Abstract
Organ regenerative therapy aims to reproduce fully functional organs to replace organs that have been lost or damaged as a result of disease, injury, or aging. For the fully functional regeneration of ectodermal organs, a concept has been proposed in which a bioengineered organ is developed by reproducing the embryonic processes of organogenesis. Here, we show that a bioengineered hair follicle germ, which was reconstituted with embryonic skin-derived epithelial and mesenchymal cells and ectopically transplanted, was able to develop histologically correct hair follicles. The bioengineered hair follicles properly connected to the host skin epithelium by intracutaneous transplantation and reproduced the stem cell niche and hair cycles. The bioengineered hair follicles also autonomously connected with nerves and the arrector pili muscle at the permanent region and exhibited piloerection ability. Our findings indicate that the bioengineered hair follicles could restore physiological hair functions and could be applicable to surgical treatments for alopecia.
Regenerative medicine is based on the principles of stem cell biology, developmental biology and regeneration and is expected to replace organ transplantation therapy1,2,3. Almost all ectodermal organs, such as hair follicles, teeth and salivary glands, are developed through reciprocal epithelial-mesenchymal interactions during embryonic organogenesis4,5. For the fully functional regeneration of ectodermal organs, a concept has been proposed in which a bioengineered organ is developed from a bioengineered organ germ, such as a tooth or a hair follicle, by reproducing the organogenesis processes6,7. These organs have a number of physiological roles and contribute to quality of life by preventing the morbidity that is associated with diseases such as caries, hypodontia8 and androgenetic alopecia9. We have demonstrated a novel concept for bioengineered mature organ replacement by which the mature tooth unit, which is generated from bioengineered tooth germ, can restore tooth functions, such as masticatory potential, periodontal ligament function for bone remodeling and responsiveness to noxious stimuli in the oral cavity3,10.
The hair follicle is made up of a permanent region, which consists of the infundibulum and isthmus, and a variable region, known as the hair shaft factory, which includes differentiated epithelial cells, the hair matrix, and dermal papilla (DP) cells11,12. After hair follicle morphogenesis, various stem cells are maintained in the follicle stem cell niches, such as epithelial stem cells (with CD34 and CD49f-positive cell markers) in the bulge region of the permanent portion13,14, neural crest-derived melanocyte precursor cells for hair pigmentation in the sub-bulge region of the follicle permanent region15, and multipotent mesenchymal precursor cells in the DP cells16. The interaction between epithelial stem cells and mesenchymal precursor cells mediates the hair cycle, which depends on the activation of these cells during telogen-anagen transition and the anagen, catagen and telogen phases12,17. The follicular epithelial stem cells retain the ability to differentiate into outer root sheaths, hair matrix, inner root sheaths, and hair shafts during the anagen phase12,13,14. The DP and the dermal sheath cells coordinate to regulate cell proliferation18, cell differentiation and the fate of the hair matrix cells and thus engender different hair types, for example, awl/auchene, guard and zigzag as pelage hairs, and vibrissa in rodents, which are distinguished by hair features such as hardness that are based on the hair shaft structure, length, and diameter, as well as the duration of the hair cycle12,19,20.
Hairs have physiological functions, such as providing thermoregulation and protection against extrinsic insults, and they act as contact sensors through the elongation of the hair shaft, the connection with surrounding muscle and nerve tissues for piloerection, through the enduring hair cycles21. To achieve the fully functional regeneration of hair follicles, many technologies have been developed to reconstruct the variable region of the hair follicle22,23 or de novo folliculogenesis via the self-assembly of epithelial and mesenchymal cells that are isolated from skin and hair follicles24,25. Recently, we have successfully demonstrated that our bioengineered tooth and hair follicle germs, which were regenerated using a three-dimensional cell manipulation method termed the organ germ method26, can orthotopically regenerate a structurally correct and fully functional tooth and hair follicle3,27. For androgenetic alopecia, autologous hair follicle unit transplantation (FUT) has clinically achieved the restoration of proper hair appearance by controlling hair type and density and hair stream via the representation of natural hair orientation through surgical implantation of hair follicles28,29. Thus, the mature bioengineered hair follicle, but not the follicle germ, transplantation model is advantageous for facilitating the replacement of bioengineered hair follicles in the proper orientation through surgical procedures such as FUT therapy28.
In our current study, a bioengineered hair follicle, which was ectopically regenerated and matured with the hair shaft, was transplanted into normal skin using the FUT method. The bioengineered hair follicle autonomously rearranged and connected to the recipient cutaneous tissues, such as the skin epithelium, nerve fibers, and the arrector pili muscle. The bioengineered hair follicles also restored inherent hair physiological functions, such as eruption and growth of the hair shafts from the skin surface, maintenance of the proper hair cycles as a result of reproduction of follicular stem cell niche, and piloerection. Our results indicated that the transplantation of the bioengineered hair follicles can be developed into a viable alternative to conventional FUT therapy for future hair regenerative therapies.
