Abstract
The possible role of β-2 adrenergic receptors in modulation of inflammatory and nociceptive conditions suggests that the β-2 adrenergic receptor agonist, salbutamol, may have beneficial anti-inflammatory and analgesic effects. Therefore, in this study, we induced inflammatory and nociceptive responses with carrageenan-induced paw edema or cotton-pellet-induced granuloma models, both of which result in oxidative stress. We hypothesized that salbutamol would prevent inflammatory and nociceptive responses by stimulating β-2 adrenergic receptors and the prevention of generation of ROS during the acute inflammation process in rats. Both doses of salbutamol used in the study (1 and 2 mg/kg) effectively blocked the acute inflammation and inflammatory nociception induced by carrageenan. In the cotton-pellet-induced granuloma test, both doses of salbutamol also significantly decreased the weight of granuloma tissue on the cotton pellets when compared to the control. Anti-inflammatory and analgesic effects of salbutamol were found to be comparable with those of indomethacin. Salbutamol decreased myeloperoxidase (MPO) activity and lipid peroxidation (LPO) level and increased the activity of superoxide dismutase (SOD) and level of glutathione (GSH) during the acute phase of inflammation. In conclusion, salbutamol can decrease acute and chronic inflammation, possibly through the stimulation of β-2 adrenergic receptors. This anti-inflammatory effect may be of significance in asthma treatment, where inflammation also takes part in the etiopathology. This study reveals that salbutamol has significant antioxidative effects, which at least partially explain its anti-inflammatory capabilities. These findings presented here may also shed light on the roles of β-2 adrenergic receptors in inflammatory and hyperalgesic conditions.
1. Introduction
Inflammatory diseases such as rheumatoid arthritis, hepatitis, and asthma are major causes of morbidity in humans. Chronic inflammation is now well known to also lead to the development of cancer [1], cardiovascular diseases [2], and neurodegenerative diseases [3]. In inflammatory diseases, the most common complaint of the patient is accompanying nociception and fever. Although nonsteroidal anti-inflammatory drugs (NSAIDs), especially indomethacin, are the drugs of choice in the treatment of inflammatory diseases [4] and are highly effective, they have a number of deleterious side effects, such as gastrointestinal ulcers and even bleeding [4, 5]. So investigators are still looking for new analgesic drugs with fewer side effects for the treatment of inflammation and nociception.
The most common mechanism for the anti-inflammatory and analgesic effects of NSAIDs is via the inhibition of prostaglandin synthesis by the COX enzyme [6]. On the other hand, some other mechanisms such as the L-arginine/nitric oxide pathway or the serotonergic system have also been suggested for analgesic effects of NSAIDs [7, 8]. In addition, Lizarraga and Chambers claim a role for the opioidergic system in the analgesic effect mechanism of NSAIDs [9]. However, recent studies now claim a role for β-2 adrenergic receptors in the anti-inflammatory and analgesic effects of NSAIDs [10, 11]. A role for adrenergic β-2 receptors has been previously demonstrated in inflammatory conditions [12, 13]. While there are some publications that indicate that activation of β-2 adrenergic receptors may be involved in the increased nociceptive sensitivity [14], and inflammatory hyperalgesia [15] recent studies suggested that β-2 adrenergic receptor activation can inhibit nociception and inflammation [16–18].
Recent literature on a possible role of β-2 adrenergic receptors in modulation of inflammatory and nociceptive conditions led us to hypothesize that β-2 adrenergic receptor agonists, such as salbutamol, may provide anti-inflammatory and analgesic relief for nociceptive inflammatory conditions. Salbutamol is a well-known drug that is commonly used in the treatment of bronchial asthma [19]. Salbutamol selectively binds to and activates β-2 adrenergic receptors on the surface of many cells. The inhibitory effect on inflammatory processes is seen primarily for CD4 cells but also for other leucocytes with a high density of β-2 receptors such as monocytes, macrophages, and Langerhans cells [20–22]. Also anti-inflammatory effects of β-2 adrenergic receptors on pulmonary inflammation models [23] support the role of β-2 adrenergic receptors in inflammatory conditions.
The binding of the β-2 receptor agonist to these cells inhibits activation of the expression of inflammatory genes and thereby their proinflammatory cytokines, such as interleukin-2 and interferon-γ. Salbutamol also inhibits superoxide generation and peroxidase release from stimulated human granulocytes [24]. These effects can be investigated for their therapeutic potential using inflammation models. For example, one hour after subcutaneous injection of carrageenan into the rat paws nociception model or inflammation model, in which vascular permeability increases and leukocyte migration occurs, involves inflammatory mediators including neutrophil-derived active oxygen species and free radicals, such as hydrogen peroxide, superoxide and the hydroxyl radical [25–27] nitric oxide, prostaglandins, and cytokines [28]. Also, neutrophil accumulation liberated proinflammatory mediators such as cytokines, including TNF-α and IL-1β, are considered to be proinflammatory agents that stimulate the cellular chemotaxis and serve to further increase tissue inflammation [29]. However, no studies have yet investigated the anti-inflammatory and analgesic potential of salbutamol in relation to these oxidative parameters.
Therefore, in the present study, we induced inflammatory and nociceptive responses with carrageenan-induced paw edema and with cotton-pellet-induced granuloma models. Both of these treatments result in oxidative stress, and we hypothesized that salbutamol would prevent inflammatory and nociceptive responses by stimulating β-2 adrenergic receptors and reducing the generation of reactive oxygen species (ROS) during acute inflammation process in rats.
2. Materials and Methods
2.1. Animals
In this study, we used a total of 90 male Albino Wistar rats obtained from the Medical Experimental Research Centre, Atatürk University (ATADEM). The animals weighed between 200 and 220 g and were fed under normal temperature conditions (22°C) in separate groups before the experiments. Animal experiments were performed in accordance with the national guidelines for the use and care of laboratory animals, and the study was approved by the local animal care committee of Atatürk University.
2.2. Chemicals
All chemicals for laboratory experimentation, including carrageenan, were purchased from Sigma Chemical (Germany). Thiopental sodium was purchased from IE Ulagay A. S. Istanbul, Turkey; indomethacin was purchased from Deva, Turkey; salbutamol was purchased from GlaxoSmithKline, and propranolol was purchased from Sanofi Aventis, Istanbul, Turkey.
