Abstract
Introduction
Immunophilin ligands provide potentially new alternatives for the treatment of erectile dysfunction (ED) which occurs after injury of the cavernous nerves (CN).
Aim
To review and update current knowledge of the neurotrophic effects and likely mechanism of action of immunophilin proteins with emphasis on the FK506-binding protein (FKBP) subfamily and the role of immunophilin ligands for the treatment of CN injury induced ED.
Methods
Review of available reports of studies investigating the effects and neurotrophic mechanisms of immunophilin ligands involved in erectile function recovery in rodent models of CN injury.
Main Outcome Measures
Erection parameters and molecular correlations associated with CN injury and functional recovery.
Results
Treatment with prototype immunosuppressive immunophilin ligands FK506 (FK) and Rapamycin (Rapa) improve erectile function in animal models of CN injury. Similarly, non-immunousuppressive analogs such as GPI-1046 and FK1706 are effective in recovery of erections after CN injury. Neuronal nitric oxide may influence the erection recovery effects of immunophilin ligands after CN injury. FKBPs 38 and 65 expression changes in the penis and its innervation coincide with the neurotrophic effects of immunophilin ligands. Antioxidative actions of immunophilin ligands contribute to their neurotrophic effects. Immunophilins are localized to nerves coursing in human prostate and penile tissue.
Conclusions
The findings support the hypothesis that immunophilin ligands, working through specific receptor mechanisms which are specific to injured CN, are potentially useful to sustain erectile function in men following radical prostatectomy.
Introduction
Erectile dysfunction (ED) continues to be a major complication following radical prostatectomy which impacts the patient’s quality of life [1, 2]. With the acknowledgment that penile neuropathy is a major pathogenic basis for ED in patients after radical prostatectomy (RP), there has been great interest to promote neuroprotective and nerve regenerative strategies for facilitating functional recovery. In this regard, investigators have sought to evaluate the neurotrophic effects of immunophilin proteins and immunophilin ligands mostly in preclinical cavernous nerve (CN) injury studies for the purposes of developing a potential basis for the treatment of neurogenic ED. In this paper, we will review the current literature in this field and briefly present new findings from our laboratory, which contribute towards understanding the mechanisms of immunophilin actions and the beneficial effects of immunophilin ligands in this setting.
Immunophilin Proteins and Ligands
Immunophilins are a large group of cellular proteins that were initially identified as targets for drugs like FK506 (FK), Cyclosporin and Rapamycin (Rapa)[3, 4]. Based on the selectivity of this binding, one of the main families of immunophilins are FK506 Binding Proteins (FKBPs), which act as receptors for FK and Rapa. Several FKBPs have been identified in various cellular and subcellular locations (cytosol, endoplasmic reticulum, mitochondria and nucleus)[5]. To date, more than 15 FKBP sequences have been identified in the human genome that are expressed in a variety of cell types, and they are designated according to their molecular weights, which range from 12 to 135 kDa [5–7]. All FKBPs have a PPIase (peptidyl-prolyl cis trans isomerase) domain that regulates the folding/unfolding of other proteins. However, the domain compositions differ in different FKBPs (eg. DNA-binding domains, calmodulin binding domain, transmembrane motifs or nuclear localization signal)[3, 6]. Reflecting the diversity of their structures, FKBPs are multifunctional proteins that act as chaperone proteins, regulate protein folding, and participate in intracellular protein trafficking. FKBPs are proposed to be the modulator proteins of receptors in a cell. For example, FKBP12 is associated with inositol 3-phosphate and ryanodine receptors present on the endoplasmic reticulum and plays a role in calcium release[8]. Similarly, FKBP12 modulates the activity of transforming growth factor (TGF)-β. On the other hand, FKBPs 52 and 51 are known to be components of unliganded steroid receptor heterocomplexes, an interaction that occurs via binding heat shock protein 90 by tetratricopeptide repeat (TPR) domains of FKBPs [9]. It has been suggested that FKBP52 plays a role in the nuclear translocation of steroid hormone receptors [10]. Moreover, FKBPs 38 and 65 are involved in the regulation of cellular functions such as transcription, apoptosis and inflammatory responses [11–13].
