Abstract
Introduction
Immune system dysfunction is considered to be one of many medical disorders found in children with autism. The primary objective of the study was to assess if blood tests reflecting humoral immunity (IgA, IgG, IgM, IgE) are useful in identifying children with regressive autism. The secondary objective was to evaluate a part of the cellular arm of immunity (CD4/CD25 Tregs, CD4/CD23 cells) in those children.
Material and methods
Using a clinical case-control design, the systemic levels of immunoglobulins and lymphocyte subpopulations analysed by flow cytometry were compared in children aged 3-6 years old with a new diagnosis of regressive autism (n = 24; mean age: 4.25 ±1.70 years; male 23/24) and in sex- and age-matched healthy children (n = 24; aged 4.25 ±2.20 years; male 23/24).
Results
The humoral immunity profile, described by three binary variables, IgA < 0.97 g/l, IgE > 36 IU/ml, and IgG > 6.3 g/l, with a sensitivity of 79% and a specificity of 83% (p < 0.0001), was able to identify children with autism. The highest risk of autism diagnosis was associated with IgA < 0.97g/l (OR – 23.0; p < 0.001). A higher number of CD19/CD23 was found in children diagnosed with autism than in the control group (36.82 ±6.72% vs. 18.20 ±3.95%; p < 0.02). No correlation between the number of CD23-positive cells and serum IgE levels was observed.
Conclusions
A subtle shift of serum immunoglobulins consisting of low-normal IgA and B cell activation expressed by an increase of CD23-positive cells may characterize children with regressive autism aged 3-6 years old.
Keywords: autism, immunoglobulins, flow cytometry, CD4/CD23
Introduction
For many years reciprocal functional connections between the brain, behaviour, and the immune system have been the subject of interest for researchers [1, 2]. Numerous recent publications have focused on immune dysfunction and autoimmunity in various neuropsychiatric disorders such as schizophrenia, paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), and obsessive compulsive disorder [3, 4]. Immune dysfunction is also considered in the aetiology and pathology of autism spectrum disorders (ASD) [5, 6].
The autistic spectrum disorders are complex developmental disorders and are a part of a broad spectrum of neurodevelopmental disorders known as pervasive developmental disorders. They are diagnosed on the basis of clinical characteristics (DSM-IV) [7]. The ASDs are characterized by impairments in social interaction, deficits in verbal and nonverbal communication, and the presence of restricted and repetitive stereotyped behaviours. They manifest within the first 3 years of life. ASDs are an extremely heterogeneous group of disorders with multiple phenotypes and subgroups which differ in the degree of behavioural disorders and vary in the response to treatment.
Immune system abnormalities in children with autism include both enhanced autoimmunity and reduced immune function. Immune abnormalities reported in autistic children include abnormal Th1/Th2 cytokine profiles, decreased lymphocyte numbers, a decreased T cell mitogen response, and an imbalance of serum immunoglobulin levels [5, 8, 9]. In addition, autism has been linked with autoimmunity and an association with immune-based genes, including human leukocyte antigen (HLA)-DRB1 and complement C4 alleles, has been described [8]. Autoimmunity is characterized by the presence of antibodies reactive to central nervous system (CNS) proteins with the potential for neuronal tissue destruction. These antibodies also include those against neuron-axon filament proteins (NAFP), cerebellar neurofilaments, myelin basic protein (MBP), the caudate nucleus, serotonin receptors, brain endothelial cells, as well as antibodies against tissue and specific antibodies targeted against gut epithelial cells [6, 8, 10–12]. These findings lead some researchers to treat autism as a chronic inflammatory disease of the brain with an origin in prenatal life [13].
According to recent findings, circulating maternal autoantibodies directed toward fetal brain proteins are highly specific for autism [14].
Other observations suggest activation of an allergic process and warrant antiallergenic treatment and elimination of certain foods from the diet of children with autism [15, 16]. Immunological phenomena associated with autistic disorders are not yet fully understood.
The primary objective of this study was to assess if commonly available blood tests reflecting the humoral immunity profile (IgA, IgG, IgM, and IgE) are able to identify recently diagnosed children with regressive autism. A secondary objective was to investigate cellular immunity (lymphocyte subtypes, CD4/CD25 T regulatory cells) in those children. Additionally, gluten-specific IgG in sera of children with autism was assessed and compared to the sera of healthy children.
