Abstract
Introduction
Trastuzumab, a recombinant humanized monoclonal antibody, is targeted against the external domain of the human epidermal growth factor receptor type 2 (HER2). It improves efficacy of HER2-positive breast cancer treatment. The authors present their experience with patients (pts) treated with trastuzumab in the aspects of cardiac complications.
Material and methods
We observed prospectively 253 women with early positive HER2 breast cancer treated with trastuzumab. Assessment of cardiovascular status, ECG and echocardiography was performed initially and every 3 months until 6th month during follow-up.
Results
Cardiac complications developed in 52 pts (20.55%) and included: asymptomatic left ventricle dysfunction (43), symptomatic heart failure (6), new asymptomatic LBBB (1); new negative T-waves in ECG (2). There was a progressive decline in left ventricular ejection fraction (LVEF) during treatment. It was more enhanced in pts with cardiac complications. Following trastuzumab termination/discontinuation LVEF increased but at month 18 still remained significantly lower than initially in both groups (61.07 ±4.84 vs. 59.97 ±5.23 – no cardiac complications; p < 0.05; 58.14 ±4.08% vs. 53.08 ±5.74% – cardiac complications; p < 0.05). During 6-month follow-up 33 out of 46 pts experienced an improvement in left ventricular status. In 13 pts in whom trastuzumab was discontinued, it was restarted; 6 of them successfully completed total therapy. Univariate analysis revealed no association between any cardiovascular risk factor and the development of cardiotoxicity.
Conclusions
One out of five treated patients discontinues trastuzumab in an adjuvant setting due to cardiac complications. LV dysfunction is the most frequent. Routine cardiac monitoring should be obligatory.
Keywords: trastuzumab, cardiotoxicity, trastuzumab-related cardiomyopathy, breast cancer, HER2 overexpression
Introduction
Breast cancer is the most frequent cancer in women and the leading cause of cancer-related deaths. In 20-30% of invasive breast cancers overexpression of human epidermal growth factor receptor type 2 (HER2) occurs, which is associated with a poor prognosis [1, 2]. Trastuzumab is a humanized monoclonal antibody that binds to the extracellular domain of HER2 receptor and inhibits carcinoma cellular proliferation [3]. Four large multicenter randomized trials revealed that trastuzumab in HER2-positive early breast cancer added to anthracycline, cyclophosphamide or paclitaxel chemotherapy resulted in 50% reduction in 3-year risk of recurrence and over 30% reduction in the death rate [4–6]. These benefits were recently confirmed by longer follow-ups [7].
But the efficacy of trastuzumab is at the cost of significant cardiotoxicity, which manifests usually as either asymptomatic left ventricular dysfunction or as symptomatic heart failure (HF). The incidence of cardiotoxicity was highest in patients receiving concurrent trastuzumab and anthracyclines (27%) with lower risk in patients receiving trastuzumab plus paclitaxel (13%) or trastuzumab alone (3-7%) (in metastatic disease) [8]. Now trastuzumab is recommended to use following, but not concurrently, anthracycline therapy to minimize cardiotoxicity.
As trastuzumab-associated cardiotoxicity is not well defined and its nature is not understood but often limits scheduled breast cancer treatment, we present our prospective observation of patients treated with trastuzumab in an adjuvant setting in the aspects of cardiac complications.
Material and methods
The study included 253 consecutive patients with early breast cancer, qualified for trastuzumab adjuvant chemotherapy, who were referred to our echo-laboratory from 1 March 2008 to 30 June 2011 for 2-dimensional echocardiography and who met the inclusion criteria. The inclusion criteria followed clinical guidelines [9, 10] and were histologically confirmed invasive HER2 positive breast cancer and LVEF > 50%. The patients were excluded if they had metastatic disease, symptoms of heart failure, LVEF < 50%, had myocardial infarction 6 months previously, or presented uncontrolled symptomatic angina pectoris, uncontrolled arrhythmia, uncontrolled hypertension or any significant valvular heart disease (mitral or aortic insufficiency).
Each participant of the study signed informed consent. The protocol was approved by the local Ethics Committee.
Cardiovascular system evaluation (history, cardiovascular risk factors, blood pressure and physical examination), electrocardiography and echocardiography were performed at baseline and repeated every 3 months until 6 months after trastuzumab termination. It was performed and interpreted by the same experienced cardiologist (GP). Parasternal and apical views were obtained using a standard echocardiograph (GE VIVID 4, transducer 1.7-4.0 MHz, USA). Left ventricular ejection fraction (LVEF) was determined from two-dimensional images according to established criteria including modified Simpson's method [11].
