Abstract
Introduction
Visfatin is an adipokine secreted by visceral adipose tissue with insulin-mimetic properties. Higher circulating visfatin levels were reported in type 2 diabetes. The aim of this study was to analyse circulating visfatin and insulin levels and the visfatin/insulin ratio in obese women with and without metabolic syndrome (MetS).
Material and methods
The study involved 92 obese women. Subjects were diagnosed with MetS according to IDF 2005 criteria. The MetS group consisted of 71 subjects (age: 52.8 ±9.4 years, body mass index [BMI]: 39.1 ±5.6 kg/m2, waist circumference: 109.6 ±11.4 cm and fat mass: 52.0 ±12.8 kg) while the non-MetS group consisted of 21 subjects (age: 51.7 ±9.5 years, BMI: 36.3 ±5.2 kg/m2, waist circumference: 104.7 ±11.0 cm and fat mass: 45.2 ±10.7 kg). In addition to anthropometric measurements and assessment of serum glucose and lipids, plasma concentrations of visfatin were estimated by enzyme-linked immunosorbent assay (ELISA) and of insulin by radioimmunoassay (RIA). Homeostatic model assessment insulin resistance (HOMA-IR) and visfatin/insulin ratio were calculated.
Results
In the MetS group significantly higher (p < 0.01) plasma concentrations of insulin and HOMA-IR values but similar visfatin levels were observed than in the non-MetS group. As a consequence of the significantly higher plasma insulin concentration the visfatin/insulin ratio was significantly lower in the MetS group (p < 0.05). The visfatin/insulin ratio correlated inversely with anthropometric parameters such as body mass, BMI, body fat and waist circumference (r = –0.41, p = 0.0003; r = –0.42, p = 0.0002; r = –0.29, p = 0.01; r = –0.23, p = 0.04, respectively).
Conclusions
We conclude that the visfatin/insulin ratio declining with increasing visceral obesity may predispose to the development of insulin resistance.
Keywords: visfatin, insulin, obesity, metabolic syndrome
Introduction
Visfatin is an adipokine predominantly secreted by visceral adipose tissue and its release is upregulated in obese animals and humans [1, 2]. Its expression in visceral and subcutaneous adipose tissue is inversely related to body mass index (BMI) changes. In normal weight, insulin sensitive subjects, visfatin is predominantly produced by subcutaneous adipose tissue [3]. In contrast, in the obese subjects, high synthesis of visfatin by macrophages in visceral adipose tissue was observed [3–6].
Visfatin may act as an autocrine, paracrine and endocrine mediator and participate in the regulation of a variety of physiological functions including cell proliferation, synthesis of nicotinamide mono- and dinucleotide and glucose homeostasis [7].
Experimental studies have revealed that visfatin has insulin-mimetic properties. This adipokine binds to the insulin receptor at a site distinct from insulin. By this mechanism visfatin reduces glucose release from hepatocytes and stimulates glucose utilization in adipocytes and myocytes [1].
Our recent study [8], similarly to others previously published [2, 9, 10], reported an increased circulating visfatin level in the obese subjects. Moreover, in type 2 diabetic patients increased circulating visfatin levels have also been observed [11–13]. Based on these results it was suggested that the increasing visfatin level may serve as a compensation for the impairment of insulin action [6]. However, data concerning the association between circulating levels of visfatin and insulin resistance are inconsistent [11–15].
The pathogenesis of insulin resistance in humans is complex. Therefore, a simple link between circulating level of visfatin and insulin resistance is difficult to establish. As previously mentioned, visfatin has insulin-mimetic properties. However, visfatin also stimulates production and release of proinflammatory cytokines by monocytes and macrophages, such as tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6). These cytokines are involved in the pathogenesis of insulin resistance [6]. Therefore, an increased visfatin level in the obese may also indirectly participate in the development of insulin resistance.
It was also found that visfatin is highly expressed in lipid-loaded macrophages in atherosclerotic lesions, particularly in plaques of symptomatic patients [16].
To shed more light on the putative role of visfatin in the development of insulin resistance in MetS, we measured the circulating levels of visfatin and insulin in a subset of obese subjects matching or not matching the International Diabetes Federation (IDF) 2005 criteria of MetS. We hypothesised that MetS is characterized by an imbalance between circulating visfatin and insulin levels.
Material and methods
Ninety-two obese women with stable weight during the last 3 months were included in the study. Their obesity had lasted for at least some years. Patients with weight loss or gain above 3 kg during 6 months, chronic inflammatory diseases, type 2 diabetes, treated hypertension, acute and gastrointestinal diseases, pregnancy, any medication, drug abuse, smoking and consumption of more than 2 alcoholic drinks per week were excluded. The study was approved by the Ethics Committee of the Medical University of Silesia. All subjects gave their informed consent for participation in the study.