FK (Tacrolimus) and Rapa (Sirolimus) are two of the prototype immunophilin ligands that are natural macrolide compounds discovered in 1984 as a product of Streptomyces tsukubaenis and in 1971 as a product of Streptomyces hygroscopius, respectively. Both drugs have been routinely used to prevent allograft rejection following transplant surgeries due to their potent immunosuppressive effects [14, 15]. The immunosuppressive effects of FK and Rapa are mediated by binding to FKBP12, one of the small cytoplasmic proteins of the FKBP family, in immune cells, thereby inhibiting their activation [14]. Moreover, both drugs can bind to other FKBPs with varying affinities, although the effects mediated by other FKBPs are not completely understood [16].
Neurotrophic Effects of Immunophilins
Beyond their expressions in immune cells and role in immunosuppression, immunophilins are found to be enriched in the nervous system. Steiner et al., discovered that the FKBP12 level in the rat brain is 10–15 fold higher than its expression in immune tissues suggesting that these receptor proteins and their ligands exert important roles in the nervous system [17]. These investigators further demonstrated regenerative effects of FK by the enhancement of neurite outgrowth in cultures of the rat PC12 cell line and sensory ganglia treated with this drug [18, 19]. Neuroprotective effects of immunophilin ligands were also demonstrated in a number of animal studies. In particular, in animal models of focal cerebral ischemia, stroke and sciatic nerve injury, treatment with FK provided protection against nerve injury and enhanced functional recovery [20–22].
Effects of Immunophilins after Cavernous Nerve Injury
Based on solid evidence from in vitro and in vivo studies of the neurotrophic effects of immunophilin ligands, the potential benefit of these drugs for the recovery of erectile function following radical prostatectomy has been investigated in animal models of CN injury. We first demonstrated in a rat model of partial CN injury that treatment with FK (1mg/kg/day, ip) improved the erectile response induced by electrical stimulation of CN at 1, 3 and 7 days after injury[23]. When the cross sections of CNs were examined under electron microscopy, we also found that FK treatment significantly increased the number of surviving unmyelinated axons at all time points after injury, clearly demonstrating the neuroprotective effects of the immunophilin ligand treatment. In other studies, it was also found that FK was highly effective in maintaining erectile function in a more severely injured rat model involving focal transection or bilateral crush of the CN [24, 25]. We have further tested the effects of Rapa in a rat model of bilateral CN injury. Treatment with Rapa (2 mg/kg/day, sc) produced an approximate 50% increase in erectile responses measured as the maximum intracavernous pressure (ICP) and total ICP at 1 and 7 days after injury (unpublished data). Currently, an ongoing clinical trial (Phase IV, randomized, double-blind, placebo-controlled) is evaluating the safety and effectiveness of FK in the prevention of ED in men following bilateral nerve-sparing RP [26].
For neuroprotective/nerve regenerative therapy, FK and Rapa have potential advantages over conventional nerve growth factors since they are non-peptides, lipophilic and selectively effective for damaged nerves. However, these immunophilin ligands have been clinically linked with a number of adverse effects when administered chronically such as immunosuppression and nephrotoxicity. To circumvent these problems, non-immunosuppressive analogs of these ligands with neurotrophic and neuroprotective effects have been developed. The non-immunosuppressive analogs of FK, GPI-1046 and FK1706, have been proven to preserve cavernous tissue structure and erectile function in rats after CN injury [27–29]. A recent clinical trial (Phase II, multi-center, randomized, double-blind, placebo-controlled, 3 arm, 12-month study) was conducted to evaluate the effects of GPI-1485 , a non-immunosuppressive analog of FK, from Guilford Pharmaceuticals (now MGI Pharma, Inc.), on erectile function in men undergoing nerve sparing RP [30]. Although administration of GPI-1485 did not reveal significance among treatment groups, it is possible that drug formulation or scheduling was suboptimal. Further investigation in this area remains warranted. Recently, two non- immunosuppressive analogs of Rapa have been developed and demonstrated to be neuroprotective in cortical neuron excitotoxicity and stroke [31]. It would be interesting to test the effectiveness of these compounds in the treatment of CN injury induced ED, which may further contribute toward understanding the role of immunophilins in neuroprotection.