The following cardiovascular risk factors were analysed: age, overweight (body mass index – BMI > 25kg m2 and < 30 kg/m2), obesity (BMI > 30 kg/m2), hypertension, smoking, sedentary lifestyle, positive family history, hypercholesterolaemia, diabetes mellitus, depression.
All patients were diagnosed with histologically confirmed, completely excised invasive breast cancer with HER2 overexpression, fulfilling criteria for adjuvant therapy with trastuzumab. Trastuzumab was initiated after completion of chemo- and radiotherapy. The loading administration dose of trastuzumab was 8 mg/kg of body weight, and the maintenance dose was 6 mg/kg triweekly for a total of 52 weeks. Trastuzumab was discontinued in patients who developed significant cardiotoxicity, which was defined as a potentially life-threatening cardiac event. Anthracycline-containing regimens used as adjuvant or neoadjuvant chemotherapy were AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2, every 3 weeks for four cycles) or in 7 patients FEC (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 600 mg/m2, every 3 weeks for more than four cycles). Docetaxel was given at 60-75 mg/m2 triweekly. Endocrine therapy was added as clinically indicated on the basis of tumour characteristics. Radiotherapy was administered in 159 (62.9%) individuals and neoadjuvant therapy according to the AT protocol was applied in 70 patients (27.7%).
HER2 status was determined by immunohistochemical staining (3+) or in case of HER2 result 2++ amplification of the HER2 gene was evaluated using the fluorescence in situ hybridization (FISH) method.
Cardiotoxicity
Significant cardiotoxicity was regarded as a potentially life-threatening cardiac event and was defined as: (1) each absolute decrease of LVEF > 15% [12], (2) absolute reduction in LVEF of 10% from the baseline value and below the level of 50% [5], (3) any symptoms or signs of heart failure. As other events that occur in the cardiovascular system during trastuzumab treatment are rare and not well known, they were not defined precisely in advance, but were evaluated individually by the cardiologist and oncologist together in the course of the treatment. In case of significant cardiotoxicity trastuzumab was terminated early. The decision regarding discontinuation of trastuzumab was made according to guidelines [9, 10] and each time it was made individually by the oncologist responsible for the treatment after consultation with the supervising cardiologist. In the majority of cases of significant cardiotoxicity, trastuzumab was discontinued, and heart failure (HF) treatment with angiotensin-converting enzyme inhibitors/angiote-nsin receptor antagonists (ACE-I/ARA) and/or β-blockers was initiated and up-titrated to the maximum tolerated doses. Additional cardiac treatment, including diuretics, anticoagulants, and antiarrhythmic drugs, was given as required by the clinical situation, based on the current standard of care [13].
Statistical analysis
Data were reported as mean ± SD. Comparisons between groups were done by unpaired Student's t-test for continuous variables and by χ2 test or Fisher's exact test as appropriate for categorical variables. Univariate regression analysis was used to identify covariates of cardiotoxicity. Statistical Analysis Systems (SPSS PC and Statistica) were used to perform the analysis. A p value less than 0.05 was considered significant.
Results
Two hundred and fifty-three women entered the study (mean age: 55 ±10 years), which was 60.19% of the total (420 women) population treated with trastuzumab in our centre from 1 March 2008 to 30 June 2011. Fourty-seven patients (11.1%) did not fulfil the entry criteria (initial LVEF < 50%) or had contraindications to trastuzumab therapy (advanced heart diseases), 18 (4.3%) refused to participate in the study, and 5 patients (1.2%) were not included because of extremely poor quality of the echocardiographic image. The remaining women were diagnosed with metastatic cancer or had echocardiography performed outside our centre. After 3 months 241, after 6 months 239, after 9 months 205, and after 12 months 142 patients had echocardiography performed. At follow-up visits at 3 and 6 months after trastuzumab termination 124 and 101 patients were assessed, respectively.
The duration of trastuzumab treatment differed between groups with and without cardiac complications. In the population with cardiac complications, the mean duration of treatment with trastuzumab was 25.3 weeks (from to 4 to 52 weeks) and for the population with no complications 51.2 weeks (from 49.3 to 53.9 weeks).