Seventy-one subjects fulfiled the IDF 2005 diagnostic criteria of MetS (MetS group), while 21 others did not (non-MetS group). Characteristics of study groups are presented in Table I.
Table I.
Characteristics of obese women fulfilling (MS) or not fulfilling (non-MS) the diagnostic criteria of metabolic syndrome (MS)
| Parameter | MetS (n = 71) | Non-MetS (n = 21) | Value of p |
|---|---|---|---|
| Age [years] | 52.8 ±9.4 | 51.7 ±9.5 | NS |
| Weight [kg] | 100.9 ±15.0 | 94.3 ±13.9 | NS |
| BMI [kg/m2] | 39.1 ±5.6 | 36.3 ±5.2 | NS |
| Body fat [kg] | 52.0 ±12.8 | 45.2 ±10.7 | < 0.05 |
| Body fat [%] | 50.8 ±6.3 | 47.4 ±6.9 | NS |
| Fat-free mass [kg] | 48.9 ±5.8 | 49.1 ±9.0 | NS |
| Fat-free mass [%] | 49.2 ±6.5 | 52.6 ±6.7 | NS |
| Waist circumference [cm] | 109.6 ±11.4 | 104.7 ±11.0 | NS |
| Systolic blood pressure [mm Hg] | 144.7 ±20.8 | 131.9 ±13.6 | < 0.05 |
| Diastolic blood pressure [mm Hg] | 86.8 ±11.9 | 79.7 ±10.4 | NS |
| Glucose [mg/dl] | 114 ±27 | 89 ±9 | < 0.005 |
| Total cholesterol [mg/dl] | 237 ±51 | 205 ±44 | < 0.05 |
| LDL cholesterol [mg/dl] | 160 ±59 | 127 ±44 | NS |
| HDL cholesterol [mg/dl] | 46 ±13 | 60 ±15 | < 0.01 |
| Triglycerides [mg/dl] | 154 ±71 | 86 ±25 | < 0.005 |
BMI – body mass index, LDL – low density lipoprotein, HDL – high density lipoprotein
Anthropometric measurements (weight, height, waist circumference) and blood pressure measurements were performed in all subjects. The BMI was calculated according to the standard formula. Body composition was assessed by the bioimpedance method using a Bodystat 1500 analyzer (Douglas, Isle of Man).
Venous blood samples were withdrawn in the morning between 8.00 a.m. and 9.00 a.m., after overnight fasting (16 h). The blood samples were collected according to the recommendations of kit manufacturers. Serum and plasma samples were stored frozen at –70°C.
Laboratory procedures
Plasma glucose, triglycerides and total cholesterol and its LDL (low density lipoproteins) and HDL (high density lipoproteins) fractions were measured by colorimetric methods using a commercially available kit (Roche, Switzerland).
Serum insulin concentration was assessed by radioimmunoassay (DPC Diagnostic Products Corporation, Los Angeles, USA) with a lower limit of sensitivity of 1.2 µIU/ml and intra- and inter-assay coefficients of variations of 5.2% and 5.8%, respectively. The HOMA-IR index was calculated with the standard formula: HOMA-IR = fasting concentration of insulin [µIU/ml] × fasting concentration of glucose [mmol/l]/22.5 (normal ratio < 2.77).
The plasma concentration of visfatin was estimated by a commercially available ELISA kit from Phoenix Pharmaceuticals (Burlingame, USA) with the lower limit of sensitivity of 2.63 ng/ml and intra- and inter-assay coefficients of variations of 5.2 and 5.8% respectively.
The visfatin/insulin ratio was calculated for plasma insulin and visfatin concentrations expressed in pmol/l (Table II).
Table II.
Plasma concentrations of visfatin and insulin and their ratios and HOMA-IR in obese women fulfilling (MS) or not fulfilling (non-MS) the diagnostic criteria of metabolic syndrome (MetS)
| Parameter | MetS | Non-MetS | Value of p |
|---|---|---|---|
| Insulin [µIU/ml] | 16.6 ±10.9 | 8.8 ±4.6 | 0.01 |
| HOMA-IR | 5.5 ±4.3 | 2.0 ±1.2 | 0.0001 |
| Visfatin [ng/ml] | 33.6 ±12.6 | 29.8 ±8.7 | NS |
| Visfatin/insulin ratio [pmol/l] | 9.3 ±7.3 | 12.5 ±4.9 | 0.005 |
HOMA-IR – homeostasis model assessment insulin resistance
Statistical analysis
All statistical analyses were performed using Statistica 8.0 software (Statsoft Poland, Krakow) Results are presented as means ± SD. The Mann-Whitney U test (two-tailed) was used for group comparisons. The univariate correlation coefficients were calculated according to Spearman. A p < 0.05 was considered as significant.