Material and methods
Subjects
According to the study protocol a case-control design was used. Children recently diagnosed with autism were recruited from the Bialystok Branch of the National Autism Society. The diagnosis of autism was made in all subjects by a team of experienced psychologists and a psychiatrist according to the diagnostic criteria for autism outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and the International Classification of Diseases, Tenth Revision [7, 17]. Children were recruited to the study from February 2009 to April 2010. The medical history was gathered and physical examinations were performed on all children. The course of pregnancy, mode of delivery, and general condition of the newborn after birth were noted from personal health books. A medical history for acute and chronic illnesses was collected from the parents. A family history of the atopic triad – atopic dermatitis, allergic rhinitis, and bronchial asthma – was included in the questionnaire. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). The inclusion criteria for the study group were: 1) a new diagnosis of regressive autism (children with regression who initially developed but subsequently lost previously acquired language and/or social skills); 2) no pharmacological or dietary treatment as well as no supplements within the last 6 weeks (in order to avoid any potential influences on immunity such as stimulating or inhibiting effects); 3) a negative history of infection in the previous 6 weeks.
One healthy child of the same age (3-6 years) and sex was matched for each child with autism. The place of residence (town/village) was taken into account in matching participants to their groups. Healthy children with normal development were recruited from two preschools (town/village; n = 18/n = 6). The inclusion criteria for the control group were the same as criteria 2 and 3 for the autism group listed above. Participants were excluded if they had an acute infection or chronic diseases, had taken medications, vitamins, or supplements, or if dietary intervention or alternative therapy was applied. Children for each case-control pair were recruited in the same allergic season and the time interval of the blood sample collection from both (the case and the control) was no longer than 4 weeks. None of the control children were recruited from families with a history of ASD. All study participants were Caucasian.
This study protocol followed ethical guidelines and was approved by the Bioethics Committee of the Medical University of Bialystok. Informed consent was obtained from the parents prior to participation.
Laboratory analysis
Blood samples were collected between 7 am and 9 am. Immediately after centrifugation the sera were aliquoted and frozen at –80°C until assayed for immunoglobulin levels. Serum IgG, IgM, and IgA concentrations were measured using the nephelometric technique (Siemens nephelometer BN II system) with a detection range of 0.06-8.00 g/l for IgA, 1.4-45.0 g/l for IgG, 0.05-6.40 g/l for IgM. The reference range of serum immunoglobulins for children aged 3 to 6 years in the children's hospital laboratory are: IgA 0.36-2.40 g/l, IgG 5.0-13.2 g/l, IgM 0.46-1.75 g/l, IgE 0-85 IU/ml. Serum IgA levels less than 7 mg/dl (0.07 g/l) were accepted as diagnostic of selective IgA deficiency and levels at least 2 SD (standard deviations) below normal for age were diagnostic of partial IgA deficiency [18]. Total IgE and gluten-specific IgG (f79) were detected using the UniCAP fluoroenzymeimmunoassay (Pharmacia, Sweden) according to the manufacturer's instructions. The reference range of IgE in the laboratory for children 3-4 years of age is 0-33 IU/ml, and for children ≥ 5 years of age is 0-85 IU/ml. Serum hsCRP and serum tumour necrosis factor α (TNF-α) were measured using a commercially available ELISA kit (Quantikine High Sensitivity Human by R&D Systems, Minneapolis, Minn., USA) and according to the manufacturer's instructions.
Assessment of peripheral blood morphology. For the assessment of the leukogram component, 2 ml of venous blood was obtained in blood collection tubes containing EDTA. A blood count analysis was performed using automated haematology analyzers (SYSMEX XT 2000i, Japan). Leukocyte count was expressed in G/L and lymphocytes were expressed as percentages (%).
Assessment of the lymphocyte subpopulation in peripheral blood. Cytometric analysis was performed on the blood which remained after the morphological assessment mentioned above. The following monoclonal antibodies were added in 10 µl amounts to 100 µl of full blood (Beckman Coulter): CD3-PC5, CD4-FITC, CD4-PC5/CD25-FITC, CD8-PE, CD19-PC5, CD19-PC5/CD23-PE.