Mechanisms of Neuroprotection
The mechanism by which immunophilin ligands elicit their neuroprotective effects is not well understood. Although FKBP12 mediates immunosuppression, its involvement in FK-induced neuroprotection is still under debate. Based on the ability of FK and other immunophilin ligands to form complexes with diverse FKBPs, the role of other FKBPs and other potential mechanisms have been explored. As a first step towards understanding the role of different FKBPs, we have examined the erectile function in rats and changes in expressions of FKBPs 38, 52 and 65 in the rat penis after CN injury. Erectile function, measured as the change in ICP to CN electro-stimulation, was significantly lower 1 day after injury compared to a sham group, whereas it was significantly higher in animals treated with FK or Rapa compared to vehicle treatment responses (unpublished data).
Penile expressions of FKBPs 38 and 65 were significantly decreased after CN injury compared to sham treatment without a significant change in FKBP52 expression in the penis in these 2 groups (unpublished data). Since FKBP38 is suggested to be involved in antiapoptotic mechanisms in the cell [32], the decreased penile expression of FKBP38 may indicate increased apoptotic changes in the penis after CN injury.
Although the exact mechanism is not known, FKBP65 has been suggested to be developmentally regulated and later in adulthood to be involved in cellular repair mechanisms [11]. The decreased expression of FKBP65 in the penis may indicate impaired repair mechanisms that may be involved in erectile impairment. However, at a 1 day interval after CN injury, FK treatment prevented the decreased expressions of FKBPs 38 and 65, and Rapa treatment prevented the decreased expressions of FKBP38 but not FKBP65 (unpublished data), suggesting that the neuroprotective effects of FK involve prevention of apoptosis and activation of cellular repair mechanisms whereas that for Rapa involves altered apoptotic pathways without affecting repair mechanisms.
The main neuronal innervation of the penis is provided via CN which arise bilaterally from the major pelvic ganglia (MPG). It has been well established that injury of a nerve induces physical and functional changes of its fibers distally in the target tissue, as well as in the ganglion cells where the nerve cell bodies are localized. Therefore, in our model of CN injury, in addition to exploring molecular changes in the penis, we have investigated FKBPs 38, 52, and 65 in the MPG of these animals. One day after CN injury, similar to findings in the penis, the expression of FKBP38 decreased in the MPG without significant expressional changes in FKBP52 or FKBP65 (unpublished data). FK treatment prevented the decrease in FKBP38 expression and further increased the FKBP65 expression, whereas Rapa treatment did not affect the expressions of either FKBP (unpublished data). These results indicate that FK may stimulate antiapoptotic and repair mechanisms not only in the penis but also in the MPG in contrast with Rapa, which appears to mediate its effect via actions on penile tissue. In our ongoing studies, these observations are extended to later time points after injury to better understand the role of immunophilins in recovery of erectile function.
Nitric oxide (NO), the major neuronal mediator of penile erection, may also participate in neurotrophic mechanisms in the penis. However, the role of neuronal NO in neuroprotection/regeneration has been controversial. Support for the beneficial effects of this molecule in physiologic nerve regeneration is offered by the finding of regenerative delay in neuronal nitric oxide synthase (nNOS)-alpha knock out (nNOS−/−) mice in a sciatic nerve injury model and also by the findings of decreased survival of ganglion cells in these mice after axotomy [33, 34]. In contrast, it has been reported that after spinal cord injury, nNOS−/− mice have a better functional recovery indicating the negative influence of neuronal NO [35]. In addition, it has also been demonstrated that nitrotyrosine immunoreactivity, a marker of protein nitration, is attenuated in nNOS−/− mice after traumatic injury [36].