Results
Both groups of obese women, MetS and non-MetS, were of similar age, and had comparable body mass, BMI, waist circumference and fat free mass (Table I). Only the content of fat mass expressed in kg was higher in the MetS than in the non-MetS group (Table I).
As expected, serum concentrations of total cholesterol, triglycerides, glucose and insulin and HOMA-IR value were higher and concentration of HDL cholesterol was lower in the MetS than in the non-MetS group (Tables I and II).
Plasma concentrations of visfatin were only slightly (by 12.8%), not significantly higher in the MetS than in the non-MetS group. As a consequence of the significantly higher plasma insulin concentration the visfatin/insulin ratio was also significantly lower in the MetS group (Table II).
There was a significant positive correlation between concentrations of visfatin and triglycerides, insulin or HOMA-IR values (r = 0.45, p = 0.04; r = 0.53, p = 0.01; r = 0.53, p = 0.01, respectively) in the non-MetS but not in the MetS group.
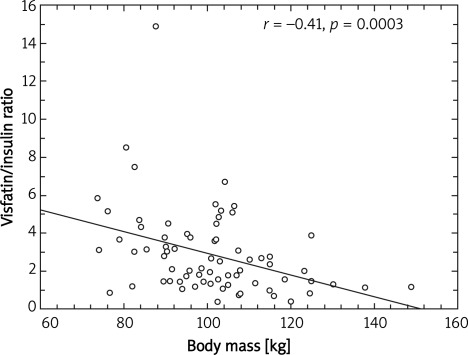
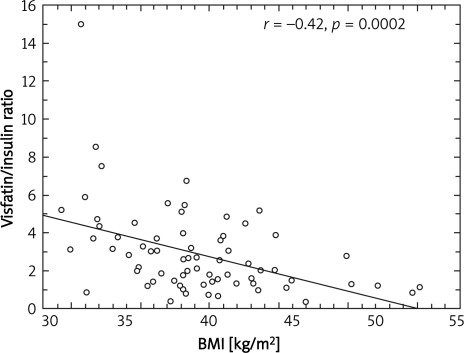
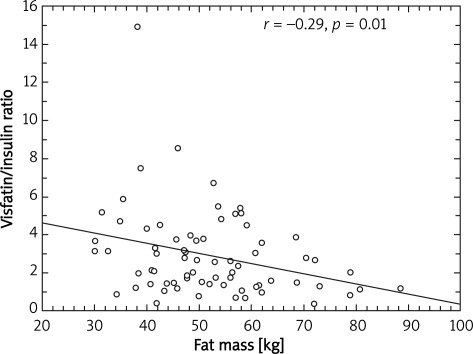
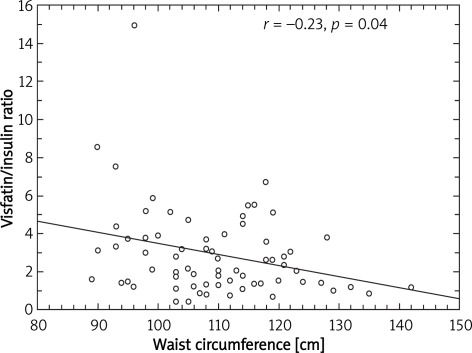
A significant negative correlation between visfatin/insulin ratio and body mass (r = –0.41; p = 0.0003) (Figure 1), BMI (r = –0.42; p = 0.0002) (Figure 2), fat mass (r = –0.29; p = 0.01) (Figure 3) and waist circumference (r = –0.23; p = 0.04) (Figure 4) only in the MetS group was found.
Figure 1.
Correlation between body mass and visfatin to insulin ratio
Figure 2.
Correlation between BMI and visfatin to insulin ratio
Figure 3.
Correlation between fat mass and visfatin to insulin ratio
Figure 4.
Correlation between waist circumference and visfatin to insulin ratio
There was no correlation between visfatin/ insulin ratio and serum concentrations of glucose and lipids in both analysed groups.
Discussion
Our results demonstrate that plasma concentration of visfatin increases along with rising insulin resistance, but the increase is less pronounced than that of insulin.