Results
Ectopic hair follicle regeneration from bioengineered hair follicle germs
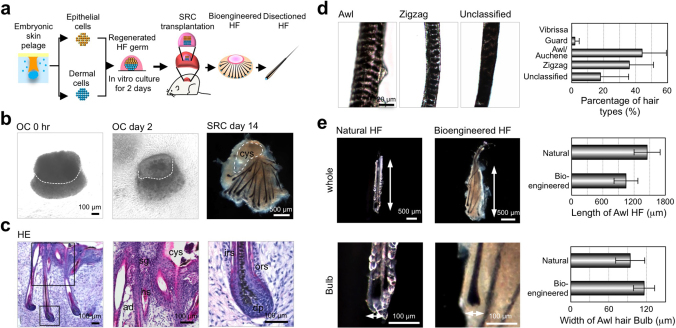
We first analyzed the structure, hair types and sizes of the bioengineered hair follicles, which were ectopically developed from the bioengineered pelage follicle germ using the organ germ method in the subrenal capsule26. Bioengineered hair follicle germs were reconstructed from 7.5×104 enzymatically dissociated epithelial cells and 7.5×104 mesenchymal cells, which were derived from skin from the backs of enhanced green fluorescence protein (EGFP)-transgenic (Tg) mouse embryos at embryonic day (ED) 18 (Fig. 1a and b left in phase contrast photographs). At 2 days after organ cultivation, a translucent zone was observed in the boundary surface between the epithelial and mesenchymal cell layers (Fig. 1b). To promote development into mature hair follicles, the bioengineered hair germs were transplanted into subrenal capsules. At 14 days after transplantation, the hair follicle germ ectopically generated mature hair follicles, which produced the black hair shaft, and the growth directions of each hair were uniform (Fig. 1b, macroscopy). The ectopic bioengineered hair follicles were histologically correct and were made of concentric epithelial layers of outer and inner root sheath (ORS and IRS) and dermal papilla (DP), which were surrounded by hair matrix at the distal end of the bioengineered hair follicles (Fig. 1c). Sebaceous glands attached to the upper portions of the bioengineered hair follicles (Fig. 1c). Morphological analyses of the regenerated hair shafts using light microscopy following a previously reported method29 revealed that the hair types were all of pelage types, including guard, awl/auchene and zigzag, but not vibrissa types (Fig. 1d, left panels). The frequency of each hair type among the bioengineered hairs was 44.0% awl/auchene, 36.2% zigzag, and 2.0% guard (Fig. 1d, right panel). We were not able to classify 15.9% of the bioengineered hairs, which exhibited dense melanin granules. These hairs were similar to the awl/auchene and zigzag types in size, although they showed typical pelage shapes (Fig. 1d). The frequencies of the hair types of the bioengineered pelage follicles were similar to those in the natural pelage follicles at ED18 because zigzag hair follicles are known to be induced after ED18~19, and the frequency of the zigzag hair type is low at ED1820. The size of the bioengineered hair follicles, which is characterized by the length of the follicle and the diameter of the hair bulb, were similar to natural anagen pelages, with no significant differences (Fig. 1e). These results indicated that the bioengineered hair follicle germs that were used to reconstruct embryonic skin epithelial and mesenchymal cells could ectopically produce pelage-type hairs.
Figure 1. Ectopic regeneration of a hair follicle via transplantation of a bioengineered hair follicle germ.
(a) A schematic representation of the method for regenerating the hair follicle via transplantation into the sub-renal capsule (SRC). (b) Morphologic and histological analysis of the bioengineered hair follicle germ. Phase contrast photographs of a hair follicle germ at 0 hr and 2 days after cultivation (left two panels). The dotted lines indicate a boundary between the epithelial and mesenchymal cells in the OC (organ culture) at 0 hr and day 2. Macro-morphological observations of the bioengineered hairs at 14 days after transplantation into the sub-renal capsule (right panel). (c) Histological analysis of the bioengineered hair follicles. The boxed area shows high-magnification in the right two panels. ad, adipocyte; cys, cyst; dp, dermal papilla; hs, hair shaft; irs, inner root sheath; ors, outer root sheath; sg, sebaceous gland. (d) Microscopic observations of the ectopically bioengineered hair shafts classified as awl, zigzag or unclassified hairs. The percentage of regenerated hair types (right). Bars represent standard deviations. (e) A comparison of the length of the hair follicle and the bulb between the natural and regenerated awl hairs.