Serious cardiac complications that resulted in early trastuzumab termination occurred in 52 patients (20.55%). Among cardiac complications associated with trastuzumab, asymptomatic left ventricle (LV) dysfunction was the most frequent, whereas severe, symptomatic heart failure (HF) (New York Heart Association [NYHA] functional class III/IV), new asymptomatic left bundle branch block (LBBB), new negative T-waves in electrocardiography (ECG) and asymptomatic right bundle branch block (RBBB) were observed much more rarely (Table I). Severe HF (NYHA III/IV) occurred in 6 patients (2.37%) – in 3 associated with LV systolic dysfunction while in 3 others LV systolic function was preserved.
Table I.
Reasons for early trastuzumab termination
| Reason for early trastuzumab termination | Patients (N = 253) | |
|---|---|---|
| n | % | |
| Cardiac complications | 52 | 20.55 |
| Asymptomatic LV dysfunction | 43 | 17 |
| Severe, symptomatic HF | 6 | 2.37 |
| Asymptomatic, new LBBB | 1 | 0.4 |
| Negative T waves V1-6 in ECG | 2 | 0.79 |
| Breast cancer recurrence (during trastuzumab treatment) | 27 | 10.67 |
| Patient withdrawal | 16 | 6.32 |
Negative T waves resumed after 6 months in both patients. Nuclear stress testing (GSPECT) was negative for ischaemia, echocardiography demonstrated no abnormalities of LV wall motion, and sequential cardiac troponin tests were negative. The LBBB was still present and still asymptomatic at the moment of the publication. This patient is still under observation in our cardiology department. Six patients with overt HF fully recovered after discontinuation of trastuzumab and implementation of heart failure therapy (ACE-I, β-blockers, temporarily diuretics) within 6 months of observation.
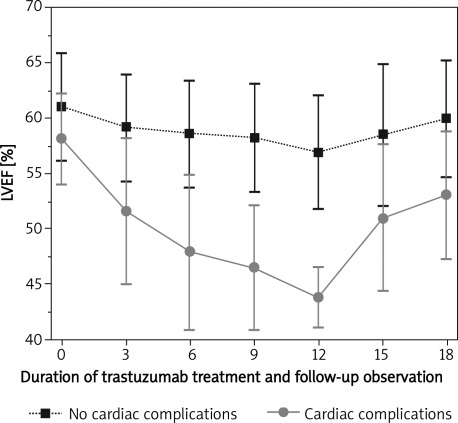
All patients had no symptoms of heart failure at baseline. Mean LVEF at baseline was 60.52 ±4.85%. Mean LVEF was significantly lower in the group with cardiac complications (58.14 ±4.08 vs. 61.07 ±4.84, p < 0.05). After 3 months of trastuzumab therapy, there was a difference in LVEF between the no cardiac complication cohort and those patients in whom cardiac complications developed (59.18 ±4.83% vs. 51.59 ±6.63%, p < 0.05). There was a progressive decline in LVEF up to 12 months in both groups, but it was more enhanced in the group with cardiac complications despite the fact that they were exposed to the medication for a shorter period. At 12 months LVEF stabilized at 56.92 ±5.17 in the group with no cardiac complications and at 43.79 ±2.67% in the group with cardiac complications, which was a significant difference (p < 0.05). Following trastuzumab termination/discontinuation LVEF increased but still remained at month 18 significantly lower in both groups as compared with baseline status (61.07 ±4.84 vs. 59.97 ±5.23 – no cardiac complications; p < 0.05; 58.14 ±4.08 vs. 53.08 ±5.74 – cardiac complications; p < 0.05; respectively) (Figure 1, Table II). The same drop of LVEF from baseline to 6 months after discontinuation of trastuzumab was observed for the whole study group (Table II).
Figure 1.
Serial monitoring of LVEF in patients with no cardiac complications compared to those with cardiac complications
Table II.
