Abstract
Long-term exposure to stress and its physiological mediators, in particular cortisol, may lead to impaired telomere maintenance. In this study, we examine if greater cortisol responses to an acute stressor and/or dysregulated patterns of daily cortisol secretion are associated with shorter telomere length. Twenty-three post-menopausal women comprising caregivers for dementia partners (n=14) and age- and BMI-matched non-caregivers provided home sampling of cortisol–saliva samples at waking, 30 min after waking, and bedtime, and a 12-hour overnight urine collection. They were also exposed to an acute laboratory stressor throughout which they provided saliva samples. Peripheral blood mononuclear cells were isolated from a fasting blood sample and assayed for telomere length. As hypothesized, greater cortisol responses to the acute stressor were associated with shorter telomeres, as were higher overnight urinary free cortisol levels and flatter daytime cortisol slopes. While robust physiological responses to acute stress serve important functions, the long-term consequences of frequent high stress reactivity may include accelerated telomere shortening.
Keywords: Cortisol, Allostasis, Allostatic load, Telomere, Cellular aging, Stress, HPA axis
1. Introduction
How does psychological stress “get under the skin” to cause deleterious health outcomes? McEwen and colleagues have put forth a helpful model describing how the wear-and-tear of repeated physiological responses to psychological stress that are part of allostasis, over time, lead to allostatic load (damage due to these fluctuations) and eventually poor health outcomes. Much population and experimental data support this model [1,2], and it is relatively well established that chronic psychological stress is harmful to health. Stress has been linked longitudinally to disease states such as metabolic syndrome, cardiovascular disease, diabetes, and other diseases of aging [3–5]. These general and pervasive effects of stress may be linked to changes proximal to the stress response, such as changes in regulation of the HPA axis, but also may have common cellular mechanisms. Telomeres may be one of the common cellular mechanisms linking chronic stress to diseases of aging. Telomeres are DNA–protein complexes that protect chromosomal DNA from damage. As mitotic cells divide, telomeres get shorter, leaving the cell vulnerable to genomic instability, end-to-end chromosome fusion, less efficient mitosis, and loss of ability for cell replenishment and thus tissue replenishment [6,7]. Even non-mitotic cells may develop shortened telomeres when chronically exposed to oxidative stress [8]. When telomeres shorten to a certain point, cells undergo senescence. Telomere shortening, therefore, represents both a marker and mechanism of biological aging [9], as the progression toward senescence can be monitored by telomere shortness and telomere dysfunction activates p53-mediated cellular damage [10].
Chronic caregiving stress has been related to shorter telomere length in both young women [11] and in older men and women [12]. Similar findings have emerged in experimental models of stress in mice [13]. Thus, telomere shortening may be one important pathway by which stress gets under the skin to promote early aging. Shorter telomere length has been related to conditions of chronic adversity, such as longer working hours [14], being single [15], lower socioeconomic status [16], major depression [17] and duration of depression [18], and childhood trauma [19,20].
Given that telomere length is associated with a variety of stressor exposures, here we conceptualize telomere length as a potential molecular-level measure of allostatic load. Allostatic load incorporates dysregulation across multiple systems and telomere length may provide an index of cumulative inputs from multiple regulatory systems. Shorter telomere length is associated with worse function across multiple regulatory systems, including greater inflammation, oxidative stress, and insulin resistance [21–23]. Moreover, the cumulative inflammatory load of being high on both interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) was related to shorter telomere length than being high on just one individually [21]. For these reasons we conceptualize telomere length as a potential summary measure of total cumulative biochemical stressor exposures [24]—in a sense a molecular measure of allostatic load.
Psychological stress activates the hypothalamic-pituitary-adrenocortical (HPA) axis, and the end product, cortisol, can be used as one index of stress reactivity. In vitro studies have demonstrated that the application of high doses of hydrocortisone to lymphocytes lowers telomerase [25], the enzyme primarily responsible for telomere maintenance. Telomerase elongates telomeric DNA to counteract shortening of telomeres and thus protects them [26]. The allostatic load model predicts that multiple patterns of altered responses to stress (strained allostasis) can contribute to allostatic load. Given the consistent associations of stress with telomere shortness, it follows that certain stress-related patterns of allostasis may also lead to telomere shortening. Relevant here, an individual might: (1) experience exaggerated responses to repeated “hits” of acute stress and therefore have excessive exposure to physiological stress mediators; or (2) have a dysregulated diurnal rhythm of stress mediators, in particular flat slope or high evening levels of cortisol.