Regeneration of hair follicles by intracutaneous transplantation
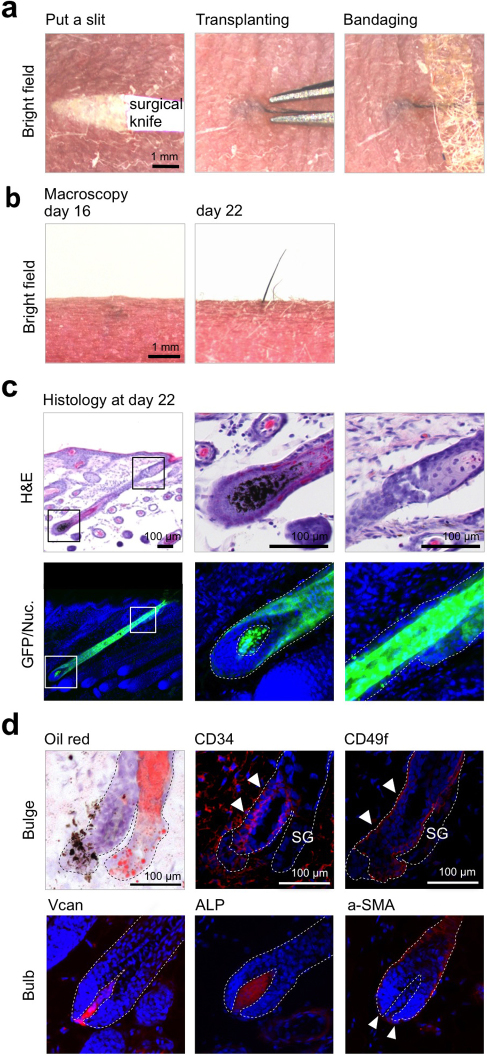
To develop hair regeneration therapies for alopecia, bioengineered hair follicles are promising for intracutaneous transplants and exhibit enduring hair functions, similarly to FUT methods28. Therefore, we investigated whether the isolated a single or a couple of follicular unit of the ectopic bioengineered hair follicles could be properly arranged to resume hair growth and to sustain the proper histological features in the host skin. We have previously established a mouse pelage FUT model for analyzing hair functions such as hair cycle and piloerection ability through the transplantation of adult natural pelage follicle into the adult skin29. A single or a couple of follicular unit of an ectopically reproduced, EGFP-labeled bioengineered hair follicle was isolated and transplanted into a small stab (Fig. 2a, left and center), which was nearly parallel to the host pelage follicle, on the back skin of a nude mouse, according to the FUT method. To connect the bioengineered hair follicle to the recipient skin epithelium, the bioengineered hair shaft held on outside eruption with bandage tape until wound healing (Fig. 2a, right). At 7 to 10 days after the orthotopic transplantation, the wound was completely healed (data not shown). In all cases, the transplanted hair shaft was completely lost at 16 days after transplantation; however, the transplant could be detected by the EGFP signal (data not shown). The eruption of the bioengineered pelage shaft was observed at 14±1.8 (n = 30) days at a frequency of 90% (n = 33; Fig. 2b).
Figure 2. Hair follicle regeneration via intracutaneous transplantation of regenerated bioengineered hair follicles.
(a) Macroscopic observations of the transplantation procedure of the regenerated hair follicles. A slit is made using a surgical knife (left), the regenerated hair follicle is transplanted using forceps (middle), and a surgical tape bandage is used to cover the transplanted hair follicle (right). (b) Macroscopic observations of a transplanted regenerated hair follicle at day 16 (left panels) and day 22 (right panels). (c) Histological analyses of the transplanted regenerated hair follicles. H&E-staining (upper panels) and fluorescent microscopy (lower panels) of the transplanted regenerated hair follicle of a serial section is shown. The boxed areas in the left panels are shown at a higher magnification in the right panels. Broken lines indicate the outermost of the hair follicle. (d) Immunohistochemical analysis of the bulge region in a transplanted regenerated hair follicle. The bulge region was analyzed by immunostaining with specific antibodies for CD34 (upper center) and CD49f (upper right), and the sebaseous gland which was located upper region of the bulge was stained with oil red (upper left). The hair bulb was analyzed by immunostaining with anti-versican (lower left) and α-SMA (arrowheads, lower right) antibodies and by enzymatic staining for ALP (lower center). The nuclei were stained using Hoechst 33258 (Nuc, blue). The arrowheads show positively staining areas.
The bioengineered hair follicle was histologically discriminated by melanin granules in the hair matrix or by EGFP fluorescence from the host hair follicles of nude mice (Fig. 2c). EGFP-labeled cells were limited in the bioengineered hair follicle (Fig. 2c, left). The bioengineered hair follicle was correctly arranged in the recipient skin and connected to the epithelial layer of the recipient skin (Fig. 2c). EGFP-labeled sebaceous glands, positively stained using oil red, regenerated in the upper portion of the bioengineered hair follicle (Fig. 2c, right and d, upper left). The follicular epithelial stem cell markers CD34 and CD49f were positively immunostained in the outer root sheath cells underneath the sebaceous gland (Fig. 2d, upper panels). DP, ALP and versican positive, and also dermal sheath, α-smooth muscle actin (α-SMA) immune-positive thin outermost mesenchymal layer were generated in the bioengineered hair bulb (Fig. 2d, lower panels). These results indicated that an ectopic bioengineered hair follicle can successfully regenerate a hair follicle that is histologically correct, with permanent and variable portions, and that is properly oriented in the skin. We suggest that our bioengineered hair follicles are appropriate for intracutaneous transplants and that they can reproduce the inherent functions of the hair follicle, such as hair cycling and piloerection.