LVEF in total population, in group with and without cardiac complications at particular time of measurements
| Time of measurement | Total population LVEF [%] | Group with no cardiac complications LVEF [%] | Group with cardiac complications LVEF [%] | Value of p No complications vs.complications |
|---|---|---|---|---|
| Baseline | 60.52 ±4.85 | 61.07 ±4.84 | 58.14 ±4.08 | 0.00024 |
| 3 months | 57.72 ±6.03 | 59.18 ±4.83 | 51.59 ±6.63 | < 0.001 |
| 6 months | 56.87 ±6.62 | 58.66 ±4.74 | 47.92 ±7.06 | < 0.001 |
| 9 months | 57.1 ±6.23 | 58.28 ±4.91 | 46.46 ±5.58 | < 0.001 |
| 12 months | 55.55 ±6.41 | 56.92 ±5.17 | 43.79 ±2.67 | < 0.001 |
| 15 months | 56.15 ±7.33 | 58.53 ±6.41 | 50.96 ±6.58 | < 0.001 |
| 18 months | 57.87 ±6.27 | 59.97 ±5.23 | 53.08 ±5.74 | < 0.001 |
Median time of trastuzumab early termination due to all kinds of cardiac complications was 25.3 weeks (from 4 to 52 weeks). Median time of trastuzumab early termination due to significant LV systolic dysfunction was 26.2 weeks (from 4 to 52 weeks). Trastuzumab was discontinued in 13 patients (including 1 due to new onset LBBB) after 3 months, in 22 (including 2 due to ST-T repolarization disturbances and 3 due to overt HF) after 6 months, in 12 (including 2 due to overt HF) after 9 months. A significant drop in LVEF was observed after 12 months in 5 patients (including 1 with overt HF). Trastuzumab was discontinued after a mean of 8 ±4 doses.
The majority of patients with cardiac complications during trastuzumab therapy were asymptomatic (88.5%). Six patients (2.37%) presented with dyspnoea (NYHA III/IV). Out of 46 patients with LV dysfunction 43 (93.5%) received heart failure medications including ACE inhibitors and/or β-blockers. Eleven patients (23.9%) were on ACE I, 10 (21.7%) on β-blockers alone and 22 (47.8%) were on both medications. Three patients did not receive any heart failure treatment. In 9 patients (19.6%) diuretics were applied and in 9 (19.6%) aldosterone antagonist (eplerenone in 1 and spironolactone in the others).
At 6 months follow-up 33 (71.7%) out of 46 patients experienced a demonstrable improvement (LVEF > 50%) in left ventricular status. In the 13 (28.26%) remaining individuals LVEF was still below 50% after 6 months, 9 (19.6%) of them showed no significant improvement in mean LVEF (37.4%) and in 4 (8.7%) there developed a further decline in mean LVEF (35.6%). Median recovery time for 33 patients with LV systolic function improvement was 9 weeks (from 4 to 28 weeks).
In 13 patients (28.26%) in whom trastuzumab was discontinued due to LVEF drop, trastuzumab was restarted with concomitant use of ACE inhibition and β-blockade. In 6 of them, total 12-month therapy was successfully completed with no recurrence of LV systolic dysfunction. In 7 others trastuzumab was discontinued again due to recurrence of LV systolic dysfunction and in those patients the therapy was not restarted again.
In 28 (60.9%) out of 46 patients with LV systolic dysfunction regional wall motion abnormalities were observed in the first echocardiography that revealed a significant drop of LVEF. In the majority, regional hypokinesis concerned the interventricular septum – 18 patients (64.29%). In 10 patients (39.1%) general hypokinesis with no regional wall motion abnormalities was observed initially. However, in the majority of cases, subsequent echocardiographies showed general hypokinesis despite initial regional abnormalities detected at the beginning.
Cardiovascular risk factors
As shown in Table III, there were no significant differences in prevalence of cardiovascular risk factors between the group of patients with and without cardiac complications. There was relatively high prevalence of hypertension (39.1%), particularly in the group of patients who developed cardiac complications (42.3%) and high prevalence of diabetes mellitus (6.3%). Numerous patients were overweight (39.1%), and sedentary (76.7%). Sedentary women were especially frequent in the group with cardiac complications (82.7%). The prevalence of hypercholesterolaemia (34.4%) and smoking (13.4%) seems to be relatively low (Table III).
Table III.