Statistical analysis
Student's t-test was applied to compare variables of a normal distribution (gestational age, birth weight, Apgar score, breastfeeding duration, BMI, CRP, WBC, IgG, IgM). The Mann-Whitney U-test was applied to compare variables of non-parametric distribution (IgA, IgE, gluten-specific IgG, peripheral eosinophilia, lymphocyte subpopulations) and the Shapiro-Wilk test to verify the statistical shape of the tested variable distribution. Lastly, the χ2 test for independence was applied to compare qualitative and categorical variables. Spearman's non-parametric rank correlation test was used to evaluate the correlation between immunoglobulin levels, age, WBC count and lymphocyte subset. Receiver operating characteristic (ROC) curves analysis was performed to determine the most appropriate cut-off points for the individual parameters: IgG, IgA, IgM, IgE, WBC count, and absolute eosinophil count. The cut-off values were used to transform continuous data into binary (0,1) variables. These variables were included in the logistic regression model to calculate the probability of the accuracy of a diagnostic value for a diagnosis of autism. Two-tailed p < 0.05 were considered statistically significant. MedCalc was used for the ROC analysis, GraphPad Prism 5.0 was used for comparative analysis, and a logistic regression model was created using Statistica 9.0 software.
Results
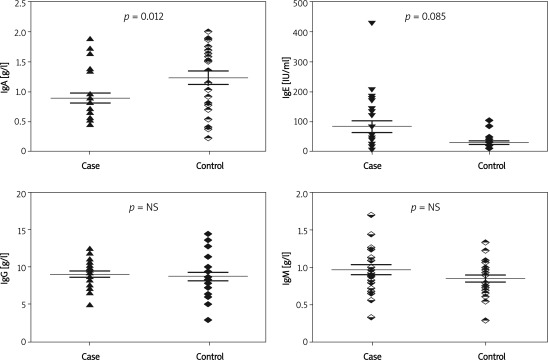
Table I shows the baseline characteristics of the study participants. Both groups had a similar perinatal history and did not differ in physical characteristics at the time of the study. Both were without clinical or laboratory (hsCRP, TNF-α) signs of infection. There was no difference in the white blood cell count in the studied groups of children although a tendency to have a higher peripheral eosinophil count (p = 0.08) was observed in children with autism. A comparison of immunoglobulin levels is presented in Figure 1. Median and interquartile range (IQR) of serum IgA was lower in children with autism compared to the controls (0.82 g/l [0.61-0.95] vs. 1.43 g/l [0.80-1.62]; p < 0.012). None of the children fulfilled the criteria of IgA deficiency or partial IgA deficiency. Lower serum IgA levels were associated with a higher white blood cell count (r = –0.50, p < 0.01) in children with autism but not in healthy children. Median and IQR levels of IgE were higher in the sera of children with autism although the difference was not significant (Table I). IgE levels above the upper limit for age were noted in 12/24 (50.0%) children with autism and in 5/24 (20.8%) of the healthy children (p = 0.035). In healthy children the total serum IgE level showed a correlation with age (r = 0.59, p < 0.05) but not in children with autism.
Table I.
Baseline characteristics of study participants
| Parameter | Autism group (N = 24) | Control group (N = 24) | Value of p |
|---|---|---|---|
| Age [years] (95% CI) | 4.25 ±1.70 (3.5-4.9) | 4.25 ±2.20 (3.3-5.1) | NS |
| Male, n (%) | 23 (95.8) | 23 (95.8) | NS |
| Urban residence, n (%) | 18 (75) | 18 (75) | NS |
| Vaginal delivery/C.S. | 19/5 | 21/3 | NS |
| Gestational age at birth [weeks] | 38.8 ±0.6 | 38.1 ±0.2 | NS |
| Birth weight [g] | 3270 ±83 | 3491 ±32 | NS |
| Apgar score | 9.6 ±0.3 | 9.4 ±0.6 | NS |
| Breast feeding duration [months] | 5.4 ±2.9 | 5.2 ±3.3 | NS |
| Familial atopy, n (%) | 7 (29.2) | 4 (16.7) | NS |
| BMI [kg/m2] | 15.4 ±0.8 | 16.7 ±1.6 | NS |
| hsCRP [mg/l] | 0.1 ±0.02 | 0.05 ±0.01 | NS |
| TNF-α [pg/ml] | 0.3 ±0.0 | 0.2 ±0.0 | NS |
| WBC [cells/Ml] (95% CI) | 8.0 ±2.3 (7.0-9.1) | 6.9 ±1.7 (6.2-7.7) | NS |
| IgA [g/l] Median (IQR) | 0.815 (0.61-0.95) | 1.43 (0.80-1.62) | 0.012 |
| IgE [g/l] Median (IQR) | 41.0 (12.0-136.5) | 20.0 (9.0-36.5) | NS (0.08) |
| Blood eosinophils [cells/μl] Median (IQR) | 220.5 (125.6-334.3) | 159.0 (107.1-202.3) | NS (0.08) |
| % WBC | 3.4 ±2.7 | 2.2 ±0.8 | |
| Patients with eosinophilia > 3% | 9 | 3 | 0.047 |
| IgG Gluten [mgA/l] Median (IQR) | 4.0 (2.53-7.64) | 5.3 (3.53-7.04) | NS |
C.S. – caesarean section, BMI – body mass index, WBC – white blood cells, TNF-α – tumour necrosis factor α
Values are mean (± SD) unless otherwise stated, IQR – interquartile range
Figure 1.