Hair cycling of the bioengineered hair follicles
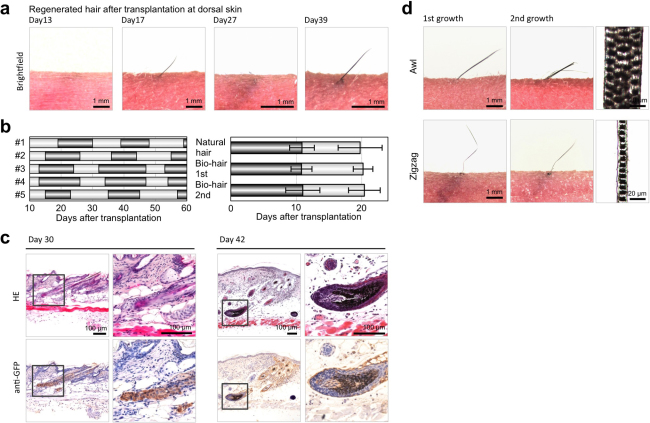
To investigate whether the bioengineered hair follicles orthotopically reproduced hair cycling, we analyzed hair cycles, including the alternating growth (hair-growing) and regression (stop-growing) phases of bioengineered hair shafts that had erupted from the bioengineered follicles for 60 days (Fig. 3). The bioengineered hairs repeatedly exhibited growth and regression (Fig. 3b). Histologically, EGFP-labeled bioengineered hair follicles in the regression phase at day 30 after transplantation corresponded to the catagen phase (Fig. 3c, left panels). In contrast, the bioengineered hair follicles at 42 days after transplantation corresponded to the anagen phase in the 2nd growth cycle (Fig. 3c, right panels). The periods of growth and regression of bioengineered hair follicles lasted 11.0 (±2.6) days and 9.4 (±2.4) days, respectively (Fig. 3b). There were no significant differences in the hair cycle periods between natural and bioengineered follicles (Fig. 3b, right). Furthermore, the bioengineered awl/auchene and zigzag pelage follicles, which were isolated from the ectopically developed bioengineered pelage follicles based on their morphological features, maintained those hair types up to the 2nd growth cycle (Fig. 3d). Thus, these results indicated that a bioengineered hair follicle can reproduce and sustain proper hair cycles and hair types consistent with the fate of the ectopically bioengineered hair follicle. It has been suggested that ectopic bioengineered hair follicles have the potential to maintain stem cells and to provide a functional stem cell system.
Figure 3. Hair cycles of transplanted regenerated hair follicle ectopically.
(a) The macroscopic analyses of various phases of the hair cycles. The bioengineered hair follicles were observed at 13 days, 17 days, 27 days and 39 days after transplantation. (b) Cycles of hair growth (dark bars) and regression (light bars) phases. The left panel shows the term of growth and regression phases of the individual regenerated hairs until 60 days after transplantation. The right panel shows the hair cycle term of natural hair (Natural hair) and the transplanted regenerated hair (Bio-hair) in the 1st and 2nd hair cycles. The error bars indicate the s.e.m. (n = 8 in natural hair, n = 5 in transplanted regenerated hair). (c) Histological and immunohistochemical analyses of the transplanted regenerated hair follicles at day 30 and day 42. H&E-staining (upper panels) and immunostaining with anti-GFP (lower panels) of a transplanted regenerated hair follicle of a serial section is shown. The boxed areas in each panel are shown at a higher magnification in the right panels. (d) Macroscopic observations of transplanted regenerated Awl hair (upper, at 1st and 2nd growth term) and zigzag hair (lower, at 1st and 2nd growth term). The right panels show macro-morphological images of each hair follicle at day 43 and day 49.
