Abstract
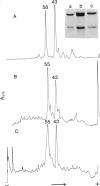
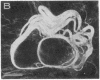
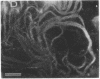
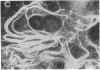
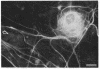
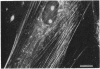
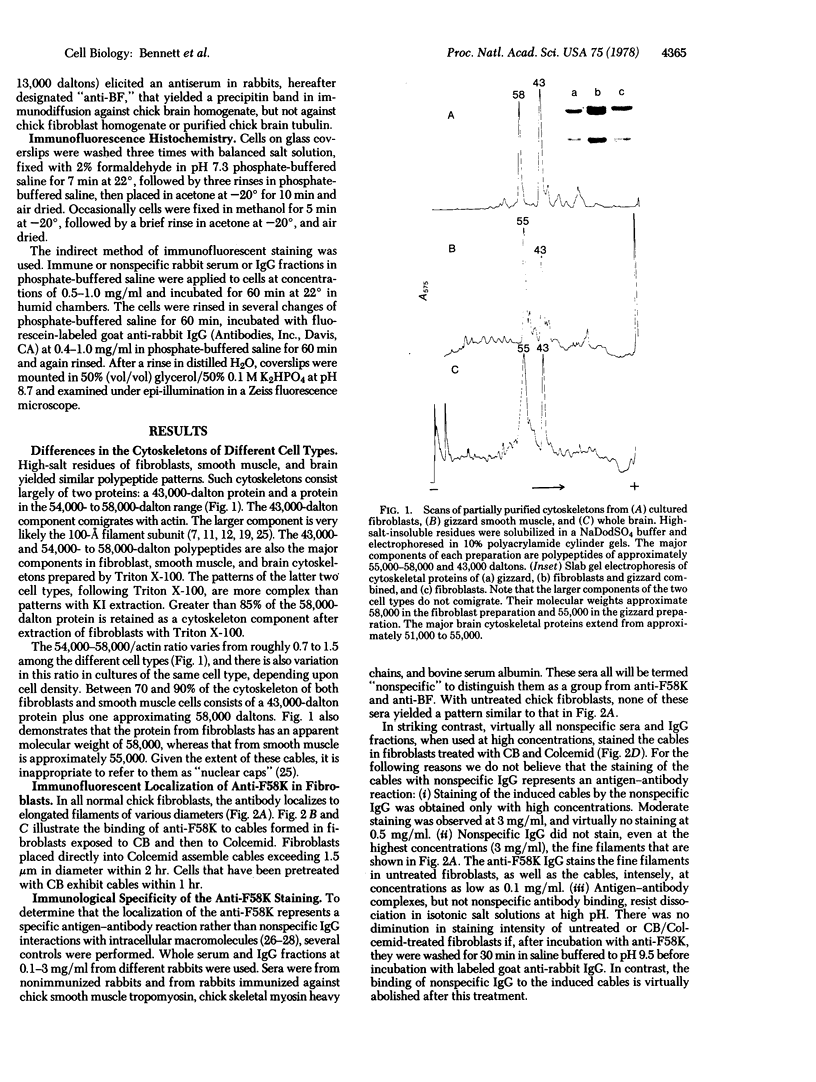
The protein subunit of 100-A filaments constitutes approximately 50% of the cytoskeleton protein of chick fibroblasts. In addition to the 43,000-dalton protein (constitutive actin) common to all cell types, fibroblast cytoskeletons contain a 58,000-dalton protein likely to be the 100-A filament subunit, whereas smooth muscle contains, instead, a 55,000-dalton protein. Additional differences among 100-A filaments are shown by immunofluorescence using antibodies angainst chick fibroblast 58,000-dalton component (anti-F58K) and against chick brain 100-A filament subunits (anti-BF). Anti-F58K binds to 100-A filaments in chick fibroblasts, presumptive myoblasts, chondroblasts, pigment cells, and neurons, but not to 100-A filaments in mouse or human fibroblasts. This antibody stains cables of 100-A filaments induced by sequentially treating cells with cytochalasin B and Colcemid. Anti-BF binds only to neurofilaments and not to 100-A filaments of other cell types studied. Absorption or antibodies with purified subunits from gizzard 100-A filaments eliminates binding of anti-F58K to the filaments of all cell types but does not diminish binding of anti-BF to neurofilaments. Various IgGs also bind nonspecifically to induced cables of 100-A filaments. The problem of nonspecific binding of labeled antibodies, as well as the problem of cell and species specificity of the 100-A filaments, is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blose S. H., Shelanski M. L., Chacko S. Localization of bovine brain filament antibody on intermediate (100 A) filaments in guinea pig vascular endothelial cells and chick cardiac muscle cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):662–665. doi: 10.1073/pnas.74.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Chi J. C., Fellini S. A., Holtzer H. Differences among myosins synthesized in non-myogenic cells, presumptive myoblasts, and myoblasts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4999–5003. doi: 10.1073/pnas.72.12.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J., Holtzer H. Response of myogenic and fibrogenic cells to cytochalasin B and to colcemid. I. Light microscope observations. J Cell Biol. 1975 May;65(2):271–285. doi: 10.1083/jcb.65.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunogenic properties of the glial fibrillary acidic protein. Brain Res. 1976 Oct 29;116(1):150–157. doi: 10.1016/0006-8993(76)90257-2. [DOI] [PubMed] [Google Scholar]