2.3. Carrageenan-Induced Paw Edema in Rats
In the first series of experiments, the anti-inflammatory effects of salbutamol and indomethacin on carrageenan-induced paw edema were studied in rats [30]. The rats were divided into 4 groups (n = 6) for experimental procedure. Three rat groups received salbutamol 1 mg/kg, salbutamol 2 mg/kg, or indomethacin 25 mg/kg by oral gavage. Rat doses of salbutamol differ from 3 μg to 60 mg/kg [31, 32]. In this study we selected 1 and 2 mg/kg doses of salbutamol [33, 34]. In acute inflammation model and hyperalgesia model we studied indomethacin at 25 mg/kg dose, which has been previously used [35–37]. The reason why we used the 25 mg/kg dose in acute experiments is that we aimed to compare the effects of salbutamol with the highest dose of the reference drug. All drugs were suspended in distilled water as vehicle. So the control group (4th group) received an equal volume of distilled water as vehicle. One hour after drug administration, 0.1 mL of 1% carrageenan was injected into the hind paw of each rat in each group. Before the carrageenan injection, the normal paw volumes of the rats were measured with a plethysmometer. The carrageenan-induced increase in the paw volume was measured four times at one-hour intervals. Namely, the paw volumes were measured for every 60 minutes times four hours after carrageenan injection [38–40]. The effects of the drugs were determined by comparing the results of the drug-treated groups with those of the control group. At the end of the experiment, paw tissues of all animals, as well as from an additional untreated group of healthy animals, were collected for biochemical examination. All of the paw tissues were immediately transferred to −80°C.
In the second series of experiments we investigated whether anti-inflammatory activity of salbutamol is related to β-2 adrenergic receptor stimulation or not. For this purpose a total of 12 rats were divided into 2 groups (n = 6). The first rat group received 40 mg/kg dose of propranolol, which was suspended in distilled water by oral gavage. One hour after propranolol administration the rat group received 2 mg/kg dose of salbutamol by oral gavage. The control group (2nd group) received an equal volume of distilled water as vehicle, and anti-inflammatory activities were determined as described above.
2.4. Carrageenan-Induced Inflammatory Paw Hyperalgesia in Rats
In this series of experiments, the analgesic effects of salbutamol and indomethacin on carrageenan-induced inflammatory paw hyperalgesia were studied in intact rats [41]. The rats were divided into 4 groups (n = 6). Drug administration and carrageenan treatment were repeated exactly as described in Section 2.3. Prior to carrageenan injection, the normal nociceptive thresholds of the rats were measured with a Basile algesimeter that measures mechanical reflex threshold. Carrageenan-induced decrease in the nociceptive threshold was measured three times at one-hour intervals. Namely, the nociceptive thresholds were measured for every 60 minutes times three times after carrageenan injection. The analgesic effects of the drugs were determined by comparing the results of the drug-treated groups with those of the control group.
2.5. Cotton Pellet Granuloma Test
In this part of experiment, we used 24 rats divided into 4 groups to examine the effects of salbutamol and indomethacin on the proliferative phase of inflammation [42]. For this purpose we used the cotton pellet test, which is a chronic inflammation model used for evaluating the antiproliferative effects of drugs [42, 43]. In this model, a short time after the initiation of acute inflammation, proliferative cells developed and inflammation became chronic. Monocyte-macrophages infiltration and fibroblast proliferation occur in chronic inflammation [44]. Also in the cotton-pellet-induced chronic inflammation model, cotton pellet, which we applied in interscapular area, induced a chronic inflammation process. In this process monocyte migration, liquid accumulation, apoptosis, damage and so on will occur in the surrounding tissue of the pellets and these accumulations will produce a granulation tissue that covers the pellets. Salbutamol at 1 mg/kg and 2 mg/kg doses was administered to the first two groups of rats, and 5 mg/kg of indomethacin [45] was given orally with the aid of gavages to a third group. The reason why we used 5 mg/kg dose of indomethacin in chronic administration is that high dose (25 mg/kg) indomethacin has quite harmful effects on stomach resulting in stomach bleeding and even death in chronic administrations. All drugs were suspended in distilled water as vehicle. The control group received an equal volume of distilled water. Thirty minutes after the administration of drugs, rats were anesthetized with 20 mg/kg of thiopental sodium. Cotton pellets, weighing 7 ± 1 mg and prepared under sterile conditions, were then implanted subcutaneously (sc) in the interscapular area. Drugs were administered once a day for a period of 7 days. On the 8th day, rats were euthanized with a high-dose (50 mg/kg) of thiopental sodium. Cotton pellets with the granuloma tissue that involves migrated monocytes, accumulated liquid, and fibroblasts were removed and weighed. Effects of the drugs on chronic inflammation were determined by comparing the results obtained for the test groups with the results of the control group.
2.6. Biochemical Estimations
After the macroscopic analyses, superoxide dismutase (SOD) and myeloperoxidase (MPO) enzyme activities and the glutathione (GSH) and lipid peroxidation (LPO) levels in rat paw tissues were determined. To prepare the tissue homogenates, whole paw tissues were ground with liquid nitrogen in a mortar. The ground tissues (0.5 g each) were then treated with 4.5 mL of appropriate buffer. The mixtures were homogenized on ice using an ultraturrax homogenizer (IKA-Germany) for 15 min. Homogenates were filtered and centrifuged by using a refrigerated centrifuge at 4°C. Then, these supernatants were used for determination of the enzymatic activities. All assays were carried out at room temperature in triplicate.
2.6.1. Superoxide Dismutase Activity
As outlined by Sun et al. [46] superoxide dismutase estimation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with nitro blue tetrazolium to form formazan dye. Superoxide dismutase activity was then measured at 560 nm as the degree of inhibition of this reaction and was expressed as millimoles per minute per milligram tissue (mmol/min/mg tissue).
2.6.2. Myeloperoxidase Activity
Myeloperoxidase activity was measured according to a modified method of Bradley et al. [47]. The homogenized samples were frozen and thawed three times and centrifuged at 1500 g for 10 min at 4°C. Myeloperoxidase activity in the supernatant was determined by adding 100 mL of the supernatant to 1.9 mL of 10 mmol/L phosphate buffer (pH 6.0) and 1 mL of 1.5 mmol/L o-dianisidine hydrochloride containing 0.0005% (wt/vol) hydrogen peroxide. The changes in absorbance at 450 nm for each sample were recorded on a UV-Vis spectrophotometer. Myeloperoxidase activity in tissues was expressed as micromoles per minute per milligram tissue (μmol/min/mg tissue).