Reproduction of piloerection of the bioengineered hair follicle
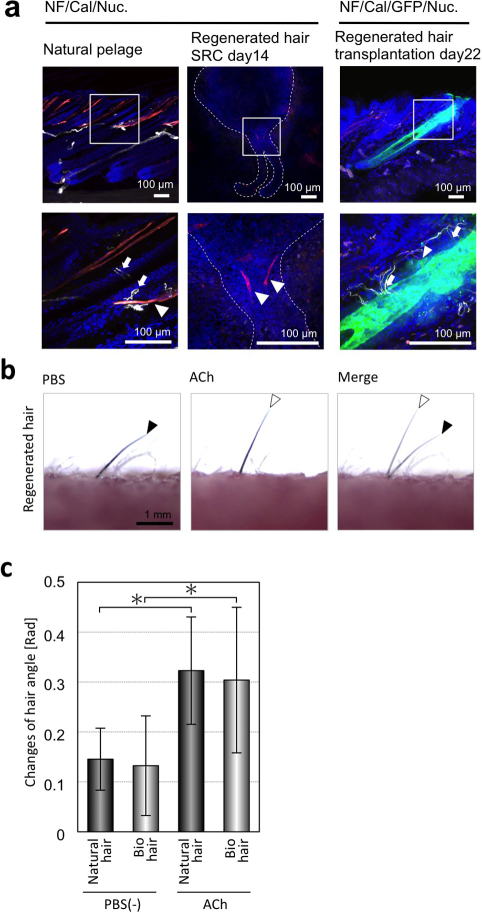
To achieve functional hair follicle regeneration, it is essential that the engrafted follicle is able to autologously connect with the arrector pili muscle and make nerve connections and that it exhibits the ability for piloerection30. We thus performed immunohistochemical staining to analyze the connections between the bioengineered hair follicles and the arrector pili muscle and nerve fibers using antibodies against calponin for smooth muscle, troponin for striated muscle and neurofilament H for nerve fibers. At 14 days after transplantation into the sub-renal capsule, calponin-positive filamentous cells were detected in the upper region of the follicles. However, nerve fibers could not been found (Fig. 4a, middle panels). In contrast, both the arrector pili muscle and the nerve fibers connected to the bulge region of a bioengineered hair follicle that was orthotopically transplanted into the skin, similarly to natural pelage (Fig. 4a, left and right panels).
Figure 4. Piloerection ability of ectopically transplanted regenerated hair follicles.
(a) Immunohistochemical analysis of the regenerated hair follicles in the sub-renal capsule at day 14 (center) and 22 days after transplantation into the back skin (right) and natural back skin (left). Specific antibodies against calponin (Cal, to detect smooth muscle, red) and neurofilament H (NF, white) were used. The boxed areas in each panel are shown at a higher magnification in the lower panels. The nuclei were stained using Hoechst 33258 (Nuc, blue). The arrows and arrowheads show positively stained calponin and NF-H areas. (b) Analyses of the piloerection ability of the transplanted regenerated hair follicles following an intradermal injection of ACh. The positions of the hair shafts (black arrowheads in left) moved after this treatment (white arrowheads in center) and merged (right). (c) Assessment of hair angle changes associated with regenerated hair (light bars) and natural hair (dark bars) before (PBS(-)) and after the administration of ACh. The error bars represent the standard deviation (n = 6). P<0.05 (*) was regarded as statistically significant.
Finally, we investigated the piloerection ability of the bioengineered hair follicles in the skin. Acetylcholine (Ach; 1 µg/site) was administered intradermally in the vicinity of the engrafted follicles, and the angles of the hair shafts before and after treatment were calculated (Fig. 4b). ACh injection led to a significantly increased angle of piloerection in the bioengineered pelage compared to a control (Fig. 4b and c). In contrast, an anti-cholinergic agent, atropine (AT), inhibited this effect (Fig. 4c). These results indicated that the bioengineered hair follicles reproduced the piloerection ability equivalent to that of natural follicles.
Discussion
In this study, we demonstrated that bioengineered hair follicles generated by ectopic transplantation can functionally replace orthotopic FUT therapy. The bioengineered hair follicles reproduced the hair cycle through the rearrangement of stem cell niches for epithelial stem cells and mesenchymal precursor cells in the DP. The bioengineered hair follicle autonomously connected properly to the arrector pili muscle and nerve fibers and showed piloerection in the skin environment. These findings indicated the potential of functional hair follicle regenerative therapy for alopecia by using the bioengineered hair follicles for transplantation.
Currently, to fully restore organ functions, the replacement of dysfunctional and/or missing organs in a recipient by a healthy donor organ is essential and applies to various organs, such as the heart, liver, kidneys and intestine10,31. Organ regenerative therapy, in which a fully functional mature bioengineered organ is reconstructed using a three-dimensional arrangement of various stem/progenitor cells and engrafted to restore organ functions32, is expected to emerge as an attractive alternative. For androgenetic alopecia, a hair follicle is one of the most successful targets for autologous organ replacement therapy using FUT28. Various methods of de novo hair regeneration that are centered on the common concept of reproducing the epithelial-mesenchymal interactions during organogenesis or the early anagen phase of the hair cycle have been reported22,23,24,25,26. These previous studies showed that the dissociated trichogenic epithelial and mesenchymal cells autonomously formed cell aggregates for follicle germs in the cell mixture and then generated hair follicles without regulation of the growth polarity or the density of the regenerated hair follicles in the skin24,25. In this current study, we successfully demonstrated that our bioengineered follicle germs ectopically regenerated a structurally correct hair follicle that was suitable for implantation into the back skin of mice via the FUT method. Bioengineered hair follicles are reproducible, and their quality-control can be easily managed. These findings suggest that our bioengineering technology and bioengineered follicles have the potential to be applied to hair regenerative therapy.
