Abstract
Background and objective
Standard bronchoscopic techniques (transbronchial lung biopsy and endobronchial biopsy) provide a diagnosis in 70% of patients with pulmonary sarcoidosis. Previous data suggest that endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has a high sensitivity in patients with sarcoidosis. The feasibility and utility of combining EBUS-TBNA with standard bronchoscopic techniques is unknown. The aim of this study was to evaluate the feasibility, safety and efficacy of combined EBUS-TBNA and standard bronchoscopic techniques in patients with suspected sarcoidosis and enlarged mediastinal or hilar lymphadenopathy.
Methods
Forty consecutive patients with suspected pulmonary sarcoidosis and enlarged mediastinal or hilar lymph nodes (radiographical stage I and stage II) underwent EBUS-TBNA followed by transbronchial biopsies and endobronchial biopsies under conscious sedation.
Results
Thirty-nine out of 40 patients successfully underwent combined EBUS-TBNA and standard bronchoscopy. Twenty-seven patients were diagnosed with sarcoidosis, eight had tuberculosis, two had reactive lymphadenopathy, two had lymphoma and one had metastatic adenocarcinoma. In patients with sarcoidosis, the sensitivity of EBUS-TBNA for detection of noncaseating granulomas was 85%, compared with a sensitivity of 35% for standard bronchoscopic techniques (P < 0.001). The diagnostic yield of combined EBUS-TBNA and bronchoscopy was 93% (P < 0.0001).
Conclusions
Combination of EBUS-TBNA with standard bronchoscopic techniques is safe and feasible, and optimizes the diagnostic yield in patients with pulmonary sarcoidosis and enlarged intrathoracic lymphadenopathy.
Keywords: endobronchial ultrasound, mediastinal lymphadenopathy, sarcoidosis, transbronchial biopsy
INTRODUCTION
A pathological diagnosis of sarcoidosis is required to exclude other differential diagnoses and to justify the use of immunosuppressive treatment. This may only be avoided in patients with clear evidence of bilateral hilar lymphadenopathy on CXR, and those with arthritis or erythema nodosum (Loefgren’s syndrome).1 In patients with enlarged intrathoracic lymph nodes due to suspected sarcoidosis, other diagnoses such as tuberculosis and malignancies must be excluded.
Pathological confirmation of pulmonary sarcoidosis is most commonly accomplished by flexible bronchoscopy, which has a yield of approximately 70%, with higher yields in patients with radiographically more advanced disease.2 Flexible bronchoscopy under conscious sedation permits transbronchial needle aspiration (TBNA) and transbronchial lung biopsy (TBLB). Endobronchial biopsy (EBB) is also routinely recommended as an additional procedure, and may demonstrate non-caseating granulomas even when no endobronchial disease is evident.3 Despite the use of combined TBLB and EBB, approximately one-third of bronchoscopies do not provide a diagnosis of sarcoidosis.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is gaining momentum as an important new technique for the diagnosis of enlarged lymph nodes due to sarcoidosis. Recent data from randomized trials have demonstrated its superiority to conventional TBNA with a 19-G needle, for the diagnosis of pulmonary sarcoidosis.4 Cohort studies of selected patients with radiographical stage I and stage II sarcoidosis and a high pretest probability of disease (>90%) suggest sensitivities of between 85% and 93%.5-9
There are no data available on the safety and efficacy of combining standard bronchoscopic techniques (TBLB and EBB) with EBUS-TBNA, for the diagnosis of sarcoidosis. We hypothesized that the diagnostic yield from the combination of EBUS-TBNA with standard bronchoscopy, performed during the same period of conscious sedation, would be higher than that from standard bronchoscopy alone. A prospective study was therefore performed to evaluate the safety and diagnostic yield from EBUS-TBNA, TBLB, EBB and the combination of these procedures, in consecutive patients with enlarged intrathoracic lymph nodes due to suspected sarcoidosis.
