Abstract
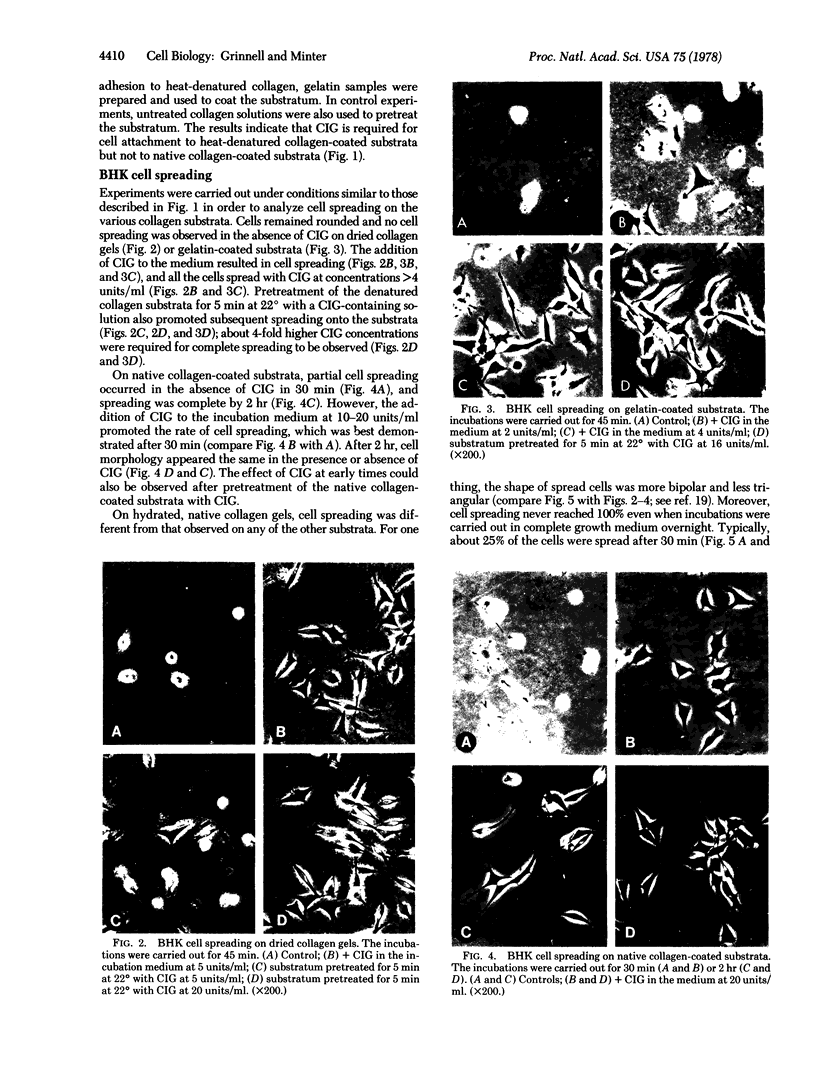
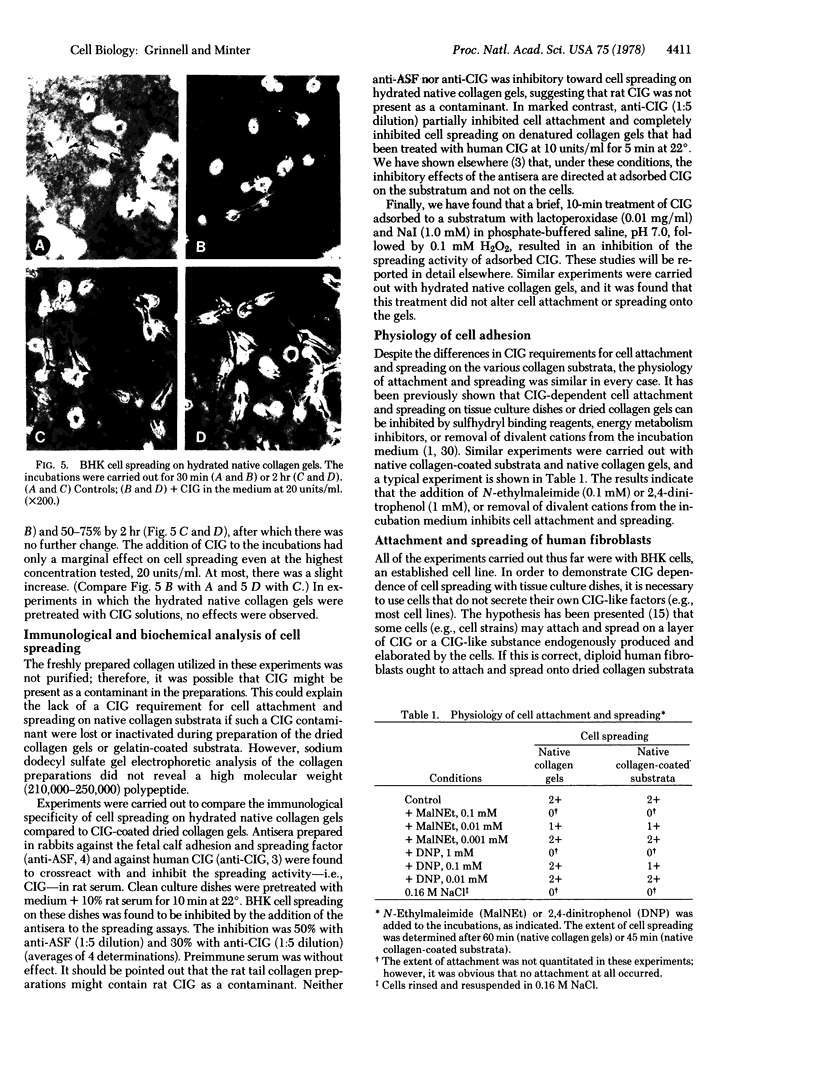
Studies have been carried out to determine the effects of cold-insoluble globulin (CIG) on the attachment and spreading of baby hamster kidney cells on various collagen substrata. Cell attachment to native collagen substrata occurred in the absence of CIG just as fast as attachment to dried collagen or gelatin substrata occurred in the presence of CIG. On the other hand, cell attachment to dried collagen or gelatin was markedly reduced in the absence of CIG. Cell spreading also occurred on native collagen in the absence of CIG; however, CIG was absolutely required for cell spreading to occur on dried collagen or gelatin. Finally, anti-CIG antiserum or lactoperoxidase treatment inhibited cell spreading on CIG-coated substrata but not on native collagen substrata. The data are discussed in terms of the interaction of fibroblasts with collagen in situ.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baier R. E. The role of surface energy in thrombogenesis. Bull N Y Acad Med. 1972 Feb;48(2):257–272. [PMC free article] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Grinnell F. Cell spreading factor. Occurrence and specificity of action. Exp Cell Res. 1976 Oct 1;102(1):51–62. doi: 10.1016/0014-4827(76)90298-6. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Hays D. G., Minter D. Cell adhesion and spreading factor. Partial purification and properties. Exp Cell Res. 1977 Nov;110(1):175–190. doi: 10.1016/0014-4827(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Hayase K., Reisher S. R., Miller B. F. Purification and separation of A and B forms of N-acetyl- -D-hexosaminidase from human aorta. Prep Biochem. 1973;3(3):221–241. doi: 10.1080/00327487308061508. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Cell attachment to collagen: the requirement for energy. J Cell Physiol. 1975 Oct;86(2 Pt 1):231–236. doi: 10.1002/jcp.1040860206. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Blout E. R. Heterogeneity of bovine fibrinogen and fibrin. J Biol Chem. 1973 Oct 10;248(19):6896–6903. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Keski-Oja J., Vaheri A. Distribution of a major surface-associated glycoprotein, fibronectin, in cultures of adherent cells. J Supramol Struct. 1977;6(4):551–557. doi: 10.1002/jss.400060408. [DOI] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Pegrum S. M., Maroudas N. G. Early events in fibroblast adhesion to glass. An electron microscopic study. Exp Cell Res. 1975 Dec;96(2):416–422. doi: 10.1016/0014-4827(75)90277-3. [DOI] [PubMed] [Google Scholar]
- ROSENBERG M. D. Microexudates from cells grown in tissue culture. Biophys J. 1960 Nov;1:137–159. doi: 10.1016/s0006-3495(60)86881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman R., Rounds D. E., Yen S. P., Rembaum A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp Cell Res. 1974 Oct;88(2):327–339. doi: 10.1016/0014-4827(74)90248-1. [DOI] [PubMed] [Google Scholar]
- Stamatoglou S. C. Ultrastructural relationship between cell and substrate coats in serum-free and serum-supplemented cultures. J Ultrastruct Res. 1977 Aug;60(2):203–211. doi: 10.1016/s0022-5320(77)80065-8. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Fibroblast surface antigen produced but not retained by virus-transformed human cells. J Exp Med. 1975 Aug 1;142(2):530–535. doi: 10.1084/jem.142.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman L. What factors determine thrombogenicity. Bull N Y Acad Med. 1972 Feb;48(2):302–310. [PMC free article] [PubMed] [Google Scholar]
- Witkowski J. A., Brighton W. D. Stages of spreading of human diploid cells on glass surfaces. Exp Cell Res. 1971 Oct;68(2):372–380. doi: 10.1016/0014-4827(71)90162-5. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Quantitation of a transformation-sensitive, adhesive cell surface glycoprotein. Decrease of several untransformed permanent cell lines. J Cell Biol. 1977 Aug;74(2):649–654. doi: 10.1083/jcb.74.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi Y., Kanaseki T. Role of microexudate carpet in cell division. Nature. 1972 Jun 2;237(5353):283–285. doi: 10.1038/237283a0. [DOI] [PubMed] [Google Scholar]










