Abstract
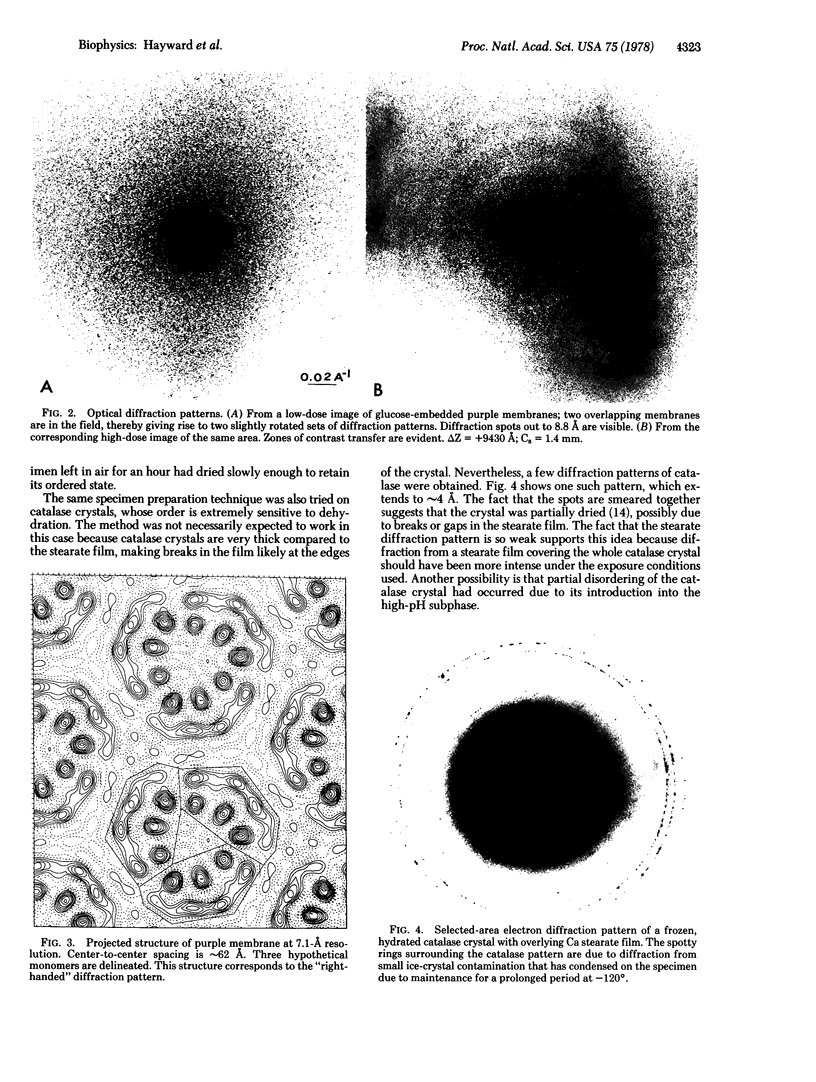
The direction of orientation of the protein bacteriorhodopsin within the purple membrane of Halobacterium halobium has been determined by selected-area electron diffraction of membranes preferentially oriented by adsorption to polylysine. Purple membrane is known to adsorb preferentially to polylysine by its cytoplasmic surface at neutral pH and by its extracellular surface at low pH. To maintain the adsorbed membranes in a well-ordered state in the electron microscope, an improved technique of preparing frozen specimens was developed. Large areas of frozen-hydrated specimens, devoid of bulk water, were obtainable after the specimen was passed through a Ca stearate film at an air-water interface. High-resolution microscopy was used to relate the orientation observed in the electron diffraction patterns to the orientation of the projected structure that is obtained from images. We have found that the three-dimensional structure determined by Henderson and and Unwin [Henderson, R. & Unwin, P.N.T. (1975) Nature 257, 28--32] is oriented with the cytoplasmic side uppermost--i.e., the helices fan outward on the cytoplasmic side of the membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Keefer L. M., Bradshaw R. A. Structural studies on Halobacterium halobium bacteriorhodopsin. Fed Proc. 1977 May;36(6):1799–1804. [PubMed] [Google Scholar]
- Kuo I. A., Glaeser R. M. Development of methodology for low exposure, high resolution electron microscopy of biological specimens. Ultramicroscopy. 1975 Jul;1(1):53–66. doi: 10.1016/s0304-3991(75)80007-6. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Stoeckenius W. Comparison of purple membrane from Halobacterium cutirubrum and Halobacterium halabium. Biochim Biophys Acta. 1976 Apr 5;426(4):703–710. doi: 10.1016/0005-2736(76)90135-8. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Glaeser R. M. Electron microscopy of frozen hydrated biological specimens. J Ultrastruct Res. 1976 Jun;55(3):448–456. doi: 10.1016/s0022-5320(76)80099-8. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]