METHODS
Patients
Forty consecutive patients with enlarged intrathoracic lymph nodes (≥1 cm in short axis) and suspected sarcoidosis were recruited from three London Hospitals (The Whittington Hospital, 12; The North Middlesex Hospital, 14 and University College London Hospital (UCLH), 14) between August 2008 and July 2009, and procedures were performed at UCLH. Informed written consent was obtained from all patients and the institutional review board approved this prospective study. In all patients, pathological confirmation was clinically required, to exclude other diseases or to support systemic treatment of sarcoidosis. All patients underwent CXR, CT or PET, and on the basis of the clinical details and radiological findings were suspected to have stage I or stage II sarcoidosis (Fig. 1a). Lymph node location was described according to the American Thoracic Society lymph node map proposed by Mountain and Dresler.10 Patients underwent sequential EBUS-TBNA followed by TBLB and EBB under conscious sedation with up to 5 mg midazolam and 75 °g of fentanyl, as well as topical anaesthesia with 2% and 4% lidocaine. At insertion of the standard bronchoscope, additional 2% lidocaine was applied to the vocal cords and bronchial tree as required. All procedures were conducted in an ambulatory care setting, without an anaesthetist being present. In all cases, EBUS-TBNA was performed before standard bronchoscopy in order to avoid airway contamination following TBLB and EBB.
Figure 1.
(a) Contrast-enhanced CT showing enlarged bilateral hilar and subcarinal lymphadenopathy due to sarcoidosis. (b) Endobronchial ultrasound image demonstrating transbronchial needle aspiration of subcarinal lymph node with a 22-G needle. (c) Non-caseating granuloma (low power, May-Grunwald-Giemsa stain) consistent with sarcoidosis, obtained by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). (d) Multinucleate giant cell (high power, May-Grunwald-Giemsa stain), obtained by EBUS-TBNA. LN, lymph node.
EBUS-TBNA procedure
The technique for performing EBUS-TBNA has been described previously.11 Briefly, an integrated linear ultrasound fibre-optic bronchoscope (BF-UC160F-OL8, Olympus, Tokyo, Japan), which scans parallel to the direction of insertion of the bronchoscope, was used. The scope offers endobronchial views (at a 35° forward oblique angle) and when in contact with airway wall, the 7.5 MHz convex ultrasound transducer provides imaging of parabronchial structures (Fig. 1b). A balloon may be inflated around the tip of the scope in order to maintain contact with the airway wall. Once the target lymph node had been located (and vascular structures excluded with the Doppler function), a compatible 22-G needle was placed in the working channel of the EBUS scope. The tip of the sheath of the needle was visible on the endobronchial view, and the needle was then allowed to pierce the airway wall and enter the lymph node using the jabbing technique under direct ultrasound guidance (Fig. 1b). Suction was applied and the needle moved to and fro within the lymph node. A minimum of four passes per node were planned, in accordance with previous data.7 Samples were smeared directly onto slides and air-dried before being transferred to the laboratory for cytological analysis. If histological cores were obtained, these were placed in formalin. Samples were not evaluated on site.
Standard bronchoscopic procedure
After the EBUS scope was withdrawn, it was immediately replaced with a standard flexible videobronchoscope. Additional topical lidocaine was applied when required. TBLB was performed from the lobe that was demonstrated to be abnormal on imaging.12 In patients with normal lung parenchyma (stage I sarcoidosis), TBLB was performed from the most convenient location, at the operator’s discretion. Four to six TBLB were taken per patient, in order to maximize the amount of diagnostic tissue as recommended by current guidelines.13 Fluoroscopy was not utilized and all TBLB were performed by experienced bronchoscopists (N.N., H.L.B., S.M.J.), who each perform more than 100 bronchoscopies per year. After the completion of TBLB, EBB was performed. Areas of endobronchial cobblestoning were sampled preferentially. Where no macroscopic endobronchial abnormalities were evident, the operator selected a suitable area for biopsy. At least four EBB were taken to maximize diagnostic yield. BAL was also performed in selected patients, depending on the clinical details. All patients underwent routine post procedure CXR in order to detect pneumothorax. The mean duration of the entire procedure was 46 min. The mean duration for EBUS was 22 min, while the mean duration for standard bronchoscopic procedures was 25 min.
