Abstract
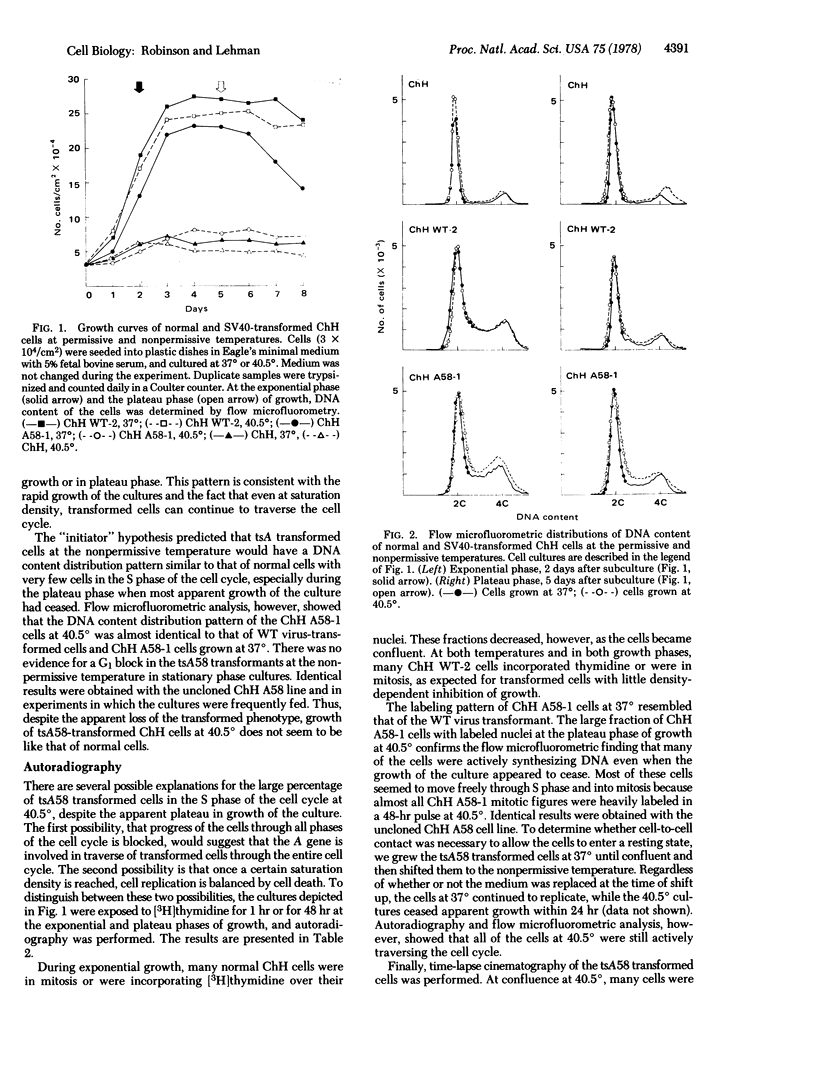
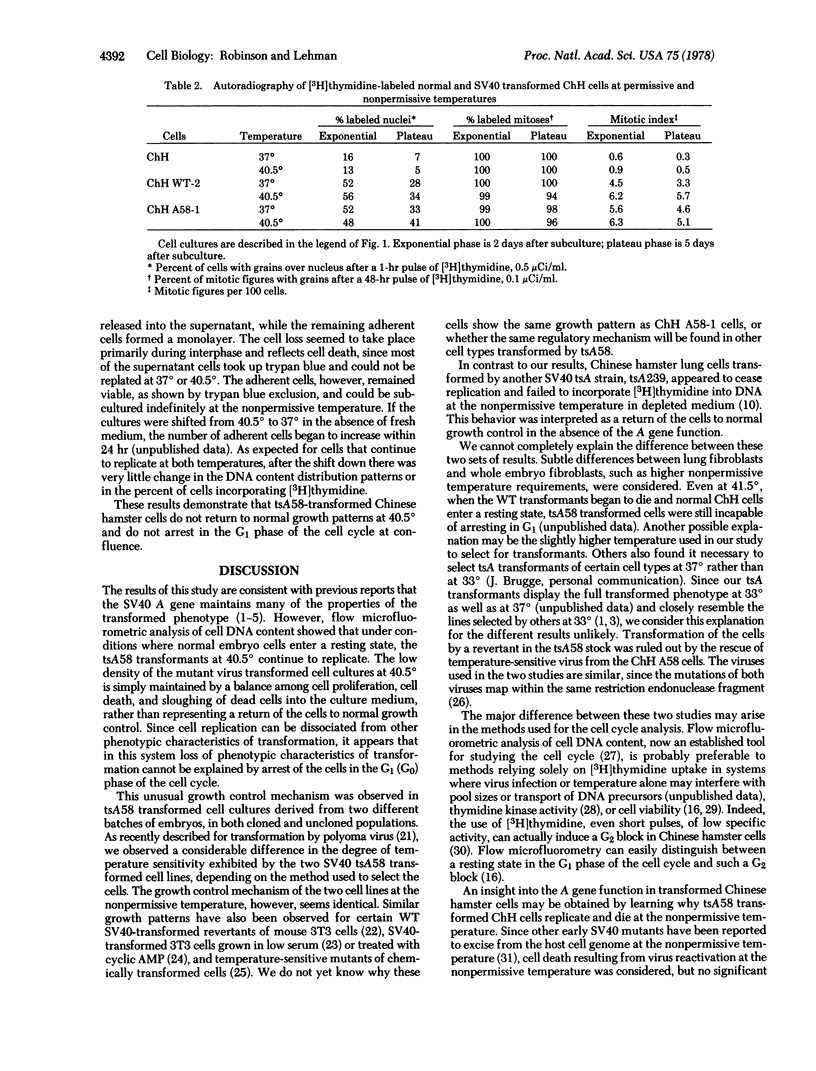
Replication of two Chinese hamster embryo cell lines transformed by an early temperature-sensitive mutant of simian virus 40, tsA58, was examined by flow microfluorometry and autoradiography of [3H]thymidine-labeled cells in order to determine whether transformed cell DNA synthesis is initiated by the virus A gene. At the permissive temperature (37 degrees), cells transformed by the mutant were like the wild-type virus transformants in appearance, colony-forming ability, high saturation density, and rapid replication. At the nonpermissive temperature (40.5 degrees), the tsA58 transformed cells resembled normal embryo fibroblasts and seem to return to normal growth patterns. Although both mutant transformed cell lines at 40.5 degrees appeared to cease growth at low saturation density, the cells did not enter a resting state, but continued to replicate. The cultures were maintained at low densities by a balance among cell replication, cell death, and sloughing of dead cells into the supernatant. These results suggest that the simian virus 40 A gene function effected by the tsA58 mutation does not prevent Chinese hamster embryo transformed cells from entering a resting state, although the gene may control other phenotypic characteristics of transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Martin R. G. SV40 transformation of mouse brain cells: critical role of gene A in maintenance of the transformed phenotype. J Cell Physiol. 1976 May;88(1):65–76. doi: 10.1002/jcp.1040880109. [DOI] [PubMed] [Google Scholar]
- Bartholomew J. C., Yokota H., Ross P. Effect of serum on the growth of Balb oT3 A31 mouse fibroblasts and an SV40-transformed derivative. J Cell Physiol. 1976 Jul;88(3):277–286. doi: 10.1002/jcp.1040880303. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Basilico C. Transformation by polyoma virus alters expression of a cell mutation affecting cycle traverse. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2540–2544. doi: 10.1073/pnas.72.7.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Defendi V., Lehman J. M. Transformation of hamster embryo cells in vitro by polyoma virus: morphological, karyological, immunological and transplantation characteristics. J Cell Physiol. 1965 Dec;66(3):351–409. doi: 10.1002/jcp.1030660313. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Trkula D., Kit S. T antigen and initiation of cell DNA synthesis in a temperature-sensitive mouse line transformed by an SV40tsA mutant and in heterokaryons of the transformed cells and chick erythrocytes. Somatic Cell Genet. 1978 Jan;4(1):95–110. doi: 10.1007/BF01546495. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Williams J. R., Nagle W. A., Brown J. A., Belli J. A., Lett J. T. Perturbations in cell cycle progression from radioactive DNA precursors. Nature. 1975 Dec 18;258(5536):633–636. doi: 10.1038/258633a0. [DOI] [PubMed] [Google Scholar]