Critical issues to be considered in hair regenerative therapy include whether the bioengineered hair follicles can regenerate normal inherent traits and enduringly maintain those of physiological functions according to their fate determination11,33. Hair follicles repetitively reproduce and regress to maintain their constitutive hair functions, such as protection against the ambient environment, thermoregulation, and maintenance of sensory functions21,30,34,35. In autologous FUT therapy for androgenetic alopecia, an occipital normal hair follicle enduringly reproduces the normal traits of the hair shaft, including strength, diameter, shape, and color, and the duration of the hair cycle independently of surrounding skin environment29,28. The maintenance of hair traits and the enduring hair cycle are attributed to follicular stem cells and their niches, which are formed through follicular morphogenesis during embryonic organogenesis and are retained over the lifetime of the animal2,11,13,14. A bulge region of hair follicle in which the follicular epithelial stem cell niche is located provides stem cells not only to the hair follicle but also to the sebaceous gland and the epidermis after traumatic injury via connections with the basal layers of the outer root heath and the skin epidermis2,11,13,36. The follicular mesenchymal components, the DP and the dermal sheath coordinate to induce epithelial stem cells in the variable region of the hair follicle and to regulate the various types of hair shafts18,19,22,23. Therefore, the essential issues of functional hair regeneration are the arrangement of the bioengineered hair follicles in the skin, the reproduction of follicular stem cell niches and the maintenance of the hair cycle4. In our current study, we showed that the ectopic bioengineered pelage follicle connected to the epidermal layer of the skin, reproduced the stem cell niche and the hair cycle equivalent to the natural pelage, and repeatedly produced the same hair types during the hair cycles. Our findings suggest the possibility that the ectopic bioengineered hair follicle can regenerate enduring hair follicles and sustain the fate of hair types.
In the skin, hair follicles, pelage and vibrissa derive their piloerection ability and whiskering movements from positionally proper connections with selective muscle tissue, smooth muscle in the case of the pelage and striated muscle for the vibrissa, and they coordinately function as a sensory organ30,33,34,35,37. The sensory nerve and arrector pili muscle connections are selectively located around the bulge region of the permanent portion of the pelage follicle and develop at the bulbous hair peg stage during follicular morphogenesis35,37. The sensory nerves also play an essential role for maintaining the epithelial stem cell niche through a Hedgehog pathway38. It is assumed that the sensory nerve ending is induced and attached by merkel cells in the hair follicle30,34,35. Furthermore, it was reported that the arrector pili muscle was induced by the bulged epithelial cells of the pelage, in which it regioselectively secreted an extra cellular matrix protein, nephronectin37. Thus, critical issues for consideration in hair regenerative therapy include whether the bioengineered hair follicle can establish coordinating functions in adult skin through autologous correct connections between the nerve and muscle29. In our current study, we provide evidence that the bioengineered pelage properly connected to nerves and the arrector pili muscle at appropriate locations in the bulge region, and they exhibited ACh-induced piloerection ability. Our findings suggest that the orthotopic transplantation of the bioengineered hair follicles can restore hair functions and thus are applicable to future surgical treatments for alopecia.
In conclusion, this study demonstrated fully functional regeneration using ectopically bioengineered hair follicles transplanted via the FUT method that are practical for clinical therapies. Our study has made substantial advances in the development of a novel therapeutic method for hair follicle regenerative therapy for alopecia and organ replacement regenerative therapy. Future studies of in vitro culture systems that can generate bioengineered hair follicles with hair shafts from bioengineered hair follicle germs are expected to promote therapeutic systems like FUT in the clinic.
Methods
Preparation and cultivation of bioengineered hair germ
E18 mouse embryonic back skin was aseptically treated with 4.8 U/ml dispase II (BD, Franklin Lakes, NJ) and divided into epithelial and mesenchymal layers. To prepare the epithelial cells, the epithelial layer was treated with 100 U/ml collagenase (Worthington, Lakewood, NJ) twice for 40 min and treated with 0.25% trypsin (Invitrogen) for 10 min at 37°C. To prepare the mesenchymal cells, the dermal layer was treated with 10,000 U/ml collagenase (Worthington) for one hour at 37°C. Debris and undissociated tissues were removed from the dissociated epithelial cells using a cell strainer (35 µm mesh, BD). The bioengineered hair follicle germs were reconstituted between the epithelial and dermal cells portions according to the organ germ method, as reported previously26. The bioengineered hair germs were placed on a cell culture insert (0.4 mm pore diameter; BD), and then incubated at 37°C for 2 days in DMEM10.
Animals
C57BL/6 mice were purchased from CLEA Japan Inc. (Tokyo, Japan). C57BL/6-TgN (act-EGFP) mice were purchased from Japan SLC Inc. (Shizuoka, Japan). C57BL/6-TgN (act-EGFP) OsbC14-Y01-FM131 mice were obtained from RIKEN Bioresource Center (Tsukuba, Japan). Mouse care and handling conformed to the NIH guidelines by the Tokyo University of Science Animal Care and Use Committee. These studies were approved by the Tokyo University of Science.