Risk factors in total population, in group with and without cardiac complications
| Risk factors | Total population (N = 253) | Group with no cardiac complications (n = 201) | Group with cardiac complications (n = 52) | Value of p No complications vs. complications |
|---|---|---|---|---|
| Age [years] | 55 ±10 | 54.8 ±9.7 | 56.8 ±9.6 | 0.366 |
| BMI [kg/m2] | 26.8 ±4.5 | 26.9 ±4.6 | 27.3 ±4.4 | 0.417 |
| Hypertension | 99 (39.1%) | 77 (38.3%) | 22 (42.3%) | 0.327 |
| Diabetes mellitus | 16 (6.3%) | 13 (6.47%) | 3 (5.8%) | 0.952 |
| Smoking | 27 (13.4%) | 27 (13.4%) | 7 (13.5%) | 0.841 |
| Hypercholesterolaemia | 87 (34.4%) | 70 (34.8%) | 17 (32.7%) | 0.931 |
| Sedentary life style | 194 (76.7%) | 151 (75.1%) | 43 (82.7%) | 0.109 |
| Overweight (≤ 25 BMI < 30) [kg/m2] | 99 (39.1%) | 80 (39.8%) | 19 (36.5%) | 0.864 |
| Obesity (BMI≥≥ 30) [kg/m2] | 59 (23.3%) | 46 (22.9%) | 13 (25.0%) | 0.410 |
| Depression | 28 (11.1%) | 21 (10.5%) | 7 (13.5%) | 0.523 |
| Positive family history | 88 (34.8%) | 68 (33.8%) | 20 (38.5%) | 0.321 |
| ACE-I/ARA (at baseline) | 78 (30.8%) | 62 (30.9%) | 16 (30.8%) | 0.172 |
| β-Blockers (at baseline) | 22 (8.7%) | 18 (9%) | 4 (7.8%) | 0.649 |
| Coronary artery disease | 10 (4%) | 10 (5%) | 0 (0%) | 1.000 |
In univariate logistic regression none of the analysed cardiovascular risk factors (age, obesity, hypertension, smoking, sedentary lifestyle, positive family history, hypercholesterolaemia, diabetes mellitus, depression) was associated with significant cardiotoxicity (Table IV).
Table IV.
Associations between dependent variable: cardiac complications vs. no cardiac complications and group of independent variables in univariate logistic regression
| Independent covariates | OR | –95% CI | +95% CI | Value of p |
|---|---|---|---|---|
| Age | 1.02 | 0.99 | 1.06 | 0.197 |
| BMI | 1.02 | 0.95 | 1.09 | 0.564 |
| Hypertension | 1.38 | 0.72 | 2.64 | 0.328 |
| Diabetes mellitus | 0.96 | 0.26 | 3.52 | 0.952 |
| Smoking | 0.96 | 0.75 | 1.24 | 0.771 |
| Hypercholesterolaemia | 1.03 | 0.53 | 2.01 | 0.931 |
| Sedentary life style | 2.63 | 0.77 | 9.09 | 0.122 |
| Overweight (≤25 BMI < 30) [kg/m2] | 1.13 | 0.57 | 1.78 | 0.243 |
| Obesity (BMI ≥ 30) [kg/m2] | 1.35 | 0.66 | 2.78 | 0.411 |
| Depression | 1.35 | 0.54 | 3.40 | 0.524 |
| Positive family history | 1.39 | 0.72 | 2.68 | 0.322 |
| ACE-I/ARA (at baseline) | 1.07 | 0.66 | 1.99 | 0.456 |
| β-Blockers (at baseline) | 1.16 | 1.19 | 1.40 | 0.329 |
Discussion
Cardiotoxicity of the treatment in oncology often limits its benefits [14]. This is why monitoring and early prevention of aggressive anti-tumour treatment cardiac complications is of clinical interest.
The current study provides insight into the common experience of trastuzumab use in real life situations common in oncology and cardiology clinics. In that aspect, it differs from most published clinical trials. In a prospectively evaluated population of 253 HER2 positive, early breast cancer women treated with trastuzumab in the adjuvant setting, nearly 21% required discontinuation of the medication due to cardiac complications. In 18% of patients significant LV systolic dysfunction was the reason for trastuzumab discontinuation. The majority of complications were asymptomatic. Only 6 patients complained of significant shortness of breath, and 3 of them had preserved LV systolic function.
The asymptomatic nature of cardiac complications makes monitoring of trastuzumab therapy safety necessary. This issue has been addressed and regulated by a few guidelines [9, 10]. They suggest that detection of heart damage due to trastuzumab therapy is best accomplished via sequential measurements of LV function, either by multiple-gated acquisition scans (MUGA) or by echocardiography techniques. In addition to imaging methods, the literature suggests that measurement of plasma markers, such as brain natriuretic peptide (BNP) as a marker of LV stretch and cardiac troponins as markers of myocardium disintegration, may be used to predict and to detect cardiac dysfunction during treatment with trastuzumab [15].