- Gelfant S. A new concept of tissue and tumor cell proliferation. Cancer Res. 1977 Nov;37(11):3845–3862. [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Kase K., Hahn G. M. Differential heat response of normal and transformed human cells in tissue culture. Nature. 1975 May 15;255(5505):228–230. doi: 10.1038/255228a0. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Defendi V. Changes in deoxyribonucleic acid synthesis regulation in Chinese hamster cells infected with simian virus 40. J Virol. 1970 Dec;6(6):738–749. doi: 10.1128/jvi.6.6.738-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood M. P., Rothschild H., Wilson M. Early viral functions of in (ts)-1501 temperature-sensitive SV40 mutant. Nature. 1977 Apr 21;266(5604):720–722. doi: 10.1038/266720a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Stein S. Resting state in normal and simian virus 40 transformed Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1655–1659. doi: 10.1073/pnas.73.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondovì B., Finazzi Agrò A., Rotilio G., Strom R., Moricca G., Rossi Fanelli A. The biochemical mechanism of selective heat sensitivity of cancer cells. II. Studies on nucleic acids and protein synthesis. Eur J Cancer. 1969 May;5(2):137–146. doi: 10.1016/0014-2964(69)90060-7. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. Effects of cyclic AMP on SV3T3 cells in culture. Nat New Biol. 1972 Dec 6;240(101):179–181. doi: 10.1038/newbio240179a0. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Levine A. J. The requirement of simian virus 40 gene A product for the stimulation of cellular thymidine kinase activity after viral infection. Virology. 1976 Aug;73(1):206–215. doi: 10.1016/0042-6822(76)90075-1. [DOI] [PubMed] [Google Scholar]
- Schulman N., Hall E. J. Hyperthermia: its effect on proliferative and plateau phase cell cultures. Radiology. 1974 Oct;113(1):209–211. doi: 10.1148/113.1.209. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A., Kraemer P. M. A method for comparing effects of different synchronizing protocols on mammalian cell cycle traverse. The traverse perturbation index. J Cell Biol. 1972 Sep;54(3):638–645. doi: 10.1083/jcb.54.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A. Unique techniques for cell analysis utilizing mithramycin and flow microfluorometry. Exp Cell Res. 1975 Jun;93(1):235–239. doi: 10.1016/0014-4827(75)90445-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Weinstein I. B. Temperature-sensitive mutants of chemically transformed epithelial cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):214–218. doi: 10.1073/pnas.72.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]