We wanted to evaluate whether nNOS and neuronal NO signaling exert beneficial or detrimental effects on the recovery of erectile function after CN injury and influences the effects of FKBPs and immunophilin ligands. nNOS−/− mice do preserve an approximately 10% nNOS activity in the penis, as we have recently published [37]. We performed unilateral CN injury in wild type (WT) and nNOS−/− mice and then evaluated these mice for erection physiology at 1, 3 and 7 days after injury. Both in WT and nNOS−/− mice, erectile response (measured as maximum ICP) significantly decreased 1 day after injury compared to sham treatment responses (unpublished data). At days 3 and 7 after injury, erectile responses remained low in WT animals, whereas in nNOS−/− mice, erectile responses were approximately similar to sham treatment responses (unpublished data). The improved recovery of CN stimulated erections after CN injury in nNOS−/−mice, relative to WT mice erection recovery, suggests that neuronal NO may act deleteriously in nerve functional recovery after CN injury. Conceivably, neuronal NO exerts a pathologic role in nitrosative and oxidative damage after CN injury, distinct from its role in physiologic penile erection. This concept is consistent with known neuropathic actions of NO in the face of neuronal diseases or injury [35, 36].
We have demonstrated that baseline expressions of FKBPs 38 and 52 were higher, whereas FKBP65 expression was lower in the nNOS−/− mouse penis compared to the WT mouse penis (unpublished data). One day after CN injury, only FKBP65 expression was increased in WT mouse penis without significant expressional changes in FKBPs 38 and 52 (unpublished data). In the nNOS−/− mouse penis, expressions of FKBPs 38 and 52 remained higher and FKBP65 remained lower, after CN injury (unpublished data). Although the exact mechanisms for the changes are not clear, differential expressions of FKBPs in nNOS−/−mouse penis compared to WT expressions suggest that the increased FKBP38 expression in nNOS−/− mice corresponds with a greater apoptotic basis in nNOS−/− mice and decreased FKBP65 expression corresponds with reduced cell repair mechanisms in these mice.
It has been demonstrated that physical disruption of neuronal tissue leads to secondary damage presumably due to oxidative stress as indicated by increased peroxynitrite which is formed by the reaction of superoxide and NO [38]. Tyrosine nitration is a protein modification mediated by peroxynitrite that leads to cytoskeletal damage and disturbances in energy production and signal transduction. Following traumatic brain injury, decreased protein nitration in the nNOS−/− mouse has been correlated with improved functional recovery [36]. We demonstrated in our rat CN injury model that nitrated protein levels increased in penile tissue after injury [39].
Evidence exists suggesting that the neuroprotection induced by immunophilin ligands involves antioxidative mechanisms. Both FK and GPI-1046 display antioxidative actions, mainly by their activation of glutathione synthesis [40, 41]. In culture systems, both GPI-1046 and FK increase intracellular glutathione content which prevents apoptotic cell death induced by oxidative stress [40–42]. To investigate the antioxidative actions of immunophilin ligands in the recovery of erectile function after CN injury, we examined the expressions of the cellular antioxidant enzyme glutathione peroxidase (GPX) and a marker of oxidative damage, nitrotyrosine (NT) [39]. In a rat model of CN crush injury, expression of NT is significantly increased in the penis after injury. However, FK treatment not only prevents the increase in NT expression, but also increases GPX expression in the penis. These findings further support the contribution of antioxidative mechanisms in the neuroprotection of immunophilin ligands.