Diagnostic criteria for sarcoidosis
Non-caseating granulomas, as identified by cytological (Fig. 1c,d) or histological analysis, together with negative mycobacterial and fungal cultures in the absence of malignancy, were deemed to be consistent with sarcoidosis. All patients were followed up clinically and radiologically for at least 6 months. The reference standard for negative EBUS-TBNA samples was considered to be surgical pathological sampling by mediastinoscopy, VATS, mediastinal lymph node dissection at thoracotomy, or clinical and radiological follow-up of at least 6-month duration. EBUS-TBNA, TBLB and EBB results were each categorized into true positives (TP), true negatives (TN) or false negatives (FN) per patient.
Statistical analysis
Standard definitions of sensitivity (TP/[TP + FN]), specificity (TN/[TN + FP]), positive predictive value (TP/(TP + FP]), negative predictive value (TN/[TN + FN]) and accuracy ([TP + TN]/[TP + TN + FP + FN]) were used. The unit of analysis was the patient. Yields from the diagnostic modalities were compared using chi-square or Fischer’s exact tests. A P-value <0.05 was taken to denote statistical significance.
RESULTS
Forty consecutive patients with suspected sarcoidosis were scheduled to undergo EBUS-TBNA, TBLB and EBB. Twenty-two patients were male and the mean age was 46 years (range 19–68). Based on radiological findings, 27 patients were considered to have stage I sarcoidosis, while 13 patients were considered to have stage II sarcoidosis. Thirty-four patients had symptoms of cough, fevers or weight loss. Six patients were asymptomatic but required a tissue diagnosis due to immunosuppression for another disorder, infection with HIV or prior malignancy. The characteristics of these patients are summarized in Table 1.
Table 1.
Characteristics of the patients with enlarged intrathoracic lymph nodes and suspected sarcoidosis
| Age range, years | 19–68 |
| Gender, n | |
| Males | 22 |
| Females | 18 |
| Ethnicity, n | |
| African or Caribbean | 11 |
| Asian | 2 |
| Caucasian | 27 |
| Symptoms, n | |
| Cough | 29 |
| Fevers | 5 |
| Weight loss | 7 |
| Asymptomatic | 6 |
| Lymph node stations sampled by |
|
| EBUS-TBNA, n | |
| 4R | 21 |
| 4L | 3 |
| 7 | 35 |
| 10R | 10 |
| 10L | 2 |
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
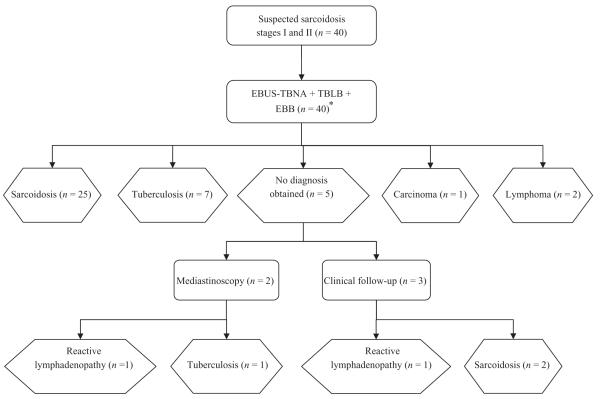
Thirty-nine patients had sequential EBUS-TBNA, TBLB and EBB under conscious sedation. One patient was unable to undergo standard bronchoscopy following EBUS-TBNA due to intolerance of sedation. Overall, 27 patients were diagnosed with sarcoidosis, eight had tuberculosis, two had reactive lymphadenopathy, two had lymphoma (diagnosed by EBUS-TBNA and confirmed by bone marrow biopsy) and one had metastatic adenocarcinoma (Fig. 2). All patients were followed up for at least 6 months and were reviewed in a multidisciplinary setting. No false positive results were obtained. EBUS-TBNA was used to sample 71 nodes in 40 patients, with a median of four passes per lymph node (range 3–5). All patients had enlarged lymph nodes sampled in station 4, 7 or 10. The mean size of the lymph nodes sampled was 24 mm (range 10–45 mm). The sensitivity of EBUS-TBNA for detecting non-caseating granulomas in patients with sarcoidosis was 85% (23/27). If it is assumed that the patient clinically diagnosed with reactive lymphadenopathy in fact had sarcoidosis, the sensitivity of EBUS-TBNA was 82% (23/28). The sensitivity of standard bronchoscopic techniques alone was significantly lower at 35% (9/26) (P < 0.001). The yield per procedure according to stage of sarcoidosis is summarized in Table 2. There was no significant difference in the diagnostic yields of EBUS-TBNA for stage I and stage II sarcoidosis. However, the sensitivity of standard bronchoscopic techniques was significantly greater for stage II (78%) compared with stage I (12%) disease (P = 0.001).