Transplantation of the bioengineered hair follicle germ into the sub-renal capsule
To develop and mature bioengineered hair follicles, bioengineered hair follicle germ was transplanted into the sub-renal capsules of 8-week-old C57BL/6 mice, as previously described26. At 14 days after sub-renal engraftment, mature bioengineered hair follicles were harvested and dissected into a single or a couple of follicular unit via stereomicroscopic observation.
Intracutaneous transplantation of the bioengineered hair follicles
Six-week-old Balb/c nu/nu mice were anesthetized with pentobarbital by intraperitoneal injection. Shallow stab wounds were made using a 20G Ophthalmic V-Lance (Alcon Japan, Tokyo, Japan) on the back skin of the nude mice, and the hair follicle was intracutaneously grafted. The hair shafts were held for eruption from the wound and were covered with surgical bandage tape (Nichiban, Tokyo, Japan).
Immunohistochemistry
Paraffin sections (5 μm) were stained with hematoxylin-eosin and observed using Axioimager A1 (Carl Zeiss) with AxioCAM MRc5 (Carl Zeiss) microscopes. For fluorescence immunohistochemistry, the frozen sections (10 μm) were blocked in 1% BSA (SIGMA) and 0.01% Triton X-100 (SIGMA) in TBS for 1 hour at room temperature and then incubated overnight in primary antibodies at 4°C. The primary antibodies included versican (rabbit, Milipore, Billerica, MA), αSMA (rabbit, Epitomics, Burlingame, CA), integrin α6 (rat, abcam, Cambridge, MA), and CD34 (rat, abcam) in blocking solution. The primary antibodies were detected using the secondary antibody goat anti-IgG (H+L) Alexa Fluor 594 highly cross-absorbed (Invitrogen) in blocking solution for 3 hour at room temperature together with Hoechst 33258 (Dojindo, Kumamoto, Japan). For fluorescence immunohistochemistry, the frozen sections (100 μm) were blocked in 1% BSA and 0.5% Triton X-100 (SIGMA) in TBS for 2 hour at room temperature and were then incubated overnight with primary antibodies at 4°C. The primary antibodies included neurofilament-H (rat, CHEMICON), calponin (rabbit, abcam), and TNNI1 (rabbit, abcam) in blocking solution. Primary antibodies were detected using the following secondary antibodies: goat anti- IgG (H+L) Alexa Fluor 633 (Invitrogen) and goat anti-IgG (H+L) Alexa Fluor 594 (Invitrogen) in blocking solution for 3 hour at room temperature together with 4 µg/ml of Hoechst 33258 (Dojindo) in PBS(-). All fluorescence microscopy images were captured under a confocal microscope (LSM 780; Carl Zeiss).
Piloerection of the transplanted hair
To investigate whether the bioengineered hairs could reproduce piloerection ability, the effects of a neurotransmitter agent and piloerection inhibitors were examined using a method reported previously29,39. Statistical significance was determined using an unpaired Student's t-test. All analyses were conducted using the Common Gateway Interface Program (twk, Saint John's University).
Author Contributions
T. Tsuji and K.T. designed the research plan; K.A., N.I., H.T., A.I., T.K., T.H., K.N., H.T. and S.N. performed the experiments; K.A., K.T., A.S. and T. Tsuji discussed the results; and K.A., K.T. and T. Tsuji wrote the paper.
Acknowledgments
We thank M. Okabe (Osaka University) for providing the C57BL/6-TgN (act-EGFP) OsbC14-Y01-FM131 mice.