In the current study, we tested whether patterns of HPA axis dysregulation that are hypothesized to contribute significantly to development of allostatic load are associated with immune cell aging as measured by telomere shortness. We assessed the magnitude of diurnal cortisol slope as well as several other patterns that may indicate altered allostasis in the form of exaggerated reactivity or slow recovery from daily stress arousal. We exposed participants to an acute psychological stressor in the laboratory and measured their salivary cortisol response. Exaggerated response may indicate greater stress responding to the minor hassles of daily life. We also measured nocturnal cortisol output to assess basal activity during the quiescent period of the diurnal rhythm. We predicted that greater reactivity to acute psychological stress and signs of diurnal dysregulation such as a flatter rhythm or greater nocturnal output of cortisol would be associated with shorter telomere length.
2. Materials and methods
2.1. Participants
To draw from a population experiencing a wide range of chronic psychological stress, the study sample was drawn from a community sample and comprised 14 women caring for a partner with dementia and 9 non-caregiving women of similar age and BMI. Caregivers were recruited via flyers and ads in the community, from the University of California, San Francisco (UCSF) Memory and Aging Clinic, and from community organizations for dementia. Their matched non-caregivers were recruited through flyers and ads in the community and referrals of friends from caregiver participants. The resulting sample did in fact have a wide range of stress as measured by the Perceived Stress Scale [27]. Normal values for adults from a poll of a representative U.S. sample are available [28]. The stress score for a typical sample of adult women ranges from 7.1 to 20.3. In this sample, the stress score ranged from 7.6 to 23. To control for effects of menopausal status on cortisol reactivity, all participants were postmenopausal, and the women ranged in age from 51 to 79 (M=62, SD=6.46). Other exclusion criteria included the presence of major medical conditions such as heart disease, cancer, or diabetes, use of medications containing agents known to affect cortisol levels (e.g., hydrocortisone, DHEA), and regular smoking. Three women were on SSRI medication; the pattern of results shown below do not change with the subtraction of these women. The sample was predominantly white (82%), with the remaining self-reporting as Asian (11%), black (5%) and Latina (2%). The educational attainment was 38% college educated, 29% advanced degree, 19% some college or technical school, 9% high school and 5% an AA degree.
2.2. Procedures
All procedures were approved by the University of California, San Francisco Committee on Human Subjects Research. After providing informed consent, participants provided a fasting morning blood sample for separation of peripheral blood mononuclear cells (PBMCs) and measurement of telomere length. Participants were weighed and measured to obtain Body Mass Index (BMI), calculated as weight (kg) divided by height (meters) squared. Participants were then instructed to provide three saliva samples per day at waking, waking+30 min, and bedtime for the next three consecutive days to measure diurnal cortisol rhythm. They were also instructed to collect their urine on the last night of saliva sampling. They were then scheduled to return approximately one week later for the laboratory stress session.
2.2.1. Urine sampling
Urine was collected over 12 h. Participants were instructed to void to clear their bladder at the beginning of the collection (usually 20:00). They then voided into a specimen hat (and saved this in a bottle in a cold cooler) at all later times before bed and during the night when they naturally woke to urinate, and in the morning, until 12 h had passed (usually until 08:00). The urine was either collected by study staff or delivered by the participant the following morning. It was immediatey aliquoted and frozen at −80 C for batch analysis. Total free cortisol was used as the measure of overnight urinary cortisol secretion. Participants had to provide at least 200 mL of urine to be included for data analysis, and the urinary cortisol values were adjusted for flow-rate (volume).
2.2.2. Saliva sampling
Each saliva sample was collected in 2 mL SaliCaps tubes (IBL Hamburg, Germany) via the passive drool method. Participants were instructed to collect the first sample while still in bed and to not eat, drink, brush their teeth, or engage in vigorous activity between the first two morning samples and 20 min prior to the bedtime sample. They were also requested to refrain from alcohol prior to and for the duration of each sampling day.