- Davison P. F., Hong B. S., Cooke P. Classes of distinguishable 10 nm cytoplasmic filaments. Exp Cell Res. 1977 Oct 15;109(2):471–474. doi: 10.1016/0014-4827(77)90033-7. [DOI] [PubMed] [Google Scholar]
- Davison P. F., Hong B. S. Sturctural homologies in mammalian neurofilament proteins. Brain Res. 1977 Oct 7;134(2):287–295. doi: 10.1016/0006-8993(77)91074-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Lamelin J. P., Vassalli P., Majno G., Bouvier C. A., Cruchaud A., Lüscher E. F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973 Sep;72(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A., Burridge K. Characterization of the intermediate (10 nm) filaments of cultured cells using an autoimmune rabbit antiserum. Cell. 1978 Feb;13(2):249–261. doi: 10.1016/0092-8674(78)90194-0. [DOI] [PubMed] [Google Scholar]
- HOLTZER H., HOLTZER S. The in vitro uptake of fluorescein labelled plasma proteins. I. Mature cells. C R Trav Lab Carlsberg. 1960;31:373–408. [PubMed] [Google Scholar]
- Holtzer H., Croop J., Dienstman S., Ishikawa H., Somlyo A. P. Effects of cytochaslasin B and colcemide on myogenic cultures. Proc Natl Acad Sci U S A. 1975 Feb;72(2):513–517. doi: 10.1073/pnas.72.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Strahs K., Biehl J., Somlyo A. P., Ishikawa H. Thick and thin filaments in postmitotic, mononucleated myoblasts. Science. 1975 May 30;188(4191):943–945. doi: 10.1126/science.1138363. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S. Antibodies to tubulin in normal nonimmunized animals. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3997–4001. doi: 10.1073/pnas.74.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Linder E., Virtanen I., Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100 A) filaments. Nature. 1977 Jul 21;268(5617):240–241. doi: 10.1038/268240a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Holtzer H. Cytochalasin B: effects on cell morphology, cell adhesion, and mucopolysaccharide synthesis (cultured cells-contractile microfilaments-glycoproteins-embryonic cells-sorting-out). Proc Natl Acad Sci U S A. 1972 Jan;69(1):253–257. doi: 10.1073/pnas.69.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W. Immunological and ultrastructural studies of neurofilaments isolated from rat peripheral nerve. J Cell Biol. 1977 Jul;74(1):226–240. doi: 10.1083/jcb.74.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci. 1977 Feb;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Goldman R. D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Wisniewski H., Shelanski M. L., Terry R. D. Effects of mitotic spindle inhibitors on neurotubules and neurofilaments in anterior horn cells. J Cell Biol. 1968 Jul;38(1):224–229. doi: 10.1083/jcb.38.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]