Comparison of serum immunoglobulin levels of IgA, IgE, IgG and IgM in children with autism (case) and in healthy (control) children (mean with SEM, two-tailed p)
The groups did not differ in mean serum IgM levels (0.96 ±0.33 g/l vs. 0.86 ±0.23 g/l) or serum IgG levels (9.02 ±1.78 g/l vs. 8.75 ±2.62 g/l). The values of both immunoglobulins were within the reference standards for age. The subclass of IgG containing gluten-specific IgG was comparable in both groups.
Diagnostic accuracy was determined by area under curve (AUC) from receiver operating characteristic (ROC) analyses. The cut-off values for the respective variables are presented in the table with the results of the ROC analyses (Table II). The humoral immunity profile, described by three binary variables, IgA < 0.97 g/l, IgE > 36 IU/ml, and IgG > 6.3 g/l, with a sensitivity of 79% and a specificity of 83% (p < 0.0001), was able to identify children with autism (Table III). The highest risk of autism diagnosis was associated with IgA < 0.97 g/l (OR – 23.0; 95% CI 3.41-159.00; p < 0.001).
Table II.
Receiver operating characteristics of serum immunoglobulin levels in distinguishing between children with autism and control children – sensitivity and specificity for cut-off values of evaluated parameters
| Parameter | Cut-off value | Sensitivity (95% CI) | Specificity (95% CI) | AUC ± SE (95% CI) |
|---|---|---|---|---|
| Serum IgA [g/l] | 0.97 | 66.7 (44.7-84.3) | 79.2 (57.8-92.8) | 0.681 ±0.077 (0.531-0.808) |
| Serum IgE [IU/ml] | 36 | 79.2 (57.8-92.8) | 58.3 (36.7-77.9) | 0.646 ±0.08 (0.495-0.778) |
| Serum IgG [g/l] | 6.3 | 20.8 (7.2-72.2) | 95.8 (78.8-99.3) | 0.635 ±0.08 (0.483-0.769) |
| Serum IgM [g/l] | 1.08 | 87.5 (67.6-97.2) | 33.3 (15.7-55.9) | 0.586 ±0.083 (0.435-0.726) |
| Eosinophil count [cells/ul] | 244.4 | 91.7 (73.0-98.7) | 50.0 (23.1-70.9) | 0.648 ±0.08 (0.496-0.780) |
AUC – area under the ROC curve, CI – confidence interval, SE – standard error
Table III.
Logistic regression results with serum IgA, IgE, and IgG as the outcome variable
| Variables included in the regression model | Regression coefficient | SE | Wald statistic | Value of p | OR | 95% CI for OR lower-upper |
|---|---|---|---|---|---|---|
| IgA< 0.97 g/l | −3.151 | 0.095 | 10.903 | 0.00096 | 23.0 | 3.41-159.89 |
| IgE> 36 IU/ml | 2.233 | 0.922 | 5.861 | 0.01649 | 9.3 | 1.45-59.91 |
| IgG> 6.3 g/l | 2.924 | 1.388 | 4.438 | 0.03516 | 18.6 | 1.14-305.20 |
OR – odds ratio
Mean values (95% CI) of peripheral blood lymphocyte subpopulations in the examined groups of children are shown in Table IV. The groups did not differ in white cell counts, in number of CD3, CD4, CD8, CD19, NK, T regulatory cells (CD4/CD25), or in the CD4/CD8 ratio. Children diagnosed with autism differed in having a higher number of CD19/CD23 from children in the control group. No correlation between the number of CD23-positive cells and serum IgE levels was observed.
Table IV.