The saliva samples were stored in the participant’s freezer at 4 ° C until the kit was returned to study staff on ice, where it was stored at −80 ° C. Samples were shipped for analysis on dry ice and assayed in batch at Dr. Kirscbaum’s lab at Dresden University of Technology in Germany.
2.2.3. Acute lab stressor
In the laboratory testing session, all participants were run between 1400 and 1700 hrs to control for the diurnal rhythmicity of cortisol. There, participants were exposed to a modified Trier Social Stress Test [TSST; 29]. This is a widely used, standardized laboratory stressor designed to elicit psychological stress and cortisol responses. The 20-minute stress task was comprised of four 5-minute stressful periods, including introduction to evaluators and task instructions, speech preparation period, a videotaped public speaking task in front of two evaluative, non-responsive audience members, and lastly a serial subtraction task. Saliva samples were taken at baseline (0 minutes), 15 (after the speech task), 20 (after the stressor ended), 30 (to capture cortisol peak), 50 (short term recovery), and 90 min (long-term recovery) as described elsewhere [30]. Finally, participants were debriefed and compensated.
2.2.4. Cortisol and telomere length assays
Salivary cortisol levels were measured using a commercial chemiluminescence immunoassay (CLIA; IBL Hamburg, Germany). Inter-and intraassay coefficients of variation were below 8%.
Blood samples were collected in 10-mL heparin tubes (Becton-Dickinson, Franklin Lakes, NJ). Telomere length was assayed in PBMCs isolated using density-gradient centrifugation (with Ficoll-Paque PLUS) and frozen at −80 °C. DNA isolation was performed using a standardized and quality-controlled Qiagen). DNA was analyzed for telomere length using quantitative polymerase chain reaction (qPCR) as previously described [31] with the following modifications. The primers for the telomere qPCR were tel1b [5′-CGGTTT(GTTTGG)5GTT-3′] and tel2b [5′-GGCTTG(CCTTAC)5CCT-3′], each used at a final concentration of 900 nM. Single-copy gene (human β-globin) qPCR primers were: hbg1 [5′-GCTTCTGACACAACTGTGTTCACTAGC-3′], used at a final concentration of 300 nM, and hbg2 [5′-CACCAACTTCATCCACGTTCACC-3′], used at a final concentration of 700 nM. The final reaction mix was: 20 mM Tris–HCl, pH 8.4; 50 mM KCl; 200 nM each dNTP; 1% DMSO; .4× Sybr Green I; 44 ng Escherichia coli DNA; .8 U Platinum Taq DNA polymerase (Invitrogen) per 22 μl reaction; 1.5–20 ng genomic DNA. Tubes containing 40, 13.3, 4.4, and 1.5 ng of reference DNA from Hela cells were included in each qPCR run so that the quantity of targeted templates in each research sample could be determined relative to a single reference DNA sample by the standard curve method. All qPCRs were carried out on a MX3000P (Stratagene, La Jolla, CA) real-time PCR instrument. To adjust for batch-to-batch variation, the same four control DNA samples covering the normal range of T/S ratios were included on each of six independent runs. A conversion factor was calculated based on the average T/S ratio of the four control DNA samples in each run compared to the established T/S ratio. The coefficient of variation of the telomere length method was 5%.
2.2.5. Data analysis
To develop the conversion factor for the calculation of approximate base pair telomere length from the T/S ratio, the T/S ratios of a set of genomic DNA samples from primary human cell line IMR90 at different population doubling were determined. The terminal restriction fragment length of these DNA samples was measured by Southern blot analysis to create the TRF and T/S ratio plot. The slope of the plot was used as the conversion factor.