References
- Brockes J. P. & Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 310, 1919–1923 (2005). [DOI] [PubMed] [Google Scholar]
- Watt F. M. & Hogan B. L. Out of Eden: Stem cells and their niches. Science. 287, 1427–30 (2000). [DOI] [PubMed] [Google Scholar]
- Ikeda E. et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Nat. Acad. Sci. USA. 106, 13475–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M., Wu P., Plikus M., Jiang T. X. & Bruce W. R. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr. Top. Dev. Biol. 72, 237–274 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J. & Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 262, 195–205 (2003). [DOI] [PubMed] [Google Scholar]
- Sharpe P. T. & Young C. S. Test-tube teeth. Sci Am. 293, 34–41 (2005). [DOI] [PubMed] [Google Scholar]
- Duailibi S. E., Duailibi M. T., Vacanti J. P. & Yelick P. C. Prospects for tooth regeneration. Periodontol 2000. 41, 177–187 (2006). [DOI] [PubMed] [Google Scholar]
- Nieminen P. Genetic basis of tooth agenesis. J. Exp. Zool. B. Mol. Dev. Evol. 312, 320–42 (2009). [DOI] [PubMed] [Google Scholar]
- Randall V. A. et al. Hormones and hair growth: variations in androgen receptor content of darmal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. J. Invest. Dermatol. 101, 114S–120S (1993). [DOI] [PubMed] [Google Scholar]
- Oshima M. et al. Functional Tooth Regeneration Using a Bioengineered Tooth Unit as a Mature Organ Replacement Regenerative Therapy. PLoS ONE. 6, e21531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. H. The secret life of the hair follicle. Trends Genet. 8, 55–61 (1992). [DOI] [PubMed] [Google Scholar]
- Stenn K. S. & Paus R. Controls of hair follicle cycling. Physiol. Rev. 81, 449–94 (2001). [DOI] [PubMed] [Google Scholar]
- Oshima H., Rochat A., Kedzia C., Kobayashi K. & Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 104, 233–45 (2001). [DOI] [PubMed] [Google Scholar]
- Blanpain C., Lowry W. E., Geoghegan A., Polak L. & Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118, 635–48. (2004). [DOI] [PubMed] [Google Scholar]
- Nishimura E. K., Granter S. R. & Fisher D. E. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 307, 720–24 (2005). [DOI] [PubMed] [Google Scholar]
- Jahoda C. A., Whitehouse J., Reynolds A. J. & Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 12, 849–59 (2003). [DOI] [PubMed] [Google Scholar]
- Greco V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 4, 155–69 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao M. et al. Contact between dermal papilla cells and dermal sheath cells enhances the ability of DPCs to induce hair growth. J. Invest. Dermatol. 130, 2707–18 (2010). [DOI] [PubMed] [Google Scholar]
- McElwee K. J. et al. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Invest. Dermatol. 121, 1267–75 (2003). [DOI] [PubMed] [Google Scholar]
- Driskell R. R., Giangreco A., Jensen K. B., Mulder K. W. & Watt F. M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 136, 2815–23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M. Morphogenesis of epithelial appendage. in Molecular basis of epithelial appendage morphogenesis. (ed. Chuong, C. M.) (R. G. Landes Company, Austin, Texas) (1998). [Google Scholar]
- Jahoda C. A., Horne K. A. & Oliver R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 311, 560–62 (1984). [DOI] [PubMed] [Google Scholar]
- Jahoda C. A., Oliver R. F., Reynolds A. J., Forrester J. C. & Horne K. A. Human hair follicle regeneration following amputation and grafting into the nude mouse. J Invest Dermatol. 107, 804–07 (1996). [DOI] [PubMed] [Google Scholar]
- Weinberg W. C. et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J. Invest. Dermatol. 100, 229–36 (1993). [DOI] [PubMed] [Google Scholar]
- Lichti U., Anders J. & Yuspa S. H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 3, 799–810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K. et al. The development of a bioengineered organ germ method. Nat Methods. 4, 227–30 (2007). [DOI] [PubMed] [Google Scholar]
- Toyoshima K. E. et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat commun. 3, 784 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger W. et al. Hair Transplantation, Fifth Edition (2010). [Google Scholar]
- Sato A. et al. Single follicular unit transplantation reconstructs arrector pili muscle- and nerve-connections and restores functional hair follicle piloerection in preparing. J. Dermatol. 39, 1–6 (2012). [DOI] [PubMed] [Google Scholar]
- Peters E. M. et al. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J. Invest. Dermatol. 116, 236–45 (2001). [DOI] [PubMed] [Google Scholar]
- Fiegel H. C. et al. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J. Cell Mol. Med. 12, 56–66 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C. M., Cotsarelis G. & Stenn K. Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol. 127, 2098–2100 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner H., Requena L., Rütten A. & Mentzel T. Spindle cell predominant trichodiscoma: a fibrofolliculoma/trichodiscoma variant considered formerly to be a neurofollicular hamartoma: a clinicopathological and immunohistochemical analysis of 17 cases. Am. J. Dermatopathol. 28, 1–8 (2006). [DOI] [PubMed] [Google Scholar]
- Dorfl J. The innervation of the mystacial region of the white mouse: A topographical study. J. Anat. 142, 173–184 (1985). [PMC free article] [PubMed] [Google Scholar]
- Peters E. M. et al. Developmental timing of hair follicle and dorsal skin innervation in mice. J. Comparative Nerology. 448, 28–52 (2002). [DOI] [PubMed] [Google Scholar]
- Ito M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 447, 316–20 (2007). [DOI] [PubMed] [Google Scholar]
- Fujiwara H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 144, 577–89 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I., Guevara E., Brain B. C., Loomis C. A. & Joyner A. L. Nerve-Derived Sonic Hedgehog Defines a Niche for Hair Follicle Stem Cellls Capable of Becoming Epidermal Stem Cells. Cell Stem Cell. 6, 552–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann K. The isolated pilomotor muscles as an in vitro preparation. J. Physiol. 169, 603–20 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]