Other non-myopathic cardiac complications associated with trastuzumab therapy were: LBBB in 1, negative T-waves in 2, RBBB in 2 patients. They were rare, all asymptomatic and the last one was not the reason for trastuzumab discontinuation. The authors found a few instances of non-myopathic cardiac events after trastuzumab specifically described. There was a case of a 19-year-old woman, in whom during infusion of the fifth dose of trastuzumab chest pain occurred and ECG revealed new T wave inversions in the anterior precordial and lateral leads [16]. There were two examples of heart conduction abnormalities after trastuzumab [17] and there was also a report about ventricular tachycardia associated with trastuzumab in a patient with preserved LV systolic function that resulted in sudden cardiac death [18].
The incidence of left ventricular dysfunction in our study is consistent with that reported in the BCIRG 006 trial (18%) [19] and is higher than in the HERA trial (3.05%) [5]. It is slightly less than in retrospective observations by Wadhwa et al. [20], Tarantini et al. [21] and McArthur and Chia [22], who reported 24%, 23% and 22% LV systolic dysfunction during trastuzumab treatment, respectively.
The incidence of LV dysfunction during trastuzumab treatment has usually been reported higher in a metastatic (up to 34%) [23, 24] than in an adjuvant [25, 26] setting. The incidence of cardiotoxicity seems to be higher outside of clinical trials, in observational studies like ours.
The differences in the incidence of LV dysfunction in our and other clinical observations might have resulted from the fact that different definitions of cardiotoxicity were used in particular trials. For example, Wadhwa et al. defined cardiotoxicity as a decline in LVEF of at least 10% and below 55% and not below 50% as in our study [20], which might be one of the reasons for higher LV dysfunction incidence in his report.
Symptomatic heart failure events have been observed much less frequently than asymptomatic LV dysfunction. 1.9% in BCIRG (Breast Cancer International Research Group) 006, 4% in NSABP (National Surgical Adjuvant Cancer Treatment Group) B-31 and 0.6% in the HERA (HERceptin Adjuvant) trial of trastuzumab-treated patients reported severe symptoms of HF (NYHA III/IV) [5]. Tarantini et al. reported 3% symptomatic heart failure incidence in a cohort of 499 women with HER positive early breast cancer from 10 Italian institutions treated with trastuzumab observed retrospectively [20]. But all women in this observation were in NYHA functional class II. Such incidence is consistent with that in our study, in which 2.37% of patients complained of severe HF symptoms.
The majority of complications were observed between the 3rd and 6th month (mean after 8 ±4 doses) but later they occurred as well. An LVEF drop that fulfilled the criteria of significant cardiotoxicity was observed after 12 month in 5 patients. In the retrospective study by Wadhwa et al. mean duration of treatment for the population with LV dysfunction was 25.7 ±12.9 weeks, which is consistent with the duration of treatment for that group in our observation – 25 ±12 weeks [20]. In the NSABP-3 trial, the majority of patients developed cardiac complications between the 3rd and 12th month [27], whereas in the population observed by Tarantini et al. LV dysfunction was detected mainly during the first 3 months of therapy with a stable trend thereafter (5% new LV dysfunction episodes every 3 months during therapy) [10].
We found no differences in prevalence of cardiovascular risk factors between the group of patients with and without cardiac complications. Also no risk factor was associated with cardiotoxicity in univariate logistic regression analysis. It might be surprising, as the presence of pre-existing cardiovascular risk factors is regarded as a predictor for the development of therapy-induced cardiovascular injury. However, the association of cardiovascular risk factors with trastuzumab-related cardiotoxicity is not apparent and not fully explained. Particular trials have obtained different results for particular cardiovascular risk factors. In the NSABP B-31 and NCTTG N9831 trials age ≥ 50 years and requirement for hypertension medications were risk factors for heart failure in the course of trastuzumab treatment in univariate analysis [27, 28]. In the NSABP B-31 trial there was no association between trastuzumab-mediated cardiotoxicity and smoking, positive family history, or hypoglycaemic and hypolipaemic medications [27]. In the HERA trial overweight and obesity were risk factors for cardiac toxicity but age, hypertension, dyslipidaemia and previous heart disease did not increase the risk of trastuzumab-related cardiotoxicity [25]. Age, smoking and hypertension were independent predictors of trastuzumab-related cardiomyopathy in Wadhwa's observational study [20].
Nevertheless, in the retrospective study by Tarantini et al. [21], as in ours, no traditional risk factor (age, body mass index, systemic hypertension, diabetes mellitus) was associated with the development of trastuzumab-induced cardiotoxicity.