Lymphocyte subpopulations of peripheral blood expressed as percentages in children diagnosed with regressive autism and in healthy children
| Parameter | Autism group (N = 24) Mean ± SD (95% CI) | Control group (N = 24) Mean ± SD (95% CI) | Value of p |
|---|---|---|---|
| White cell count [G/l] | 8.39 ±0.56 (7.25-9.54) | 8.12 ±0.51 (7.06-9.17) | 0.7210 |
| CD3 | 65.21 ±2.38 (60.35-70.07) | 66.99 ±2.24 (62.36-71.34) | 0.2605 |
| CD4 | 36.91 ±0.96 (34.93-38.89) | 39.95 ±2.37 (34.91-45.00) | 0.2699 |
| CD8 | 25.02 ±1.47 (22.03-28.02) | 22.71 ±1.37 (19.85-25.56) | 0.1466 |
| NK | 10.57 ±0.83 (8.88-12.26) | 9.24 ±1.18 (6.80-11.67) | 0.3201 |
| CD4/CD8 | 1.85 ±0.30 (1.24-2.46) | 1.99 ±0.20 (1.56-2.42) | 0.7874 |
| CD4/CD25 | 10.55 ±2.10 (6.13-14.97) | 8.09 ±1.15 (5.55-10.64) | 0.3784 |
| CD19 | 18.66 ±1.02 (16.57-20.76) | 19.41 ±2.37 (14.49-24.32) | 0.8311 |
| CD19/CD23 | 36.82 ±6.72 (22.89-50.73) | 18.10 ±3.95 (9.90-26.29) | 0.0134 |
Discussion
It was found that autistic children between 3 and 6 years old differed in the humoral immunity profile and B cell activity from healthy age-matched controls. The pattern of serum immunoglobulins in children with autism included three components: IgA < 0.97 g/l, IgE > 36 IU/ml, and IgG > 6.3 g/l. The immunoglobulin levels analysed together had higher sensitivity and specificity and were more able to distinguish children with autism from their healthy peers than specific immunoglobulins considered separately. Of these 3 components, IgA had the most important contribution in the humoral immunity profile in children with autism.
A recent study estimated that serum IgA was lower in children with autism than that found in age- and sex-matched controls even though none of the children fulfilled the criteria of IgA deficiency or partial IgA deficiency. In healthy children the production of IgA matures gradually; at the age of 12 months the level of IgA in the serum accounts for about 20% of the adult value and at approximately 10 years of age reaches adult levels. The age of the studied patients did not exceed 7 years; therefore, the maturation process of IgA production could not have been completed and it could be suspected that this process may be slower in children with autism compared to healthy children. Similarly, other authors’ observations suggest that IgA in children with autism may be within the lower limits of normal or slightly below and that the prevalence of autism is low in the IgA deficient group [19, 20]. However, Warren et al. described IgA deficiency in a subset of patients with autism and found that the IgA deficit is associated with HLA-DR antigens [21].
Impaired humoral immunity manifested as low IgA may be associated with disorders of the gastrointestinal tract such as colonization with anaerobic bacteria and gastrointestinal symptoms including abdominal pain, bloating, diarrhoea, and constipation [8]. These types of gastrointestinal symptoms are described in 9.0-84.1% of children with autism compared to 9.0-37.0% of children without autism [22]. Although most truly IgA-deficient children have no clinical problems, children with the symptomatic form may suffer from diarrhoea, otitis, sinusitis, autoimmune diseases or atopic diseases. Another medical aspect of the maturational delay in IgA is that it may be associated in some children with non-IgE-mediated food allergies [23].
In this study, total serum IgE in children with autism was higher than in healthy children, but the difference did not reach the significance threshold. However, serum IgE levels above the reference range for age were significantly more common in the group of children with autism than in the control group (50.0% vs. 20.8%). IgE was also entered as a significant variable in the logistic regression model. Therefore, it appears that at least for some children with autism serum IgE levels may have clinical significance. Also, Gupta found an increase in IgE in 52.0% among 25 children with autism (13/25) [24]. There are, however, studies in which there is no increase in IgE in children with autism [9, 25]. Total IgE exceeding the norm may indicate an allergic reaction but can be a sign of infection, autoimmune disease or the presence of cancer, as well as occur in healthy people [26].
The results of this study raise the question of whether there is a link between the reduction of IgA and the increase of IgE. Overstimulation of the IgE system because of a qualitative defect in IgA production is a known phenomenon [27]. It can be speculated that this could pertain to some of the studied children. This concept, however, requires further research.