Immunophilins in Human Penile Innervation
We have recently described the expressions of various FKBPs in the lower male genitourinary tract of humans [43]. FKBPs 12, 38, 52, 65, and 135 differentially localize to both neuronal (e.g., ganglia, myelinated and unmyelinated nerves and Schwann cell nuclei) and non-neuronal structures (i.e., epithelial, endothelial and smooth muscle cells) associated with the penis and prostate [43]. All FKBPs and nNOS localize to periprostatic ganglia and nerves in penile tissue, although immunoreactivity for FKBP38 is minimal. Interestingly, FKBP52 immunoreactivity is predominant in endoneurium suggesting that the neuroprotective role of FKBP52 may not apply just to nerve cells directly but also to the supporting cells of the nervous system. All FKBPs studied localize to urethral epithelium, whereas FKBPs 12 and 65 localize to vascular endothelial cells and FKBPs 12 and 52 localize to corporal smooth muscle cells. Our findings of FKBP expressions in both neuronal and non-neuronal genitourinary tract structures suggest that immunophilins may play a role in diverse pathways important in cellular protection following local pelvic nerve injury. They also support a scientific basis for the application of immunophilin ligands for preserving lower genitourinary tract function.
Conclusions
These results suggest the involvement of multiple molecular pathways which may be targeted by immunophilin ligands for protecting or recovering lower genitourinary tract functions in the face of CN injury (Figure). Further studies to identify the molecular pathways associated with the actions of FKBPs will advance the understanding of immunophilins and the development of selective immunophilin ligands with optimal clinical profiles.
Figure.
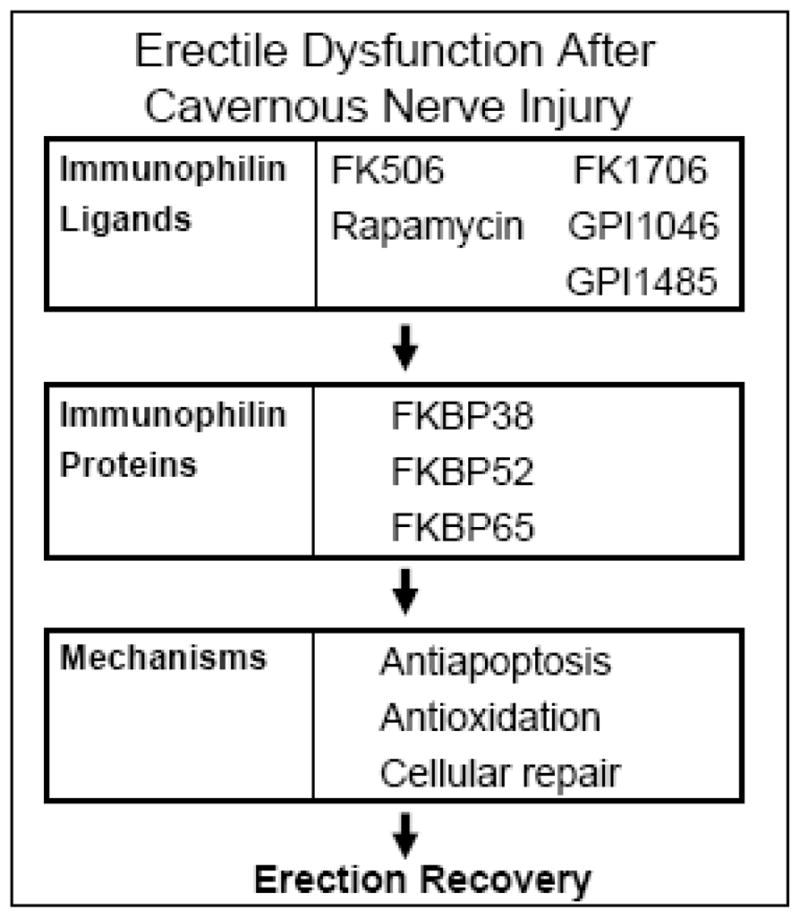
Working model of neuroprotection using immunophilin ligands.
Acknowledgments
Support from NIH/NIDDK Grant DK64679 and Prostate Cancer SPORE Developmental Project.