2.6.3. Total Glutathione (GSH) Determination
The amount of GSH in the paw tissues was measured according to the method of Sedlak and Lindsay [48]. The paw tissue homogenized in 2 mL of 50 mM Tris-HCl buffer containing 20 mM EDTA and 0.2 M sucrose, pH 7.5. The homogenate was centrifuged at 4200 rpm for 40 min at 4°C, and then the supernatant was used to determine GSH using 5,5-dithiobis(2-nitrobenzoic acid). Absorbance was measured at 412 nm using a spectrophotometer. The results of the GSH level in the rat paw tissues were expressed as nanomoles per milligram tissue (nmol/mg tissue).
2.6.4. Determination of Lipid Peroxidation Level
Lipid peroxidation levels in paw tissues were determined by estimating malondialdehyde (MDA) using the thiobarbituric acid test [49]. Briefly, the paw tissues were promptly excised and rinsed with cold saline. To minimize the possibility of interference of hemoglobin with free radicals, any adhering blood or bristles on the epidermis were carefully removed. The paw tissues were weighed and homogenized in 10 mL of 100 g/L KCl. The homogenate (0.5 mL) was added to a solution containing 0.2 mL of 80 g/L sodium lauryl sulfate, 1.5 mL of 200 g/L acetic acid, and 1.5 mL of 8 g/L 2-thiobarbiturate and 0.3 mL distilled water. The mixture was incubated at 98°C for 1 h. Upon cooling, 5 mL of n-butanol/pyridine (15 : 1) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4000 rpm. The supernatant absorbance was measured at 532 nm. A standard curve was generated using 1,1,3,3-tetramethoxypropane. All samples were measured in triplicate. The results were expressed as nmol MDA per gram wet tissue (nmol/g tissue).
2.7. Statistical Analyses
Data for acute and chronic inflammation models and acute nociceptive thresholds model were subjected to one-way analysis of variance (ANOVA) using SPSS 13.0 software. Only the data for propranolol examination were subjected to “two-independent-sample t-test.” Differences among the groups were obtained using the LSD option and were considered significant at P < 0.05. A statistical analysis of oxidative enzymes was carried out using one-way ANOVA followed by Duncan's multiple range test (DMRT) using the SPSS software package, version 13.00, and were considered significant at P < 0.05. All the results were expressed as mean ± SE.
3. Results
3.1. Carrageenan-Induced Paw Edema in Rats
As seen in Table 1, both doses of salbutamol and indomethacin significantly decreased carrageenan-induced paw edema formation in rats. The anti-inflammatory effects of 1 mg/kg dose of salbutamol were determined as 43.9%, 43.4%, 44%, and 37.5%, respectively, for the 1st, 2nd, 3rd, and 4th hours. For the same hours, a 2 mg/kg dose of salbutamol produced 44.6%, 48.1%, 48.5%, and 48.8% anti-inflammatory effects, respectively. In comparison, the anti-inflammatory effects of indomethacin were 27.7%, 40.3%, 42.7%, and 48.4%, respectively, for the same time intervals.
Table 1.
Effects of salbutamol and indomethacin on carrageenan-induced inflammatory paw edema in rats.
| Drugs | Increase in inflammatory paw volume (mL) | Anti-inflammatory effect | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st h | 2nd h | 3rd h | 4th h | 1st h | 2nd h | 3rd h | 4th h | |
| SAL-1 | 0.14 ± 0.02* | 0.28 ± 0.13* | 0.27 ± 0.05* | 0.27 ± 0.03* | 43.9 | 43.4 | 44 | 37.5 |
| SAL-2 | 0.13 ± 0.03* | 0.26 ± 0.05* | 0.25 ± 0.04* | 0.22 ± 0.03* | 44.6 | 48.1 | 48.5 | 48.8 |
| IND-25 | 0.19 ± 0.01* | 0.29 ± 0.06* | 0.28 ± 0.02* | 0.22 ± 0.02* | 27.7 | 40.3 | 42.7 | 48.4 |
| Control | 0.25 ± 0.03 | 0.49 ± 0.06 | 0.49 ± 0.02 | 0.43 ± 0.02 | — | — | — | — |
SAL-1: salbutamol 1 mg/kg; SAL-2: salbutamol 2 mg/kg; IND: indomethacin 25 mg/kg. *Significant at P < 0.05 when compared to control. (All groups received an intraplantar injection of 0.1 mL, 1% carrageenan.)
In the second series of our experiments we determined that 40 mg/kg dose of propranolol reversed the anti-inflammatory effect of salbutamol (2 mg/kg). Namely, salbutamol could not inhibit inflammation formation when β-adrenergic receptors were blocked (Figure 1).
Figure 1.
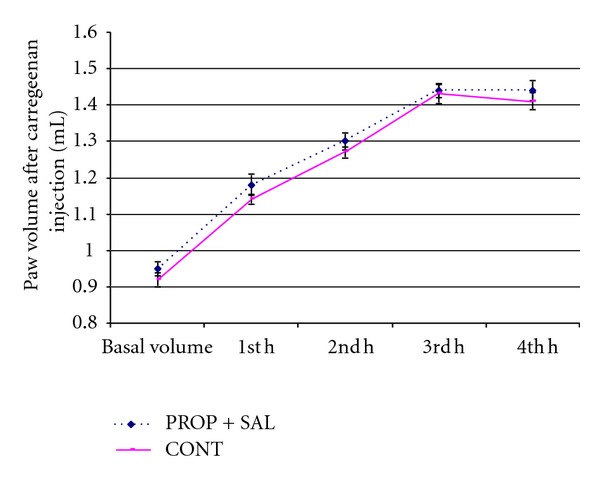
Effects of combination of salbutamol (2 mg/kg) and propranolol (40 mg/kg) (PROP + SAL) on carrageenan-induced inflammatory paw volume in rats. CONT: control.