Sensitivity to food has been suggested as a potential aetiological factor in childhood autism [28]. An IgE-dependent allergy to food and environmental antigens does not appear to be more prevalent in children with autism [25, 29]. IgE-independent reactions such as delayed hypersensitivity reactions are quite common [30]. In this study the serum gluten-specific IgG levels were assessed and the results were comparable in both groups. A higher prevalence of atopy in families of children with autism was not found; however, allergic disorders in the family and maternal autoimmune diseases were found to be more common in children with autism in other studies [28]. In the Croen study, asthma and maternal atopy were more strongly associated with autism in families with more than one ASD-affected child, thus suggesting that genes underlying atopy may also be aetiologically related to autism [31].
In a recent study, children with autism demonstrated a tendency to have a higher percentage of eosinophils among peripheral blood leukocytes. The criteria to diagnose true eosinophilia were not fulfilled; the upper confidence limit did not exceed the reference value of 400/µl. The results of this study are consistent with research by Renzoni et al. [29]. Eosinophilia in the patients did not correlate with serum IgE and could possibly have been due to factors other than allergy. Eosinophilia as a nonspecific symptom may be associated with such diseases such as parasitic infestations, non-parasitic infections, immune disorders, or endocrine diseases. Recently, a boy with autism and eosinophilic oesophagitis, which is a gastrointestinal disorder that may present with peripheral eosinophilia and increased serum IgE levels, was described [32, 33].
The IgG class antibodies are the basic antibodies produced by the immune system in response to foreign antigens. In this study no differences were found in the serum levels of IgG between the children with autism and the children in the control group. Other authors’ observations pertaining to IgG and IgM in children with autism are conflicting. Heuer et al. described decreased levels of IgG and IgM in children with autism and found that this reduction correlated with behavioural severity [34]. Enstrom et al. observed a significant increase in IgG4 subclasses and Croonenberghs et al. found increased IgG due to a rise in specific IgG subclasses IgG2 and IgG4 in subjects with autism [35, 36]. The authors note that the increased serum concentrations of IgG in autism may point towards an underlying autoimmune disorder. A family history of autoimmune disorders is more common among children with autism than healthy control children [10, 37].
Some studies indicate that the cellular arm of the immune system could be either activated or abnormal in individuals with autism [38, 39]. A reduced number of T cells was not found and the CD4/CD8 ratio was normal in this study. Lack of disorders in major T cell subpopulations can be explained by the young age of patients, short time of disease duration, and a regressive form of autism (regression after the period of normal development). According to Warren et al., only children expressing the complete syndrome of autistic disorder had significantly reduced numbers of both total T cells and CD4 cells [40]. Treg cells are regarded as the primary mediators of peripheral tolerance and play a pivotal role in the pathogenesis of autoimmune and immunosuppressive diseases. An increased number of Tregs in the studied children was not found.
In the current study, increased expression of CD19/CD23 in children diagnosed with autism was found. CD23 is a surface marker of activated B cells as well as a low-affinity Fc receptor for IgE (Fc-Epislon-RII) and is involved in the development of different kinds of diseases including, but not limited to, allergy and inflammatory or lymphoproliferative diseases (soluble CD23) [41]. CD23 is involved in the regulation of IgE responses: IgE-mediated antigen presentation, regulation of IgE synthesis, and differentiation of B and T cells [42]. CD23 is known to act as a negative regulator of IgE production and its expression is low in allergic disorders such as allergic rhinitis and allergic asthma [41, 43]. Monoclonal antibodies against the CD23 antigen inhibit IgE production by B lymphocytes and decrease total serum IgE [44]. Increased expression of CD23 in the studied children diagnosed with autism corresponded with no difference in total serum IgE level from children in the control group. This result, therefore, cannot be linked with IgE production. Moreover, a correlation between CD23 and serum IgE level was not observed. The lack of a difference in levels of serum TNF-α between both studied groups can argue against a relationship between increased expression of CD23 and inflammation in those children. However, an isolated marker is insufficient to make such a conclusion. Assessment of other inflammatory indices, and especially measurement of serum soluble CD23 (sCD23) concentrations, could provide more data to understand the findings. sCD23 is implicated in the up-regulation of IgE synthesis and elevated concentrations of circulating sCD23 are commonly associated with inflammatory or lymphoproliferative diseases, such as rheumatoid arthritis, asthma, and chronic lymphoblastic leukaemia [45]. It would be interesting to know if sCD23 correlates with serum IgE in children with autism with elevated serum IgE. CD23 is expressed on B-cells following cell activation by antigens. The results of this study suggest that B-cell activation in children diagnosed with autism takes place.