Q-Q plots indicated non-normality in all cortisol variables, which was corrected using a natural log transformation. Any outliers that were more than three standard deviations from the mean or any values not in biologically feasible ranges were excluded. The following cortisol variables were derived to test the hypotheses. The area-under-the-curve formula with respect to ground formula [32] was used to quantify total cortisol secretion for both diurnal salivary daily cortisol and cortisol response during the TSST. The cortisol awakening response (CAR) was measured by subtracting the wakeup cortisol value from the wakeup+30 min value. Cortisol slope was calculated by subtracting the waking value from the bedtime value on each day. Thus negative numbers indicated negative slopes. The CAR and slope values were each averaged across the three days to get a more reliable summary measure. To test the hypotheses, linear regression models were estimated with telomere length as the outcome variable, each predictor variable in the step 1, and age and BMI entered in step 2 as is customary in telomere length analyses. Finally, caregiver status was entered in step 3 to test for group differences.
3. Results
3.1. Descriptive statistics
Descriptive statistics appear in Table 1. Given that caregiver status was not a moderator in this study, and that there were too few in each group to analylze separately in a reliable fashion, we collapse findings across groups and show the demographics of the whole sample. There were no differences in descriptive variables by caregiver status.
Table 1.
Descriptive statistics.
| Mean | SD | Min–max | |
|---|---|---|---|
| BMI | |||
| Total | 25.44 | 4.40 | 18.80–32.10 |
| Telomere length (base pairs) | |||
| Total | 5310.10 | 566.91 | 4174.16–6787.33 |
| Cortisol stress reactivity AUC (nmol/mL) | |||
| Total | 659.59 | 262.08 | 241.95–1132.20 |
| Daily salivary cortisol AUC (nmol/mL) | |||
| Total | 3412.13 | 518.04 | 2438.92–4679.57 |
| Cortisol awakening response (nmol/mL) | |||
| Total | 200.58 | 227.62 | −307.08–557.04 |
| Cortisol slope | |||
| Total | −11.09 | 8.00 | −23.96–10.89 |
| Nocturnal 12-hr urinary cortisol (μg/mL) | |||
| Total | 1.04 | 1.12 | .22–3.60 |
3.2. Cortisol response
Zero-order correlations among each of the cortisol variables as well as telomere length are displayed in Table 2. In general, there were few interrelationships among the different aspects of HPA axis function. Diurnal cortisol AUC is derived mathematically from data points that include the CAR and slope, and thus these correlations are to be expected.
Table 2.
Zero-order correlations between cortisol parameters and telomere length.
| Daily salivary cortisol (AUC) | Cortisol awakening response | Cortisol slope | Cortisol stress reactivity (AUC) | PBMC telomere length | |
|---|---|---|---|---|---|
| Nocturnal 12-hr urinary cortisol | −.04 | −.14 | −.07 | .24 | −.64* |
| Daily salivary cortisol (AUC) | .58* | .29* | −.04 | .20 | |
| Cortisol awakening response | .30 | .18 | .05 | ||
| Cortisol slope | .01 | −.40* | |||
| Cortisol stress reactivity (AUC) | −.46* |
p < .05.
To test the hypotheses derived from the allostatic load model, we tested whether each aspect was related to shorter telomere length controllling for age, BMI, and caregiver status. Table 3 provides regression estimates at each step of the analysis. The unstandardized b coefficients represent the difference in telomere base pairs. We first examined whether greater cortisol secretion in response to acute stress was related to shorter telomere length.
Table 3.
Regression analyses for cortisol parameters and telomere length.