3.2. Carrageenan-Induced Paw-Hyperalgesia in Rats
As seen in Table 2, both doses of salbutamol and indomethacin significantly prevented the carrageenan-induced decrease in nociceptive thresholds in rat paws till the 3rd hour of carrageenan injection. In the 1st hour after carrageenan injection, the 1 and 2 mg doses of salbutamol and 25 mg/kg dose of indomethacin produced 57%, 61.1%, and 71.6% analgesic effects, respectively. The same doses of the drugs produced 44.2%, 47.8%, and 55% analgesic effects in the 2nd hour. In the 3rd hour the analgesic effects of salbutamol were lower than those in 1st and 2nd hours (36.9% for 1 mg/kg dose and 42.9% for 2 mg/kg dose). In the 3rd hour, indomethacin produced a 58.5% analgesic effect.
Table 2.
Effects of salbutamol and indomethacin on carrageenan-induced inflammatory paw nociception in rats.
| Drugs | Decrease in nociceptive threshold (g) | Analgesic effect (%) | ||||
|---|---|---|---|---|---|---|
| 1st h | 2nd h | 3rd h | 1st h | 2nd h | 3rd h | |
| SAL-1 | 18.2 ± 1.3* | 25.7 ± 2.4* | 30.2 ± 3.3* | 57 | 44.2 | 36.9 |
| SAL-2 | 16.7 ± 2.7* | 24.0 ± 4.8* | 27.3 ± 4.1* | 61.1 | 47.8 | 42.9 |
| IND | 12.2 ± 2.2* | 20.7 ± 4.4* | 19.8 ± 5.1* | 71.6 | 55 | 58.5 |
| Control | 42.8 ± 5.4 | 46.0 ± 2.5 | 47.8 ± 2.7 | — | — | — |
SAL-1: salbutamol 1 mg/kg; SAL-2: salbutamol 2 mg/kg; IND: indomethacin 25 mg/kg. *Significant at P < 0.05 when compared to control. (All groups received an intraplantar injection of 0.1 mL, 1% carrageenan.)
3.3. Cotton Pellet Test in Rats
On the 8th day, mean weights of moist pellets removed from the rat groups administered salbutamol (1 and 2 mg/kg) and indomethacin (5 mg/kg) and the control group were 94.0 ± 4.1 mg, 116.7 ± 3.0 mg, 41.3 ± 3.4 mg, and 168.7 ± 4.3 mg, respectively. According to these results, the effects of salbutamol 1 mg/kg, salbutamol 2 mg/kg, and indomethacin on chronic inflammation were evaluated as 44.3%, 30.8%, and 75.5%, respectively (Table 3).
Table 3.
Effects of salbutamol and indomethacin cotton pellet granuloma test.
| Drugs | Dose (mg/kg) | Initial weight of the cotton pellets (mg) | Wet weight of cotton pellets that were removed after 8 days (mg) | Inhibition in granuloma formation (%) |
|---|---|---|---|---|
| Salbutamol | 1 | 7 ± 1 | 94.0 ± 4.1** | 44.3 |
| Salbutamol | 2 | 7 ± 1 | 116.7 ± 3.0* | 30.8 |
| Indomethacin | 5 | 7 ± 1 | 41.3 ± 3.4** | 75.5 |
| Control | — | 7 ± 1 | 168.7 ± 4.3 | — |
*Significant at P < 0.05 when compared to control, **significant at P < 0.01 when compared to control.
3.4. Biochemical Analyses
Carrageenan injection to rat paws produced a significant increase in MPO activity and LPO level. However both doses of salbutamol significantly prevented the carrageenan induced increase in theses parameters (P < 0.05). Indomethacin administration also significantly decreased (P < 0.05) the MPO activity and LPO level when compared to control group that received carrageenan alone. The 2 mg/kg dose of salbutamol was more effective in decreasing MPO activity and LPO level than the 1 mg/kg dose of salbutamol or the 25 mg/kg dose of indomethacin (Figures 3 and 4). Carrageenan treatment resulted in a significant decrease in the activity of SOD and level of GSH, which were increased by salbutamol and indomethacin administration (P < 0.05). The 2 mg/kg dose of salbutamol was the best of the three drug treatments in terms of increasing SOD activity and GSH level (Figures 2, 3, 4, and 5).
Figure 3.
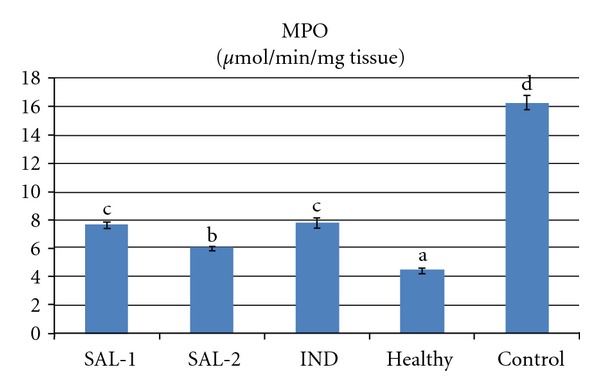
Effects of salbutamol (SAL) and indomethacin (IND) on myeloperoxidase (MPO) activity in carrageenan-injected paw tissues. Means in the same column by the same letter are not significantly different and the means in the same column by different letters demonstrate significant differences between the groups according to the Duncan test (α = 0.05). In the above figure, the letter for the means in SAL-1 and IND columns is the same: “c.” This demonstrates that values in these lines are not statistically different from each other. However, the lines with the letter “c” are statistically significant from the lines with the letters “a,” “b,” and “d.”
Figure 4.
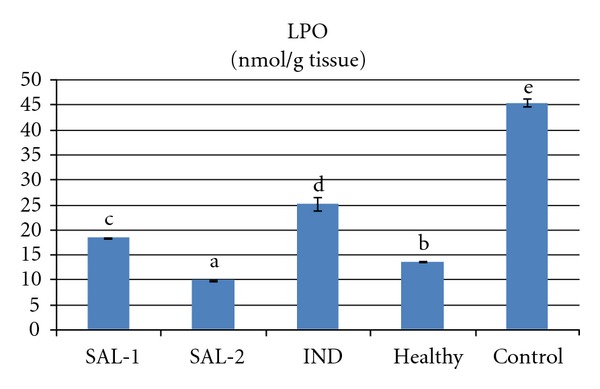
Effects of salbutamol (SAL) and indomethacin (IND) on lipid peroxidation (LPO) level in carrageenan-injected paw tissues. Means in the same column by the same letter are not significantly different and the means in the same column by different letters demonstrates significant differences between the groups according to the Duncan test (α = 0.05). In the above figure, all columns have different letters. This demonstrates that values in these columns are statistically different from each other.