Women with breast cancer are regarded as a population of high cardiovascular risk [29]. Women with breast cancer are often sedentary [30], overweight and obese [31]. Heart diseases and breast cancer have many cardiovascular risk factors in common. Recent data suggest that physical inactivity increases the risk of breast cancer among white women by 2% to 15% [32], while overweight and obesity are associated with 34% and 63% increase of breast cancer risk, respectively [33]. This is consistent with our observation, in which numerous patients were overweight (39.1%) and were sedentary (76.7%), especially in the group with cardiac complications (82.7%). There was also relatively high prevalence of hypertension (39.1%), particularly in the group who developed cardiac complications (42.3%), and prevalence of diabetes mellitus (6.3%). The prevalence of hypercholesterolaemia (34.4%) and smoking (13.4%) seems to be relatively low in our cohort as for the Polish population.
The mechanisms of trastuzumab-related cardiotoxicity remain uncertain. Genetic background has been suggested by some studies [34, 35]. The clinical picture and the lack of diagnostic structural findings on myocardial biopsy samples suggest a totally different mechanism of myocardial damage from that of anthracyclines [36]. There are two different hypothesis of trastuzumab mechanism of cardiac damage in the literature. Some preclinical data indicate that inhibition of the myocardial HER2 receptor leads to changes in the tertiary structure of the cardiac contractile apparatus, which seems likely to be a reversible effect [37]. Others suggest that trastuzumab induces apoptosis and cardiomyocytes’ death, which is likely a progressive and rather irreversible condition [38]. Both mechanisms may play a role in cardiotoxicity and numerous external factors may decide which predominates and whether heart damage is reversible.
The limitations of our study are its small sample size and short follow-up. Longer term follow-up is required to answer some unanswered questions concerning the nature of trastuzumab cardiotoxicity. (The follow-up of the population of our study is being conducted.) We analysed only the association of cardiovascular risk factor with trastuzumab-related cardiotoxicity, while numerous other situations that accompany cancer treatment (radiotherapy, left side of breast cancer, other anticancer medications and their dosages, time from anthracycline completion to trastuzumab initiation) are known to influence cardiac damage during treatment in oncology. They were not included in the analysis.
It is difficult to compare the incidence of asymptomatic LV dysfunction in our population with that in the cohorts of other studies as different LVEF criteria of thresholds for trastuzumab cardiotoxicity were used in particular studies. Also the modes of LV function evaluation and LVEF calculation were different in particular studies (echocardiography, MUGA, CT, MRI). There is no international agreement about the universal thresholds of LV function for definition of trastuzumab cardiotoxicity [39].
In conclusion, nearly one in four patients will discontinue trastuzumab treatment due to cardiac complications, among which LV dysfunction is the most frequent. Although cardiac complications of trastuzumab therapy are frequent, they seem not to be harmful. Most of them are transient, asymptomatic and reversible, although there have been reports on rare cases of progressing LV dysfunction and HF. Nevertheless, longer follow-up is needed to confirm that cardiotoxicity associated with trastuzumab therapy does not affect long-term outcome. Prior to institution of trastuzumab therapy, all patients should be evaluated for cardiovascular status. As the majority of complications are asymptomatic, routine cardiac monitoring should be performed during trastuzumab treatment.
