Google Scholar]
- 33.Malik AQ. SKC. Computation of solar ultraviolet radiation for Brunei Darussalam. Sci Technol Vis. 2007;3:23–32 [Google Scholar]
- 34.Xinli W, Wei G, John D, Becky O, George J, James S. Dependence of erythemally weighted UV radiation on geographical parameters in the United States. : Wei G, Susan LU, Remote sensing and modeling of ecosystems for sustainability IV. San Diego, CA: SPIE; 2007:667903 [Google Scholar]
- 35.Carnevale V, Modoni S, Pileri M, Di Giorgio A, Chiodini I, Minisola S, Vieth R, Scillitani A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporos Int. 2001;12:1026–30 [DOI] [PubMed] [Google Scholar]
- 36.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43 [DOI] [PubMed] [Google Scholar]
- 37.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21 [DOI] [PubMed] [Google Scholar]
- 38.Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr. 2007;98:593–9 [DOI] [PubMed] [Google Scholar]
- 39.Grant WB, Garland CF, Holick MF. Comparisons of estimated economic burdens due to insufficient solar ultraviolet irradiance and vitamin D and excess solar UV irradiance for the United States. Photochem Photobiol. 2005;81:1276–86 [DOI] [PubMed] [Google Scholar]
- 40.Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, Chandler J, Rosenberg E, Papanicolaou DA. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010;91:985–91 [DOI] [PubMed] [Google Scholar]
- 41.Amir E, Simmons CE, Freedman OC, Dranitsaris G, Cole DE, Vieth R, Ooi WS, Clemons M. A phase 2 trial exploring the effects of high-dose (10,000 IU/day) vitamin D(3) in breast cancer patients with bone metastases. Cancer. 2010;116:284–91 [DOI] [PubMed] [Google Scholar]
- 42.Wielders JP, Wijnberg FA. Preanalytical stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: solid as a rock. Clin Chem. 2009;55:1584–5 [DOI] [PubMed] [Google Scholar]
- 43.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Eriksen EF. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.