Footnotes
Conflict of Interest: None
References
- 1.Montorsi F, Burnett AL. Erectile dysfunction after radical prostatectomy. BJU Int. 2004;93:1–2. doi: 10.1111/j.1464-410x.2004.04542.x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]
- 3.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Snyder SH, Lai MM, Burnett PE. Immunophilins in the nervous system. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 5.Rulten SL, Kinloch RA, Tateossian H, Robinson C, Gettins L, Kay JE. The human FK506-binding proteins: characterization of human FKBP19. Mamm Genome. 2006;17:322–331. doi: 10.1007/s00335-005-0127-7. [DOI] [PubMed] [Google Scholar]
- 6.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 7.Galat A. A note on clustering the functionally-related paralogues and orthologues of proteins: a case of the FK506-binding proteins (FKBPs) Comput Biol Chem. 2004;28:129–140. doi: 10.1016/j.compbiolchem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Aghdasi B, Ye K, Resnick A, Huang A, Ha HC, Guo X, Dawson TM, Dawson VL, Snyder SH. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci U SA. 2001;98:2425–2430. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies TH, Sanchez ER. FKBP52. Int J Biochem Cell Biol. 2005;37:42–47. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, et al. Essential role for Co-chaperone FKBP52 but not FKBP51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson CE, Schaub T, Coleman EJ, Davis EC. Developmental regulation of FKBP65. An ER-localized extracellular matrix binding-protein. Mol Biol Cell. 2000;11:3925–3935. doi: 10.1091/mbc.11.11.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edlich F, Weiwad M, Erdmann F, Fanghanel J, Jarczowski F, Rahfeld JU, Fischer G. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. Embo J. 2005;24:2688–2699. doi: 10.1038/sj.emboj.7600739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang CB, Feng L, Chia J, Yoon HS. Molecular characterization of FK-506 binding protein 38 and its potential regulatory role on the anti-apoptotic protein Bcl-2. Biochem Biophys Res Commun. 2005;337:30–38. doi: 10.1016/j.bbrc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 16.Weiwad M, Edlich F, Kilka S, Erdmann F, Jarczowski F, Dorn M, Moutty MC, Fischer G. Comparative analysis of calcineurin inhibition by complexes of immunosuppressive drugs with human FK506 binding proteins. Biochemistry. 2006;45:15776–15784. doi: 10.1021/bi061616p. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JP, Dawson TM, Fotuhi M, Glatt CE, Snowman AM, Cohen N, Snyder SH. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992;358:584–587. doi: 10.1038/358584a0. [DOI] [PubMed] [Google Scholar]
- 18.Lyons WE, Steiner JP, Snyder SH, Dawson TM. Neuronal regeneration enhances the expression of the immunophilin FKBP-12. J Neurosci. 1995;15:2985–2994. doi: 10.1523/JNEUROSCI.15-04-02985.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons WE, George EB, Dawson TM, Steiner JP, Snyder SH. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc Natl Acad Sci U SA. 1994;91:3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold B, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995;15:7509–7516. doi: 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman EE, Grosskreutz CL. The Effects of FK506 on Retinal Ganglion Cells after Optic Nerve Crush. Invest Ophthalmol Vis Sci. 2000;41:1111–1115. [PubMed] [Google Scholar]
- 22.Bavetta S, Hamlyn PJ, Burnstock G, Lieberman AR, Anderson PN. The Effects of FK506 on Dorsal Column Axons Following Spinal Cord Injury in Adult Rats: Neuroprotection and Local Regeneration. Experimental Neurology. 1999;158:382–393. doi: 10.1006/exnr.1999.7119. [DOI] [PubMed] [Google Scholar]
- 23.Sezen SF, Hoke A, Burnett AL, Snyder SH. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med. 2001;7:1073–1074. doi: 10.1038/nm1001-1073. [DOI] [PubMed] [Google Scholar]
- 24.Burnett AL, Becker RE. Immunophilin ligands promote penile neurogenesis and erection recovery after cavernous nerve injury. J Urol. 2004;171:495–500. doi: 10.1097/01.ju.0000089775.88825.ec. [DOI] [PubMed] [Google Scholar]