Immune system abnormalities reported in children with an autistic disorder suggest that immune factors may be involved in the initiation or the evolution of neurological disorders [39]. This study confirms that children with autism can have a subtle difference in the humoral and cellular immunity profile at the moment of diagnosis than age-matched controls. However, these results are not without limitations, one being the number of subjects. This is especially visible in the logistic regression as a wide confidence interval for the odds ratio. Additionally, increasing the number of children in the control group so that the case to control ratio is 1 : 2 or more would have provided more informative results.
In conclusion, although extreme deviations and evident pathologies were not found, the pattern of humoral and cellular immunity in the studied children with autism can be described as low-normal IgA and high-normal IgE with B-cell activation manifesting as increased expression of a low affinity receptor for IgE. An increased number of CD23-positive cells was not previously described in children with autism. Identification of an antigen responsible for the increase of CD23 expression requires further research. Irrespective of the cause, this subtle shift of serum immunoglobulins and B-cell activation may be related to inflammatory or allergic reactions and autoimmunity described in children with autism.
Acknowledgments
The authors would like to sincerely thank all the children and parents who participated in this study. This study is part of a project funded by the Ministry of Science and Higher Education No. N N407 53 1538.
References
- 1.Cohen JJ. Immunity and behavior. J Allergy Clin Immunol. 1987;79:2–5. doi: 10.1016/s0091-6749(87)80005-2. [DOI] [PubMed] [Google Scholar]
- 2.Ader R, Felten D, Cohen N. Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol. 1990;30:561–602. doi: 10.1146/annurev.pa.30.040190.003021. [DOI] [PubMed] [Google Scholar]
- 3.Lang B, Dale RC, Vincent A. New autoantibody mediated disorders of the central nervous system. Curr Opin Neurol. 2003;16:351–7. doi: 10.1097/01.wco.0000073937.19076.d5. [DOI] [PubMed] [Google Scholar]
- 4.Moscavitch SD, Szyper-Kravitz M, Shoenfeld Y. Autoimmune pathology accounts for common manifestations in a wide range of neuro-psychiatric disorders: the olfactory and immune system interrelationship. Clin Immunol. 2009;130:235–43. doi: 10.1016/j.clim.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–41. doi: 10.1016/s0074-7742(05)71013-8. [DOI] [PubMed] [Google Scholar]
- 6.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 7.Association AP. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 2000. text revision (DSM-IV-TR) [Google Scholar]
- 8.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 9.Castellani ML, Conti CM, Kempuraj DJ, et al. Autism and immunity: revisited study. Int J Immunopathol Pharmacol. 2009;22:15–9. doi: 10.1177/039463200902200103. [DOI] [PubMed] [Google Scholar]
- 10.Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24:664–73. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- 11.Enstrom AM, Lit L, Onore CE, et al. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23:124–33. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrente F, Ashwood P, Day R, et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry. 2002;7:375–82, 34. doi: 10.1038/sj.mp.4001077. [DOI] [PubMed] [Google Scholar]
- 13.Padhye U. Excess dietary iron is the root cause for increase in childhood autism and allergies. Med Hypotheses. 2003;61:220–2. doi: 10.1016/s0306-9877(03)00126-9. [DOI] [PubMed] [Google Scholar]
- 14.Braunschweig D, Ashwood P, Krakowiak P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–31. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jyonouchi H. Food allergy and autism spectrum disorders: is there a link? Curr Allergy Asthma Rep. 2009;9:194–201. doi: 10.1007/s11882-009-0029-y. [DOI] [PubMed] [Google Scholar]
- 16.Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD003498.pub3. CD003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinhausen HC, Erdin A. Abnormal psychosocial situations and ICD-10 diagnoses in children and adolescents attending a psychiatric service. J Child Psychol Psychiatry. 1992;33:731–40. doi: 10.1111/j.1469-7610.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 18.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 19.Santaella ML, Varela Y, Linares N, Disdier OM. Prevalence of autism spectrum disorders in relatives of patients with selective immunoglobulin A deficiency. P R Health Sci J. 2008;27:204–8. [PubMed] [Google Scholar]