Figure 2.
Flowchart showing confirmation of diagnoses in 40 patients with suspected stage I and stage II sarcoidosis. *One patient was unable to undergo standard bronchoscopy after EBUS-TBNA. EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; TBLB, transbronchial lung biopsy; EBB, endobronchial biopsy.
Table 2.
Diagnostic yields of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), standard bronchoscopy and the combination in patients with sarcoidosis
| Number of patients with positive diagnosis (%) |
|||||
|---|---|---|---|---|---|
| EBUS-TBNA | Transbronchial lung biopsy (TBLB) |
Endobronchial biopsy (EBB) |
Standard bronchoscopy— TBLB and EBB |
Combined EBUS-TBNA + standard bronchoscopy |
|
| Stage I sarcoidosis (n = 18) | 16 (89) | 2 (12)† | 0 (0)† | 2 (12)† | 17 (94)† |
| Stage II sarcoidosis (n = 9) | 7 (78) | 6 (67) | 3 (33) | 7 (78) | 8 (89) |
| Overall (n = 27) | 23 (85)* | 8 (31) | 3 (11) | 9 (35)† | 25 (93)** |
P < 0.001 comparing yield from EBUS-TBNA with those from standard bronchoscopy
P < 0.0001 comparing yields from combined EBUS-TBNA and standard bronchoscopy with those from standard bronchoscopy alone.
One patient with stage I sarcoidosis did not undergo standard bronchoscopy after EBUS-TBNA.
In patients with negative EBUS-TBNA findings, non-caseating granulomas were detected by TBLB of radiologically normal lung parenchyma in one patient, and by EBB of apparently normal endobronchial mucosa in one patient. The sensitivity of EBUS-TBNA combined with standard bronchoscopic techniques for the diagnosis of sarcoidosis was 93% (25/27), which was significantly greater than that of standard bronchoscopic techniques alone (P < 0.0001). The overall diagnostic accuracy for EBUS-TBNA was 88% (35/40) and the combination of EBUS-TBNA with standard bronchoscopic techniques had a diagnostic accuracy of 93% (37/40). One patient experienced a pneumothorax, requiring overnight admission but not intercostal drainage.
DISCUSSION
In this prospective cohort study of patients with suspected sarcoidosis, the combination of EBUS-TBNA with standard bronchoscopic techniques optimized diagnostic yield and resulted in a higher diagnostic accuracy compared with bronchoscopy alone. Thirty-nine out of 40 patients were able to undergo the combined procedure under conscious sedation. The current British Thoracic Society guidelines13 do not mention the usefulness of EBUS-TBNA for the diagnosis of sarcoidosis. However, this study provided further evidence that EBUS-TBNA is an important minimally invasive approach that may be combined with standard bronchoscopy and considered a first-line investigation in patients with suspected sarcoidosis and enlarged intrathoracic lymph nodes.