Figure 2.
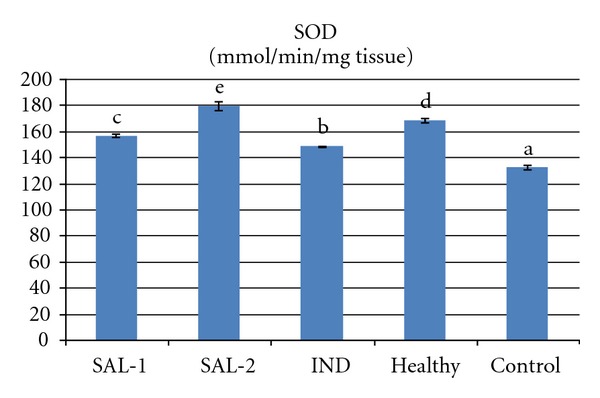
Effects of salbutamol (SAL) and indomethacin (IND) on superoxide dismutase (SOD) activity in carrageenan-injected paw tissues. Means in the same column by the same letter are not significantly different and the means in the same column by different letters demonstrate significant differences between the groups according to the Duncan test (α = 0.05). In the above figure, all columns have different letters. This demonstrates that values in these columns are statistically different from each other.
Figure 5.
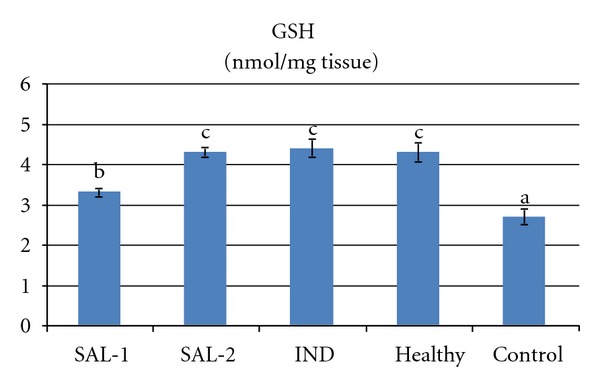
Effects of salbutamol (SAL) and indometacin (IND) on reduced glutathione (GSH) level in carrageenan-injected-paw tissues. Means in the same column by the same letter are not significantly different and the means in the same column by different letters demonstrate significant differences between the groups according to the Duncan test (α = 0.05). In the above figure, the letter for the means in SAL-2, IND, and healthy columns is the same: “c.” This demonstrates that values in these lines are not statistically different from each other. However, the lines with the letter “c” are statistically significant from the lines with the letters “a” and “b.”
4. Discussion
This study investigated the protective effect of salbutamol, a β-2 adrenergic receptor agonist drug used in bronchial asthma, on acute (carrageenan-induced) and chronic (cotton pellet induced) inflammation models and on a carrageenan-induced nociception model. Tissue levels and activities of LPO, GSH, MPO, and SOD were used to estimate antioxidant effects.
Our study demonstrated that both doses of salbutamol (1 and 2 mg/kg) effectively reduced the acute inflammation and inflammatory nociception associated with carrageenan injection. Carrageenan application is known to produce an inflammatory response that peaks at three hours, resulting in hyperalgesia [50]. In this experimental inflammation model, levels of inflammatory mediators have been reported to increase fourfold between the 1st and 3rd hours after a carrageenan injection and then to remain high for several hours thereafter [51]. In the present study, the preventive effect of salbutamol on inflammation and related nociception formation was comparable with that of indomethacin, a potent anti-inflammatory drug. Both salbutamol and indomethacin exerted anti-inflammatory and analgesic effects; however, it is known that indomethacin has quite harmful effects on stomach tissue such as ulcer, perforation, and even bleeding. The ratio of occurrence for the side effects is approximately 35–50%, and as a result of present side effects 20% of patients are forced to stop indomethacin therapy [52]. Long-acting beta agonists have also side effects in high doses on cardiovascular system by activating sympathetic system. However, these side effects are not severe in salbutamol usage because it has a short half-life [53]. So salbutamol may be safer than indomethacin in inflammation treatment.
Salbutamol selectively activates the β-2 adrenergic receptors and is clinically used for treatment of acute and chronic asthma [19]. In our previous studies, we suggested that β-2 adrenergic receptors may play a role in the suppression of inflammation as stimulation of these receptors would produce anti-inflammatory and, consequently, analgesic effects [10, 11]. There is also some evidence that catecholamines suppress immune cell functions in inflammatory tissues and that they produce this suppressive effect via activation of β-adrenergic receptors [12]. In another study, adrenergic agents were shown to suppress the immune response (the production of TNF-α) via the direct stimulation of β-adrenergic receptors on inflammatory immune cells [13]. Also some recent studies suggested that β-2 adrenergic receptor activation can inhibit nociception and inflammation [16–18]. These previous reports concerning the roles of β-2 adrenergic receptors in inflammatory conditions support a preventive role for salbutamol in inflammatory and related hyperalgesic conditions, as postulated in the present study.