| Model | Unstandardized coefficients
|
t | Sig. | ||
|---|---|---|---|---|---|
| B | Std. error | ||||
| 1 | Constant | 8162.184 | 1201.623 | 6.793 | .000 |
| Age | −14.585 | 12.985 | −1.123 | .276 | |
| BMI | −25.892 | 24.353 | −1.063 | .302 | |
| Caregiver status | −317.340 | 232.722 | −1.364 | .190 | |
| Cortisol stress Reactivity AUC (nmol/mL) | −1.143 | .419 | −2.729 | .014 | |
| 2 | Constant | 5650.124 | 1386.152 | 4.076 | .001 |
| Age | −13.252 | 14.536 | −.912 | .372 | |
| BMI | −11.971 | 30.048 | −.398 | .694 | |
| Caregiver status | −35.015 | 250.531 | −.140 | .890 | |
| Daily salivary Cortisol AUC (nmol/mL) | .245 | .236 | 1.037 | .312 | |
| 3 | Constant | 6347.198 | 1240.559 | 5.116 | .000 |
| Age | −13.109 | 14.388 | −.911 | .372 | |
| BMI | −9.066 | 28.745 | −.315 | .755 | |
| Caregiver status | 4.420 | 246.201 | .018 | .986 | |
| Cortisol awakening Response (nmol/mL) | −9.442 | 261.334 | −.036 | .971 | |
| 4 | Constant | 5455.221 | 1189.353 | 4.587 | .000 |
| Age | −11.878 | 13.136 | −.904 | .376 | |
| BMI | −4.290 | 26.307 | −.163 | .872 | |
| Caregiver status | 185.012 | 235.893 | .784 | .441 | |
| Cortisol slope | −334.055 | 146.604 | −2.279 | .033 | |
| 5 | Constant | 7728.077 | 1487.152 | 5.197 | .003 |
| Age | −15.021 | 11.460 | −1.311 | .247 | |
| BMI | −52.940 | 31.185 | −1.698 | .150 | |
| Caregiver status | −174.848 | 351.079 | −.498 | .640 | |
| Nocturnal 12-hr Urinary cortisol (nmol/mL) | −357.850 | 114.865 | −3.115 | .026 | |
As hypothesized, greater cortisol response to acute stress was related to shorter telomeres controlling for age, BMI, and caregiver status. Next, we tested whether diurnal slope, CAR, and total salivary daily cortisol were also related to telomere length controlling for age, BMI, and caregiver status. While total salivary cortisol secretion was not related to telomere length, total nocturnal urinary free cortisol was, such that greater total overnight urinary free cortisol was associated with shorter telomere length. The cortisol awakening response was not associated with telomere length. The cortisol slope, however, was significantly related to telomere length, such that flatter slopes were associated with shorter telomere length.
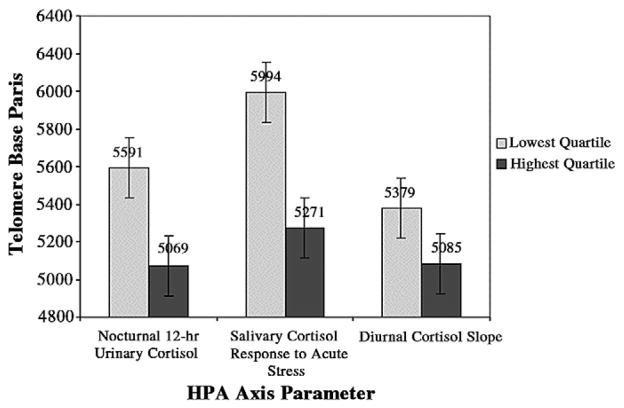
For illustrative purposes only, Fig. 1 depicts telomere length in base pairs of the lowest and highest quartile of the three significant cortisol parameters.
Fig. 1.
Telomere length in base pairs of the lowest and highest quartile of the three significant cortisol parameters. Standard error bars are shown.
4. Discussion
The cost of repeated stress exposure on physiology takes many forms of allostatic load, and telomere length has recently been proposed as a measure regulated in part by cumulative exposure to stress, and thus a potential marker of allostatic load [33]. Here, we tested whether PBMC telomere length was related first to aspects of HPA axis regulation, including greater cortisol reactivity to a novel standardized acute stressor, and second to a number of measures indicative of HPA axis function. As hypothesized, shorter telomere length was related to greater cortisol secretion to the acute stressor, to greater overnight cortisol secretion, and to flatter cortisol slopes throughout the day. These findings are in accord with the in vitro study [25] showing that high doses of hydrocortisone exposure decrease activity of telomerase in PBMCs. We did not find associations between telomere length and total daytime salivary cortisol secretion or the cortisol awakening response in this sample.