An association between insulin resistance and visfatin level was not established. Our previously published study [8] revealed an increased plasma visfatin level in obese women, and a significant positive correlation between plasma glucose (but not insulin) and visfatin concentrations in this group. Serum concentrations of insulin correlated positively with those of visfatin only in normal weight subjects. Therefore, we hypothesized that an increased circulating visfatin level in the obese may be one of the compensatory mechanisms at the early stage of the development of insulin resistance. The reported positive correlation between plasma visfatin levels and HOMA-IR supports our hypothesis [13]. Chen et al. [13] proposed that elevated visfatin levels in subjects with type 2 diabetes mellitus may reflect the impairment of visfatin signalling in target tissues (visfatin resistance) or the response to hyperglycaemia or to hyperinsulinaemia. However, the observed associations between circulating visfatin and insulin levels or HOMA-IR values only in the non-MetS group, and only slightly (not significantly) higher visfatin levels despite markedly increased concentrations of insulin and HOMA-IR values in the MetS group, seem to confirm our theory rather than that of Chen et al. [13].
As insulin resistance and visfatin release from visceral adipose tissue increase with rising adiposity [3, 4], we should expect an association between BMI or other anthropometric measurements with circulating visfatin levels. However, the reported data of the associations between BMI and plasma visfatin level are inconsistent. Some [2, 3], but not all [5] studies have shown significant positive correlations between these parameters. In the present study we did not observe any correlation between anthropometric parameters and plasma visfatin levels either in all study subjects or in the study groups.
Perhaps the proportion of the circulating visfatin and insulin molecules, expressed as the visfatin/insulin ratio, is more important than visfatin alone for prevention of the development of insulin resistance and MetS in the obese. Decreased visfatin/insulin ratio in MetS and the negative correlation between visfatin/insulin ratio and anthropometric parameters correspond to the insulin resistance in the obese with MetS. This may suggest that the increase of visfatin production in obese subjects with MetS is insufficient to prevent the development of insulin resistance. To the best of our knowledge, the only study that has assessed this issue was a paper by Panidis et al. [6]. In this study the visfatin/insulin ratio was analysed in normal weight women with polycystic ovary syndrome and healthy women with normal weight, overweight and obesity without concomitant diseases. The results presented in this study showed a significantly higher visfatin/insulin ratio in non-MetS than in MetS obese women.
Our study has several limitations including the cross-sectional study design, the small number of non-MetS subjects and the method of insulin resistance assessment. We did not apply the reference method of insulin resistance measurement, i.e. metabolic clamp technique.
In conclusion, we conclude that the visfatin/ insulin ratio declining with increasing visceral obesity may predispose to the development of insulin resistance.
References
- 1.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: aprotein secreted by visceral fat that mimics the effect of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 2.Bernt J, Klöting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot – specific mRNA expression in humans. Diabetes. 2005;54:2911–6. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 3.Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipid and inflammation. J Clin Endocrinol Metab. 2007;92:666–72. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagano C, Pilon C, Olivieri M, et al. Reduced plasma visfatin/pre-B cell colony – enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–70. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, Li TC, Li CI, et al. The relationship between visfatin levels and anthropometric and metabolic parameters: association with cholesterol levels in women. Metabolism. 2007;56:1216–20. doi: 10.1016/j.metabol.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Panidis D, Farmakiotis D, Rousso D, et al. Plasma visfatin levels in normal weight women with polycystic ovary syndrome. Eur J Intern Med. 2008;19:406–12. doi: 10.1016/j.ejim.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Aeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15:1851–62. doi: 10.2174/092986708785133004. [DOI] [PubMed] [Google Scholar]
- 8.Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, et al. Serum concentration of visfatin in obese women. Metabolism. 2007;56:1131–4. doi: 10.1016/j.metabol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstedt A, Pihlajamaki J, Sopasakis VR, et al. Visfatin is an adipokine but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91:1181–4. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 10.Haider DG, Schindler K, Schaller G, et al. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 11.Dogru T, Sonmez A, Tasci I, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24–9. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–8. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 13.Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-b cell colony- enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–9. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 14.Alghasham AA, Barakat YA. Serum visfatin and its relation to insulin resistance and inflammation in type 2 diabetic patients with and without macroangiopathy. Saudi Med J. 2008;29:185–92. [PubMed] [Google Scholar]
- 15.Chu CH, Lee JK, Wang MC, et al. Change of visfatin, C-reactive protein concentrations, and insulin sensitivity in patients with hyperthyroidism. Metabolism. 2008;57:1380–3. doi: 10.1016/j.metabol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Filipatos TD, Randeva HS, Derdemezis CS, Elisaf ME, Mikhailidis DP. Visfatin/PBEF and atherosclerosis-related diseases. Curr Vasc Pharmacol. 2010;8:12–28. doi: 10.2174/157016110790226679. [DOI] [PubMed] [Google Scholar]