The effects of salbutamol and indomethacin on chronic phases of inflammation in the cotton pellet granuloma test of intact rats were also supportive of this hypothesis. The cotton pellet test is a chronic inflammation model used for evaluating the antiproliferative effects of drugs [43]. Both doses of salbutamol significantly decreased the weight of cotton pellets when compared to the control. A short time after the initiation of acute inflammation, proliferative cells developed and inflammation became chronic. Prevention of collagen fiber formation and suppression of mucopolysaccharides are indicators of the antiproliferative effect of anti-inflammatory agents [54]. Monocyte infiltration and fibroblast proliferation occur in chronic inflammation instead of neutrophil infiltration and exudation [44]. Activated monocyte-macrophages are blood cells that have antitumor and antimicrobial functions in addition to phagocytotic functions against pathogens [55]. Salbutamol selectively binds to and activates β-2-adrenoceptors, which are molecules on the surface of many cells, such as CD4 cells, leukocytes, monocytes, macrophages, and Langerhans cells [20–22]. The binding of a β-2 receptor agonist to these cells results in stimulation of the receptor and inhibition of expression of inflammatory genes. This prevents the production of proinflammatory cytokines, such as interleukin-2 and interferon-γ [24, 56], and effectively suppresses inflammation. Therefore, the primary effects of salbutamol on the chronic phase of inflammation may be associated with its effects on the β-2 adrenergic receptors located on the monocytes and macrophages that comprise the basic components of chronic inflammation. In contrast to our hypothesis Oliveira et al. and Pelegrini-da-Silva et al. suggested that serotonin-induced inflammatory hyperalgesia and temporomandibular joint inflammation are mediated by sympathetic amines-dependent mechanism that involves the activation of peripheral β-2 adrenergic receptors [15, 57]. However, expression of β-2 adrenergic receptors within the nociceptive system suggested their potential implication in nociception and pain and studies suggesting that β-2 adrenergic receptor agonists may potentially offer an alternative therapy to antidepressant drugs for the chronic treatment of neuropathic pain [17, 18, 58]. These data bring up such a dilemma: “Are central β2 adrenergic receptors involved in the analgesic effects of salbutamol or is it only consequence of the reduction in the inflammatory process?”. In this point, previous studies which claimed that surgical stress induces sympathetically activated release of endogenous opioids from inflammatory cells and subsequent analgesia via activation of peripheral opioid receptors [59] make us hypothesize that salbutamol shows its antihyperalgesic effects by inhibition of inflammation and peripheral sympathetic activation. Also in our study propranolol, a β-adrenergic receptor antagonist, administration reversed the anti-inflammatory effects of salbutamol suggesting that salbutamol mediated its anti-inflammatory effects via β-2 adrenergic receptors. However, future studies comparing effects of both peripheral and central β-2 adrenergic receptors are required for a better understanding.
An acute inflammatory process is comprised of inflammation mediators including neutrophil-derived ROS, nitric oxide [60, 61] prostaglandins, and cytokines [62]. Also neutrophil accumulation liberated proinflammatory mediators such as cytokines, including TNF-α and IL-1β, are considered to be proinflammatory agents that stimulate the cellular chemotaxis and serve to further increase tissue inflammation [29]. ROS play an important role in the pathogenesis of many diseases, such as rheumatoid arthritis, local or systemic inflammatory disorders, ischemia-reperfusion injury, atherosclerosis, cancer, and respiratory distress syndrome [61, 63–65]. In respiratory diseases such as asthma, selective stimulation of β-2 adrenergic receptors results in NO production [66], which suggests that salbutamol produces its bronchodilator effects by stimulating β-2 adrenergic receptors, resulting in activated NO production [67–69]. Salbutamol also inhibits superoxide generation and peroxidase release from stimulated human granulocytes [24]. However, the effects of salbutamol on other parameters related to oxidative stress in inflammatory conditions have not yet been evaluated in detail in inflammatory conditions.
Our study investigated effects of salbutamol on some oxidative parameters such as MPO and SOD activities and LPO and GSH levels during the acute phase of inflammation. In inflamed tissues, activities of MPO and LPO are significantly increased. In the present case, both doses of salbutamol and indomethacin decreased the carrageenan-induced aggravation of MPO and LPO. MPO is an enzyme found primarily in azurophilic granules of neutrophils and is used as a marker for tissue neutrophil content. Its inhibition implies the presence of anti-inflammatory activity [47, 70]. Tissue MPO activity is a sensitive and specific marker for acute inflammation and reflects polymorphonuclear cell infiltration of the parenchyma. In accordance with the literature [71, 72], MPO activity in the present study significantly increased in the paw at the 4th hour after carrageenan injection when compared to healthy control rats. A variety of anti-inflammatory drugs (e.g., diclofenac, indomethacin, naproxen, piroxicam, and tenoxicam) have been shown to similarly depress the increases in myeloperoxidase activity during inflammation [73, 74].
Lipid peroxidation has been reported to increase in inflammatory conditions [38, 75, 76]. As a marker of oxidative damage, lipid peroxidation indicates changes in membrane fluidity and permeability and thus increases in rates of protein degradation, which will eventually lead to cell lysis [77]. Increased concentrations of LPO in tissue have been reported in the carrageenan-induced inflammation model [78]. In our study, LPO content was high in carrageenan-induced inflamed paws; however, salbutamol administration prevented this increase in the LPO content of the paws.
Tissue damage related to oxidative stress can be reversed via SOD enzyme and GSH. The action of these parameters limits the cytotoxic effects of toxic free radicals [79, 80]. In our study, salbutamol also significantly increased both GSH content and SOD activity in inflammatory paws compared to control paws. In many laboratory models and in a few clinical trials, SOD has proven to be therapeutically useful in protecting injured tissues (e.g., by ischemia, inflammation, hyperoxia, etc.) from one of these active oxygen species, the superoxide radical [80]. Preventive effects of salbutamol on superoxide generation and peroxidase release from stimulated human granulocytes [24] also supports our results. GSH has pleiotropic roles including the maintenance of cells in a reduced state, serving as an electron donor for certain antioxidative enzymes (e.g., glutathione peroxidase), and in the formation of conjugates with some harmful endogenous and xenobiotic compounds via catalysis of glutathione s-transferase [79]; thus, the ameliorating effects of salbutamol on GSH demonstrated a further beneficial effect of its administration. These results may also suggest that salbutamol attenuated the carrageenan-induced inflammation by preventing oxidative stress.
In conclusion, salbutamol, a bronchodilator agent used for asthma treatment, can effectively decrease both acute (carrageenan-induced) and chronic (cotton-pellet-induced) inflammation and propranolol reversed the anti-inflammatory effects of salbutamol. Stimulation of β-2 adrenergic receptors may be the underlying mechanism responsible for the observed anti-inflammatory effects. Since inflammation also takes part in asthma etiopathology, these observations may be of clinical relevance. Salbutamol also exerted significant antioxidative effects, which could at least partially explain the mechanism underlying its anti-inflammatory effects. This study may also shed light on the roles of β-2 adrenergic receptors in inflammatory and algesic conditions.