References
- 1.Hudziak RM, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci. 1987;84:7159–63. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Force T, Krause DS. Van Etten. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: amilestone in the treatment of HER-2 positive early. Oncologist. 2006;11(Suppl 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 7.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 9.Mackey JR, Clemons M, Cote MA, et al. Cardiac management adjuvant trastuzumab therapy: recommendation of the Canadian Working Group. Current Oncol. 2008;15:24–35. doi: 10.3747/co.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AL, Barlow M, Barrett-Lee PJ, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684–92. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: areport from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of chocardiography, abranch of the European Society of Cardiology. J Am Soc Echocardiography. 2005;18:1440–6. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Carver JR, Carver JR. Management of trastuzumab-related cardiac dysfunctio. Prog Cardiovasc Dis. 2010;53:130–9. doi: 10.1016/j.pcad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: full text (update 2005). The task force for the diagnosis and treatment of CHF of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki PJ, Hutka M. Cardiotoxicity of 5-fluorouracil in ayoung colorectal cancer patient – case report and review of literature. Arch Med Sci. 2009;5:277–80. [Google Scholar]
- 15.Sparano JA, Brown DL, Wolff AC. Predicting cancer therapyinduced cardiotoxicity. The role of troponins and other markers. Drug Saf. 2002;25:301–11. doi: 10.2165/00002018-200225050-00001. [DOI] [PubMed] [Google Scholar]
- 16.Olin RL, Desai SS, Fox K, Davidson RI. Non-myopathic cardiac events in two patients treated with trastuzumab. Breast J. 2007;13:211–2. doi: 10.1111/j.1524-4741.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 17.ATu CM, Chu KM, Yang SP, Cheng SM, Wang WB. Trastuzumab (Herceptin)-associated cardiomyopathy presented as new onset of complete left bundle-branch block mimicking acute coronary syndrome: acase report and literature review. Am J Emerg Med. 2009;27:903e1–3. doi: 10.1016/j.ajem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira M, Nave M, Gil N, Passos-Coelho JL. Sudden death during adjuvant trastuzumab therapy of breast cancer. Ann Oncol. 2010;21:901. doi: 10.1093/annonc/mdp587. [DOI] [PubMed] [Google Scholar]
- 19.Slamon D, Eiermann W, Robert N. Proceedings of San Antonio breast cancer symposium (SABCS) San Antonio: Breast Cancer Research and Treatment; 2006. -006 oboB BCIRG 006:2nd interim analysis chase III randomised trial comparing doxorubicine and cyclophosphamid followed by docetaxel (AC/T) with doxorubicine and cyclophosphamid followed by docetaxel and trastuzumab (AC/ETH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. [Google Scholar]
- 20.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: aretrospective study. Breast Cancer Res Treat. 2009;117:357–64. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 21.Tarantini L, Cioffi G, Gori S, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. 2012;18:113–9. doi: 10.1016/j.cardfail.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 22.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med. 2007;357:94–5. doi: 10.1056/NEJMc070065. [DOI] [PubMed] [Google Scholar]
- 23.Guarneri V, Lenihan DJ, Valero V, et al. Phase II study of weekly paclitaxel and trastuzumab in anthracycline- and taxane-pretreated patients with HER2-overexpressing metastatic breast cancer. Br J Cancer. 2004;90:36–40. doi: 10.1038/sj.bjc.6601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 26.Kelly H, Kimmick G, Dees EC, et al. Response and cardiac toxicity of trastuzumab given in conjunction with weekly paclitaxel after doxorubicin/cyclophosphamide. Clin Breast Cancer. 2006;7:237–43. doi: 10.3816/CBC.2006.n.035. [DOI] [PubMed] [Google Scholar]
- 27.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in arandomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–9. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 28.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–8. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–41. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist's recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: asingle-blind, randomized controlled trial. Ann Behav Med. 2004;28:105–13. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 31.Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after abreast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774–82. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke CA, Purdie DM, Glaser SL. Population attributable risk of breast cancer in white women associated with immediately modifiable risk factors. BMC Cancer. 2006;6:170–81. doi: 10.1186/1471-2407-6-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 34.Hosseini M, Houshmand M, Ebrahimi A. MTHFR polymorphisms and breast cancer risk. Arch Med Sci. 2011;7:134–7. doi: 10.5114/aoms.2011.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gluba A, Pietrucha T, Banach M, Piotrowski G, Rysz J. The role of polymorphisms within paraoxonases (192 Gln/Arg in PON1 and 311Ser/Cys in PON2) in the modulation of cardiovascular risk: a pilot study. Angiology. 2010;61:157–65. doi: 10.1177/0003319709351258. [DOI] [PubMed] [Google Scholar]
- 36.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 37.Pentassuglia L, Graf M, Lane H, et al. Inhibition of ErbB2 by receptor tyrosine kinase inhibitors causes myofibrillar structural damage without cell death in adult rat cardiomyocytes. Exp Cell Res. 2009;315:1302–12. doi: 10.1016/j.yexcr.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh KK, Shukla PC, Quan A, et al. Herceptin, arecombinant humanized anti-ERBB2 monoclonal antibody, induces cardiomyocyte death. Biochem Biophys Res Commun. 2011;411:421–6. doi: 10.1016/j.bbrc.2011.06.169. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Ewer MS. Is cardiotoxicity being adequately assessed in current trials of cytotoxic and targeted agents in breast cancer? Ann Oncolbibl. 2011;22:1011–8. doi: 10.1093/annonc/mdq607. [DOI] [PubMed] [Google Scholar]