Greater cortisol reactivity to an acute lab stressor was associated with shorter telomere length, which is important for several reasons. It indirectly provides support for laboratory-based cortisol reactivity to serve as a test of the “repeated hits” pattern of allostatic load, in that greater reactivity to stressful stimuli in the lab might indicate latent tendency to overreact to other mild stressors naturalistically. This finding also demonstrates that telomere length is associated with stress reactivity specifically, not just basal output of cortisol. So far, telomere length has been related to higher overnight cortisol and catecholamines [34,35]. We note that Parks and colleagues did not observe a relation between telomere length and nocturnal cortisol [35], which may have been due to the different cell types studied (all leukocytes versus PBMCs).
We also found that nocturnal and diurnal patterning of cortisol was related to telomere length, supporting the idea from the allostatic load model that dysregulated diurnal rhythms of cortisol contribute to poor health, or are at least associated with poor health. Specifically, we found a flatter diurnal cortisol slope and greater overnight output of cortisol were related to shorter telomere length. Flattened slopes have previously been linked to chronic and acute psychological stress [36], cardiovascular disease outcomes [37], mortality from breast cancer [38], and both all-cause and cardiovascular mortality [39]. Our study suggests that the flattened diurnal cortisol rhythm may also be related to accelerated cellular aging.
Urinary cortisol provides an integrated measure of cortisol during sleeping hours—usually difficult to measure using other techniques [40]. Overnight cortisol, in contrast to diurnal cortisol, tends to be a measure of how well one’s system is able to regulate and recover from demands of the day and consolidate immune cell memory function [41]. In normal function, limbic-hippocampal and other neural networks inhibit adrenocorticotropin and adrenocortical release of cortisol nocturnally, particularly during early sleep [41,42]. After acute stress and chronic stress [41], as well as in Cushing’s disease and in older humans [42], a lack of inhibition of cortisol during sleep is observed. Our findings suggest that in a healthy sample, high nocturnal adrenocortical activity is associated with and perhaps may contribute to an accelerated rate of immune cell aging.
Telomere length was not related to all measures of cortisol secretion. Although telomere length was related to overnight cortisol secretion, it was not significantly related to total secretion throughout waking hours. This may be due to the relatively few samples that were collected during the day, generating greater variability in the diurnal cortisol AUC and missing most of daytime arousal. Unlike our urinary cortisol measure, which directly measured the total free cortisol secretion during the entire nocturnal period, diurnal cortisol was derived from only three time points using the AUC formula. Likewise, telomere length was not significantly related to the CAR. This again may have also been due to the relatively few samples collected to measure the awakening response (two samples when three or more are preferable). The different parameters of cortisol secretion were generally unrelated to one another in this study, and are thought to interrogate different aspects of HPA function [43,44]. For example, the CAR specifically is considered to be a discrete component of the circadian rhythm of cortisol, regulated by its own psychological processes and neural networks [40,45].
Here we focused on PBMCs in order to extend Choi et al.’s finding [25] on cortisol exposure and PBMCs in vitro. However, we also examined relations with whole blood telomere length (data not shown), which is comprised mostly of the short-lived granulocytes. We did not see relations across any HPA axis measures with whole blood, and it is possible this is because the cells are not exposed to blood cortisol as much as the more long-lived circulating PBMCs, which play a very active role in the early acute stress response. Choi et al. found the strongest cortisol effects on telomerase with CD8+ cells. Future studies should separate out cell types to examine if the relationships observed here are specific to subpopulations of PBMCs.
This study only captures a snapshot in time of interrelations among HPA axis parameters and telomere length. The data are consistent with the hypothesis that these cortisol measures affect telomere length, as it is unlikely that telomere length, which changes over months or years, changes HPA axis function. However, this is at best preliminary evidence that cortisol may be related to telomere length, and the true mechanism linking dysregulated allostasis to cellular aging is likely much more complex. This study was also limited by the small sample size, as well as by being confined to postmenopausal women. Researchers should generalize these findings to other populations with caution. Future studies would benefit from more intensive measurement of daytime cortisol dynamics and examining these relations longitudinally to understand temporal and potential causal dynamics of cortisol on cellular aging.