Acknowledgments
This research was conducted in the Laboratory of Pharmacology Ataturk Faculty of Medicine, University, and the Laboratory of Biochemistry and Laboratory of Pharmacology at Faculty of Pharmacy, Ataturk University.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Archives of Neurology. 2001;58(11):1790–1792. doi: 10.1001/archneur.58.11.1790. [DOI] [PubMed] [Google Scholar]
- 4.Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33(4):224–234. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PW, Tee L, McBride J, Quincey D, Liddiard GS. Long-term NSAID use in primary care: changes over a decade and NICE risk factors for gastrointestinal adverse events. Rheumatology. 2005;44(10):1308–1310. doi: 10.1093/rheumatology/kei016. [DOI] [PubMed] [Google Scholar]
- 6.Vanegas H, Schaible HG. Prostaglandins and cyclooxygenases [correction of cycloxygenases] in the spinal cord. Progress in Neurobiology. 2001;64(4):327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Reval MI, Ventura-Martinez R, Deciga-Campos M, Terron JA, Cabre F, Lopez-Munoz FJ. Involvement of serotonin mechanisms in the antinociceptive effect of S(+)-ketoprofen. Drug Development Research. 2002;57(4):187–192. [Google Scholar]
- 8.Ventura-Martinez R, Deciga-Campos M, Diaz-Reval MI, Gonzalez-Trujano ME, Lopez-Munoz FJ. Peripheral involvement of the nitric oxide-cGMP pathway in the indomethacin-induced antinociception in rat. European Journal of Pharmacology. 2004;503(1–3):43–48. doi: 10.1016/j.ejphar.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Lizarraga I, Chambers JP. Involvement of opioidergic and α2-adrenergic mechanisms in the central analgesic effects of non-steroidal anti-inflammatory drugs in sheep. Research in Veterinary Science. 2006;80(2):194–200. doi: 10.1016/j.rvsc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Suleyman H, Halici Z, Cadirci E, Hacimuftuoglu A, Bilen H. Indirect role of β2-adrenergic receptors in the mechanism of anti-inflammatory action of NSAIDs. Journal of Physiology and Pharmacology. 2008;59(4):661–672. [PubMed] [Google Scholar]
- 11.Cadirci E, Suleyman H, Hacimuftuoglu A, Halici Z, Akcay F. Indirect role of β2-adrenergic receptors in the mechanism of analgesic action of nonsteroidal antiinflammatory drugs. Critical Care Medicine. 2010;38(9):1860–1867. doi: 10.1097/CCM.0b013e3181e8ae24. [DOI] [PubMed] [Google Scholar]
- 12.Pettipher ER, Eskra JD, Labasi JM. The inhibitory effect of rolipram on TNF-α production in mouse blood ex vivo is dependent upon the release of corticosterone and adrenaline. Cytokine. 1997;9(8):582–586. doi: 10.1006/cyto.1997.0205. [DOI] [PubMed] [Google Scholar]
- 13.Szelenyi J, Kiss JP, Puskas E, Szelenyi M, Vizi ES. Contribution of differently localized alpha(2)- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic. Neuroimmunomodulation. 2000;917(36):145–153. doi: 10.1111/j.1749-6632.2000.tb05378.x. [DOI] [PubMed] [Google Scholar]
- 14.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both β2- and β3-adrenergic receptors. Pain. 2007;128(3):199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelegrini-da-Silva A, Oliveira MC, Parada CA, Tambeli CH. Nerve growth factor acts with the β2-adrenoceptor to induce spontaneous nociceptive behavior during temporomandibular joint inflammatory hyperalgesia. Life Sciences. 2008;83(23-24):780–785. doi: 10.1016/j.lfs.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Choucair-Jaafar N, Yalcin I, Rodeau JL, Waltisperger E, Freund-Mercier MJ, Barrot M. β2-Adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. British Journal of Pharmacology. 2009;158(7):1683–1694. doi: 10.1111/j.1476-5381.2009.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yalcin I, Tessier LH, Petit-Demoulière N, et al. Chronic treatment with agonists of β2-adrenergic receptors in neuropathic pain. Experimental Neurology. 2010;221(1):115–121. doi: 10.1016/j.expneurol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Yalcin I, Choucair-Jaafar N, Benbouzid M, et al. β-2 adrenoceptors are critical for antidepressant treatment of neuropathic pain. Annals of Neurology. 2009;65(2):218–225. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- 19.Prenner BM. Role of long-acting β2-adrenergic agonists in asthma management based on updated asthma guidelines. Current Opinion in Pulmonary Medicine. 2008;14(1):57–63. doi: 10.1097/MCP.0b013e3282f27121. [DOI] [PubMed] [Google Scholar]
- 20.Baramki D, Koester J, Anderson AJ, Borish L. Modulation of T-cell function by (R)- and (S)-isomers of albuterol: anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomer. Journal of Allergy and Clinical Immunology. 2002;109(3):449–454. doi: 10.1067/mai.2002.122159. [DOI] [PubMed] [Google Scholar]
- 21.Volcheck GW, Kelkar P, Bartemes KR, Gleich GJ, Kita H. Effects of (R)- and (S)-isomers of β-adrenergic agonists on eosinophil response to interleukin-5. Clinical and Experimental Allergy. 2005;35(10):1341–1346. doi: 10.1111/j.1365-2222.2005.02347.x. [DOI] [PubMed] [Google Scholar]
- 22.Leff AR, Herrnreiter A, Naclerio RM, Baroody FM, Handley DA, Muñoz NM. Effect of enantiomeric forms of albuterol on stimulated secretion of granular protein from human eosinophils. Pulmonary Pharmacology and Therapeutics. 1997;10(2):97–104. doi: 10.1006/pupt.1997.0082. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Fievez L, Cheu E, et al. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. European Journal of Pharmacology. 2010;628(1–3):171–178. doi: 10.1016/j.ejphar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Jemec GB, Ullman S, Goodfield M, et al. A randomized controlled trial of R-salbutamol for topical treatment of discoid lupus erythematosus. British Journal of Dermatology. 2009;161(6):1365–1370. doi: 10.1111/j.1365-2133.2009.09330.x. [DOI] [PubMed] [Google Scholar]