Forty patients with suspected sarcoidosis were enrolled in this study. Of these, only 27 were finally diagnosed with sarcoidosis, while eight patients were diagnosed as having tuberculosis (Fig. 2). This discrepancy illustrates the inaccuracy of clinical diagnosis and the benefit of obtaining a tissue diagnosis for these patients, particularly in areas where tuberculosis is endemic. These findings contrast with previous reports on patients with suspected sarcoidosis where the disease prevalence was 93–98% and in whom the necessity for pathological diagnosis was questioned. Prior analysis of asymptomatic patients with presumed stage I sarcoidosis suggested that invasive sampling was not required due to the low probability of alternative diagnoses.14 This paradigm may not, however, be justified in regions where tuberculosis and HIV are prevalent.
A further area of controversy is cytological assessment of lymph node aspirates to obtain a reliable diagnosis of sarcoidosis. Non-caseating granulomas have been observed in mediastinal lymph nodes as a reaction to malignancy or anthracotic pigment. However, the presence of giant cells is thought to be specific for true granulomatous disease. Given that non-caseating granulomas are observed in both tuberculosis and sarcoidosis, and that Langhans-type giant cells, although characteristic of tuberculosis, are not always easily identified, a diagnosis of sarcoidosis is often made by exclusion. Additional TBLB or EBB histological material demonstrating non-caseating granulomas adds considerable weight to a diagnosis of sarcoidosis, whereas microbiological investigations of lymph node aspirate or BAL may confirm a diagnosis of tuberculosis. In all cases, the pathological findings should be interpreted within the clinical context.
In this study, the sensitivity of standard bronchoscopic techniques with TBLB and EBB, for the diagnosis of sarcoidosis, was 35%. This was considerably lower than the sensitivity of 70% reported for a previous retrospective series. In the current study, the investigators were highly experienced in TBLB and EBB and an appropriate number of specimens were obtained from each patient, as recommended by current guidelines.13 An explanation for the apparent discrepancy is that 67% of patients in the present cohort had radiographical stage I sarcoidosis with enlarged intrathoracic lymphadenopathy only. The prevalence of parenchymal and endobronchial disease in this group of patients is lower than that in patients with higher radiographical stages of disease, resulting in a lower diagnostic yield for standard bronchoscopic procedures. A high diagnostic yield (78%) was obtained from standard bronchoscopic techniques in the nine patients with stage II disease. In this subgroup there was no statistically significant benefit from the addition of EBUS-TBNA, although this analysis was insufficiently powered to draw further conclusions.
The sensitivity of EBUS-TBNA in this study (85%) is consistent with previous findings. To date, the largest published study of patients with suspected sarcoidosis was completed in EBUS expert centres in Japan, Hong Kong and Germany.5 EBUS-TBNA was performed on 65 patients, 61 of whom had sarcoidosis. The sensitivity of the procedure was 92%. A recent retrospective study compared EBUS-TBNA, TBLB and BAL in 38 patients.8 Of these, 35 were diagnosed with sarcoidosis (31 stage I and four stage II). As observed in the present study, the sensitivity was higher for EBUS-TBNA (90.3%) than for TBLB (40%). That retrospective study did not, however, include an assessment of EBB and therefore may have underestimated the diagnostic yield from bronchoscopy.
Conventional TBNA without EBUS guidance was not used in the present study. In a recent study, conventional TBNA with a 19-G needle provided a diagnosis in 42 of 53 patients with stage I and stage II sarcoidosis.15 In another study by the same group, conventional TBNA had a sensitivity of 72% in 32 patients with stage I and stage II sarcoidosis.16 However, the diagnostic yield from conventional TBNA has been variable and in a recent randomized trial the sensitivity of conventional TBNA with a 19-G needle, in patients with suspected sarcoidosis and enlarged intrathoracic lymph nodes, was 53.8%. This was significantly inferior to the yield of 83.3% from EBUS-TBNA.4 In a European implementation study by Tournoy et al., conventional TBNA was used in 21% of cases and the diagnostic yield in patients with sarcoidosis was low (14%).17 Therefore, conventional TBNA is not commonly performed in routine practice for patients with suspected sarcoidosis, and is associated with a lower yield than EBUS-TBNA.