Finally, future studies should test other patterns of the allostatic load model. One pattern is mounting an inadequate cortisol response (hypocortisolism), thereby allowing inflammatory processes to ensue. Several studies link inflammatory markers such as IL-6 to shorter telomere length [18,46]. Thus, hypocortisolism might be associated with short telomere length to the extent it permits excessive inflammation. Examining both inflammation and HPA axis function simultaneously will aid our understanding of allostatic load, hyper- versus hypocortisolism, and telomere length.
In summary, we have provided initial evidence supporting the allostatic load model by demonstrating that strained allostasis is associated with a long-term marker of cellular aging. Specifically, greater cortisol reactivity to acute stressors, less inhibition of cortisol during sleep, and a flattened diurnal cortisol slope all predicted shorter telomere length. Future studies are needed to further test and validate whether telomere length can indeed serve as a molecular marker of allostatic load. While the exact mechanisms through which stress might erode telomeres need to be elucidated the long-term consequences of excessive cortisol exposure during reactivity and quiescent basal states may include accelerated telomere shortening.
Acknowledgments
This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131, NIMH K08 MH64110-01A1 to E. Epel. A. J. Tomiyama was supported by the Robert Wood Johnson Foundation Health and Society Scholars Program.
Footnotes
Conflict of interest
Dr. E Epel, J. Lin, and E. Blackburn are co-founders in Telome Health, Inc, a telomere measurement company.
References
- 1.McEwen B. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 3.Antoni MH, Lutgendorf SK. Psychosocial factors and disease progression in cancer. Curr Dir Psychol Sci. 2007;16:42–6. [Google Scholar]
- 4.Lin J, Epel E, Blackburn E. Telomeres, telomerase, stress, and aging. In: Benton GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioural sciences. New York: John Wiley & Sons; 2009. [Google Scholar]
- 5.Kiecolt-Glaser JK, Robles TF, Heffner KL, Loving TJ, Glaser R, McGuire L. Psycho-oncology and cancer: psychoneuroimmunology and cancer. Ann Oncol. 2002;13 (Suppl 4):165–9. doi: 10.1093/annonc/mdf655. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 7.Edo MD, Andres V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66:213–21. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 9.Epel ES. Psychological stress and metabolic stress: A recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 10.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–65. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow JD, et al. Accelerated telomere shortening in response to exposure to life stress. PNAS. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biol Lett. 2007;3:128–30. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parks CG, Deroo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occup Environ Med. 2011;68:582–9. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mainous AG, III, Everett CJ, Diaz VA, Baker R, Mangino M, Codd V, et al. Leukocyte telomere length and marital status among middle-aged adults. Age Ageing. 2011;40:73–8. doi: 10.1093/ageing/afq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25:1292–8. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2010;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–40. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 23.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ. 2004;2004:pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 24.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? (Athens)Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–5. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 28.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Oskamp S, Spacapan S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 29.Kirschbaum C, Pirke K, Hellhammer D. The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 30.Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24:531–9. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruessner J, Kirschbaum C, Meinlschmidt G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 33.Epel ES. Telomeres in a lifespan: a new ‘psychobiomarker’? Current Directions in Psychology. 2009;18:6–10. [Google Scholar]
- 34.Epel E, Lin J, Wilhelm F, Mendes W, Adler N, Dolbier C, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam E, Hawkley L, Kudielka B, Cacioppo J. Day-to-day dynamics of experience—cortisol associations in a population-based sample of older adults. PNAS. 2006;103:17058–63. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 38.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–92. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metab. 2011;96:1478–85. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolson N. Measurement of cortisol. In: Leucken LJ, Gallo LC, editors. Handbook of Physiological Research Methods in Health Psychology. Thousand Oaks: Sage; 2007. pp. 37–74. [Google Scholar]
- 41.Born J, Fehm HL. The neuroendocrine recovery function of sleep. Noise Health. 2000;2:25–38. [PubMed] [Google Scholar]
- 42.Born J, Fehm HL. Hypothalamus-pituitary-adrenal activity during human sleep: a coordinating role for the limbic hippocampal system. Exp Clin Endocrinol Diabetes. 1998;106:153–63. doi: 10.1055/s-0029-1211969. [DOI] [PubMed] [Google Scholar]
- 43.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Kudielka BM, Wust S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- 45.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004:7. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 46.O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–9. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]