- 20.Spiroski M, Trajkovski V, Trajkov D, et al. Family analysis of immunoglobulin classes and subclasses in children with autistic disorder. Bosn J Basic Med Sci. 2009;9:283–9. doi: 10.17305/bjbms.2009.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren RP, Odell JD, Warren WL, et al. Brief report: immunoglobulin A deficiency in a subset of autistic subjects. J Autism Dev Disord. 1997;27:187–92. doi: 10.1023/a:1025895925178. [DOI] [PubMed] [Google Scholar]
- 22.Wasilewska J, Jarocka-Cyrta E, Kaczmarski M. Gastrointestinal abnormalities in children with autism [Polish] Pol Merkur Lekarski. 2009;27:40–3. [PubMed] [Google Scholar]
- 23.Latcham F, Merino F, Lang A, et al. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J Pediatr. 2003;143:39–47. doi: 10.1016/S0022-3476(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S. Immunological treatments for autism. J Autism Dev Disord. 2000;30:475–9. doi: 10.1023/a:1005568027292. [DOI] [PubMed] [Google Scholar]
- 25.Bakkaloglu B, Anlar B, Anlar FY, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol. 2008;12:476–9. doi: 10.1016/j.ejpn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Nowicka U. Disorders with elevated immunoglobulin E levels [Polish] Pneumonol Alergol Pol. 2009;77:533–40. [PubMed] [Google Scholar]
- 27.Lamm ME. Cellular aspects of immunoglobulin A. In: Kunkel FJ, Da HG, editors. Advances in immunology. Elsevier: 1976. [DOI] [PubMed] [Google Scholar]
- 28.Murch S. Diet, immunity, and autistic spectrum disorders. J Pediatr. 2005;146:582–4. doi: 10.1016/j.jpeds.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Renzoni E, Beltrami V, Sestini P, Pompella A, Menchetti G, Zappella M. Brief report: allergological evaluation of children with autism. J Autism Dev Disord. 1995;25:327–33. doi: 10.1007/BF02179294. [DOI] [PubMed] [Google Scholar]
- 30.Jyonouchi H. Non-IgE mediated food allergy. Inflamm Allergy Drug Targets. 2008;7:173–80. doi: 10.2174/187152808785748119. [DOI] [PubMed] [Google Scholar]
- 31.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–7. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 32.Jarocka-Cyrta E, Wasilewska J, Kaczmarski MG. Brief report: eosinophilic esophagitis as a cause of feeding problems in autistic boy. The first reported case. J Autism Dev Disord. 2010;41:372–74. doi: 10.1007/s10803-010-1059-y. [DOI] [PubMed] [Google Scholar]
- 33.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Heuer L, Ashwood P, Schauer J, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–83. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enstrom A, Krakowiak P, Onore C, et al. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23:389–95. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croonenberghs J, Wauters A, Devreese K, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–63. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 37.Ashwood P, Van de Water J. A review of autism and the immune response. Clin Dev Immunol. 2004;11:165–74. doi: 10.1080/10446670410001722096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess NK, Sweeten TL, McMahon WM, Fujinami RS. Hyperserotoninemia and altered immunity in autism. J Autism Dev Disord. 2006;36:697–704. doi: 10.1007/s10803-006-0100-7. [DOI] [PubMed] [Google Scholar]
- 39.Krause I, He XS, Gershwin ME, Shoenfeld Y. Brief report: immune factors in autism: a critical review. J Autism Dev Disord. 2002;32:337–45. doi: 10.1023/a:1016391121003. [DOI] [PubMed] [Google Scholar]
- 40.Warren RP, Margaretten NC, Pace NC, Foster A. Immune abnormalities in patients with autism. J Autism Dev Disord. 1986;16:189–97. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- 41.Lemieux GA, Blumenkron F, Yeung N, et al. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem. 2007;282:14836–44. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acharya M, Borland G, Edkins AL, et al. CD23/FcepsilonRII: molecular multi-tasking. Clin Exp Immunol. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol. 2003;112:563–70. doi: 10.1016/s0091-6749(03)01861-x. [DOI] [PubMed] [Google Scholar]
- 44.Stokes J, Casale TB. Rationale for new treatments aimed at IgE immunomodulation. Ann Allergy Asthma Immunol. 2004;93:212–7. doi: 10.1016/S1081-1206(10)61490-1. [DOI] [PubMed] [Google Scholar]
- 45.Hibbert RG, Teriete P, Grundy GJ, et al. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med. 2005;202:751–60. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]