- 25.Mulhall JP, Muller A, Donohue JF, Golijanin D, Tal R, Akin-Olugbade Y, Kobylarz K, Cohen-Gould L, Bennett NE, Scardino P. FK506 and erectile function preservation in the cavernous nerve injury model: optimal dosing and timing. J Sex Med. 2008;5:1334–1344. doi: 10.1111/j.1743-6109.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 26.A safety and efficacy study of Prograf in the prevention of erectile dysfunction after radical prostatectomy. ClinicalTrials.gov; Available at: http://clinicaltrials.gov/ct2/show/NCT0016392. [Google Scholar]
- 27.Valentine H, Chen Y, Guo H, McCormick J, Wu Y, Sezen SF, Hoke A, Burnett AL, Steiner JP. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol. 2007;51:1724–1731. doi: 10.1016/j.eururo.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bella AJ, Hayashi N, Carrion RE, Price R, Lue TF. FK1706 enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Sex Med. 2007;4:341–346. doi: 10.1111/j.1743-6109.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi N, Minor TX, Carrion R, Price R, Nunes L, Lue TF. The effect of FK1706 on erectile function following bilateral cavernous nerve crush injury in a rat model. J Urol. 2006;176:824–829. doi: 10.1016/j.juro.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 30.Evaluate the effects of GPI 1485 on erectile function following bilateral nerve-sparing prostatectomy. ClinicalTrials.gov; Available at: http://clinicaltrials.gov/ct/show/NCT00090376. [Google Scholar]
- 31.Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng X, et al. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U SA. 2008;105:33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirane M, Ogawa M, Motoyama J, Nakayama KI. Regulation of apoptosis and neurite extension by FKBP38 is required for neural tube formation in the mouse. Genes Cells. 2008;13:635–651. doi: 10.1111/j.1365-2443.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 33.Keilhoff G, Fansa H, Wolf G. Differences in peripheral nerve degeneration/regeneration between wild-type and neuronal nitric oxide synthase knockout mice. J Neurosci Res. 2002;68:432–441. doi: 10.1002/jnr.10229. [DOI] [PubMed] [Google Scholar]
- 34.Keilhoff G, Fansa H, Wolf G. Neuronal nitric oxide synthase is the dominant nitric oxide supplier for the survival of dorsal root ganglia after peripheral nerve axotomy. J Chem Neuroanat. 2002;24:181–187. doi: 10.1016/s0891-0618(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 35.Farooque M, Isaksson J, Olsson Y. Improved recovery after spinal cord injury in neuronal nitric oxide synthase-deficient mice but not in TNF-alpha-deficient mice. J Neurotrauma. 2001;18:105–114. doi: 10.1089/089771501750055811. [DOI] [PubMed] [Google Scholar]
- 36.Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 37.Hurt KJ, Sezen SF, Champion HC, Crone JK, Palese MA, Huang PL, Sawa A, Luo X, Musicki B, Snyder SH, et al. Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U SA. 2006;103:3440–3443. doi: 10.1073/pnas.0511326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamler JS, Lamas S, Fang FC. Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 39.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Yoshioka M, Miyazaki I, Fujita N, Ogawa N. GPI1046 prevents dopaminergic dysfunction by activating glutathione system in the mouse striatum. Neurosci Lett. 2002;321:45–48. doi: 10.1016/s0304-3940(01)02547-2. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Fujita N, Yoshioka M, Ogawa N. Immunosuppressive and non-immunosuppressive immunophilin ligands improve H(2)O(2)-induced cell damage by increasing glutathione levels in NG108-15 cells. Brain Res. 2001;889:225–228. doi: 10.1016/s0006-8993(00)02851-1. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K, Fujita N, Higashi Y, Ogawa N. Neuroprotective and antioxidant properties of FKBP-binding immunophilin ligands are independent on the FKBP12 pathway in human cells. Neurosci Lett. 2002;330:147–150. doi: 10.1016/s0304-3940(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 43.Lagoda G, Sezen SF, Liu T, Hoke A, Burnett AL. FK506-binding protein localizations in human penile innervation. BJU Int. 2008;101:604–609. doi: 10.1111/j.1464-410X.2007.07290.x. [DOI] [PubMed] [Google Scholar]