In this study, a statistically significant effect of adding EBUS-TBNA to standard bronchoscopic techniques was observed, despite the small sample size. The lack of randomization in this study precludes further conclusions on the relative efficacy of standard bronchoscopy and EBUS-TBNA; however, the benefit of combining the procedures was clearly demonstrated. These results reflect the experience of a single centre which performs a relatively high number of EBUS-TBNA procedures (>200) each year. The patients were unselected and were recruited consecutively, thus reflecting clinical practice. Of note, the additional benefit of EBUS-TBNA in patients with suspected stage III and stage IV sarcoidosis cannot be extrapolated from these results and requires further clarification. As these radiographical stages are not associated with enlarged lymph nodes, it is likely that the benefit from additional EBUS-TBNA samples will be lower in these patients.
In conclusion, the combination of EBUS-TBNA with standard bronchoscopic techniques is safe and feasible, and optimizes the diagnostic yield in patients with pulmonary sarcoidosis and enlarged intrathoracic lymph nodes. EBUS-TBNA in combination with standard bronchoscopy may be considered to be a first-line investigation in patients with suspected sarcoidosis and enlarged intrathoracic lymphadenopathy.
SUMMARY AT A GLANCE.
Transbronchial and endobronchial biopsies are recommended as initial procedures for the diagnosis of pulmonary sarcoidosis. In this study of patients with suspected stage I and stage II sarcoidosis, the addition of EBUS-TBNA to standard bronchoscopic techniques was safe and resulted in a significant improvement in diagnostic yield.
ACKNOWLEDGEMENTS
We would like to thank the patients who volunteered for this study and the multidisciplinary teams at the Whittington and North Middlesex University Hospitals. Neal Navani is supported by an MRC Clinical Research Training Fellowship. S.M.J. is a Wellcome Trust Senior Fellow in Clinical Science. This study was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme (S.M.J.).
REFERENCES
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N. Engl. J. Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Bilaceroglu S, Perim K, Gunel O, et al. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch. Chest Dis. 1999;54:217–23. [PubMed] [Google Scholar]
- 3.Shorr AF, Torrington KG, Hnatiuk OW. Endobronchial involvement and airway hyperreactivity in patients with sarcoidosis. Chest. 2001;120:881–6. doi: 10.1378/chest.120.3.881. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest. 2009;136:340–6. doi: 10.1378/chest.08-2768. [DOI] [PubMed] [Google Scholar]
- 5.Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur. Respir. J. 2007;29:1182–6. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 6.Oki M, Saka H, Kitagawa C, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863–8. doi: 10.1111/j.1440-1843.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 7.Garwood S, Judson MA, Silvestri G, et al. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest. 2007;132:1298–304. doi: 10.1378/chest.07-0998. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis—comparisons with other bronchoscopic diagnostic modalities. Respir. Med. 2009;103:1796–800. doi: 10.1016/j.rmed.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Morgan RK, Eberhardt R, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann. Thorac. Surg. 2008;85:1874–8. doi: 10.1016/j.athoracsur.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–23. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 11.Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–8. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer S, Milne DG, Zeng I, et al. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax. 2009;64:436–9. doi: 10.1136/thx.2008.105031. [DOI] [PubMed] [Google Scholar]
- 13.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl. 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 14.Reich JM, Brouns MC, O’Connor EA, et al. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest. 1998;113:147–53. doi: 10.1378/chest.113.1.147. [DOI] [PubMed] [Google Scholar]
- 15.Trisolini R, Tinelli C, Cancellieri A, et al. Transbronchial needle aspiration in sarcoidosis: yield and predictors of a positive aspirate. J. Thorac. Cardiovasc. Surg. 2008;135:837–42. doi: 10.1016/j.jtcvs.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Trisolini R, Lazzari AL, Cancellieri A, et al. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2004;21:147–51. [PubMed] [Google Scholar]
- 17.Tournoy KG, Bolly A, Aerts JG, et al. The value of endoscopic ultrasound after bronchoscopy to diagnose thoracic sarcoidosis. Eur. Respir. J. 2010;35:1329–35. doi: 10.1183/09031936.00111509. [DOI] [PubMed] [Google Scholar]