- 25.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. Journal of Clinical Investigation. 1973;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga da Motta JI, Cunha FQ, Vargaftig BB, Ferreira SH. Drug modulation of antigen-induced paw oedema in guinea-pigs: effects of lipopolysaccharide, tumour necrosis factor and leucocyte depletion. British Journal of Pharmacology. 1994;112(1):111–116. doi: 10.1111/j.1476-5381.1994.tb13038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. European Journal of Pharmacology. 1996;303(3):217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 28.Gualillo O, Eiras S, Lago F, Diéguez C, Casanueva FF. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sciences. 2000;67(20):2433–2441. doi: 10.1016/s0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 29.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respiratory Research. 2001;2(2):66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 31.Fozard JR, Baur F, Wolber C, Collingwood SP. Inhibition by viozan of extravasation induced in rat trachea by capsaicin is mediated exclusively by β2-adrenoceptors. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2001;364(6):570–572. doi: 10.1007/s00210-001-0488-8. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Yamagishi R, Tsutsui M, et al. Tissue- and dose-dependent alteration of stress-inducible proteins by β2-adrenoceptor agonist, salbutamol, in rats. Journal of Toxicological Sciences. 2005;30(4):305–314. doi: 10.2131/jts.30.305. [DOI] [PubMed] [Google Scholar]
- 33.Hsu WH, Cooper CW. Hypercalcemic effect of catecholamines and its prevention by thyrocalcitonin. Calcified Tissue International. 1975;19(2):125–137. doi: 10.1007/BF02563997. [DOI] [PubMed] [Google Scholar]
- 34.Gopinath C, Gibson WA. Mesovarian leiomyomas in the rat. Environmental Health Perspectives. 1987;73:107–113. doi: 10.1289/ehp.8773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suleyman H, Cadirci E, Albayrak A, et al. Reason for the aggravation of diseases caused by inflammation and the ineffectiveness of NSAIDs on these diseases in rainy weather. Pharmacological Reports. 2009;61(3):514–519. doi: 10.1016/s1734-1140(09)70094-5. [DOI] [PubMed] [Google Scholar]
- 36.Ozbakis-Dengiz G, Halici Z, Akpinar E, Cadirci E, Bilici D, Gursan N. Role of polymorphonuclear leukocyte infiltration in the mechanism of anti-inflammatory effect of amiodarone. Pharmacological Reports. 2007;59(5):538–544. [PubMed] [Google Scholar]
- 37.Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal inflammatory drug. Current Medicinal Chemistry. 2008;15(3):278–283. doi: 10.2174/092986708783497247. [DOI] [PubMed] [Google Scholar]
- 38.Odabasoglu F, Halici Z, Cakir A, et al. Beneficial effects of vegetable oils (corn, olive and sunflower oils) and α-tocopherol on anti-inflammatory and gastrointestinal profiles of indomethacin in rats. European Journal of Pharmacology. 2008;591(1–3):300–306. doi: 10.1016/j.ejphar.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 39.Albayrak A, Polat B, Cadirci E, et al. Gastric anti-ulcerative and anti-inflammatory activity of metyrosine in rats. Pharmacological Reports. 2010;62(1):113–119. doi: 10.1016/s1734-1140(10)70248-6. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food and Chemical Toxicology. 2010;48(1):61–64. doi: 10.1016/j.fct.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives Internationales de Pharmacodynamie et de Therapie. 1957;111(4):409–419. [PubMed] [Google Scholar]
- 42.Suleyman H, Halici Z, Hacimuftuoglu A, Gocer F. Role of adrenal gland hormones in antiinflammatory effect of calcium channel blockers. Pharmacological Reports. 2006;58(5):692–699. [PubMed] [Google Scholar]
- 43.Panthong A, Kanjanapothi D, Taesotikul T, Phankummoon A, Panthong K, Reutrakul V. Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre. Journal of Ethnopharmacology. 2004;91(2-3):237–242. doi: 10.1016/j.jep.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. Journal of Ethnopharmacology. 2000;73(3):379–385. doi: 10.1016/s0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- 45.Olajide OA, Makinde JM, Okpako DT. Evaluation of the anti-inflammatory property of the extract of Combretum micranthum G. Don (Combretaceae) Inflammopharmacology. 2003;11(3):293–298. doi: 10.1163/156856003322315631. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 47.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 48.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry C. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 49.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 50.Romero A, Planas E, Poveda R, Sánchez S, Pol O, Puig MM. Anti-exudative effects of opioid receptor agonists in a rat model of carrageenan-induced acute inflammation of the paw. European Journal of Pharmacology. 2005;511(2-3):207–217. doi: 10.1016/j.ejphar.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Shaffer A, Portanova J, Seibert K, Isakson PC. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. Journal of Pharmacology and Experimental Therapeutics. 1997;283(3):1069–1075. [PubMed] [Google Scholar]
- 52.Burke A, Smyth EM, FitzGerald GA. Analgesic-antipyretic agents; pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. New York, NY, USA: McGraw-Hill; 2005. pp. 671–717. [Google Scholar]
- 53.Undem BJ. Pharmacotherapy of asthma. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. New York, NY, USA: McGraw-Hill; 2005. pp. 617–637. [Google Scholar]
- 54.Vajja BN, Juluri S, Kumari M, Kole L, Chakrabarti R, Joshi VD. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. International Immunopharmacology. 2004;4(7):901–909. doi: 10.1016/j.intimp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Nacife VP, Soeiro Mde N, Gomes RN, D’Avila H, Castro-Faria Neto HC, Meirelles Mde N. Morphological and biochemical characterization of macrophages activated by carrageenan and lipopolysaccharide in vivo. Cell Structure and Function. 2004;29(2):27–34. doi: 10.1247/csf.29.27. [DOI] [PubMed] [Google Scholar]
- 56.Wulf HC, Ullman S. Discoid and subacute lupus erythematosus treated with 0.5% R-salbutamol cream. Archives of Dermatology. 2007;143(12):1589–1590. doi: 10.1001/archderm.143.12.1589. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira MC, Pelegrini-da-Silva A, Parada CA, Tambeli CH. 5-HT acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience. 2007;145(2):708–714. doi: 10.1016/j.neuroscience.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 58.Barrot M, Yalcin I, Choucair-Jaafar N, Benbouzid M, Freund-Mercier MJ. From antidepressant drugs to beta-mimetics: preclinical insights on potential new treatments for neuropathic pain. Recent Patents on CNS Drug Discovery. 2009;4(3):182–189. doi: 10.2174/157488909789104794. [DOI] [PubMed] [Google Scholar]
- 59.Kager I, Mousa SA, Sieper J, Stein C, Pipam W, Likar R. Blockade of intra-articular adrenergic receptors increases analgesic demands for pain relief after knee surgery. Rheumatology International. 2010;31(10):1299–1306. doi: 10.1007/s00296-010-1489-z. [DOI] [PubMed] [Google Scholar]
- 60.Saha K, Lajis NH, Israf DA, et al. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. Journal of Ethnopharmacology. 2004;92(2-3):263–267. doi: 10.1016/j.jep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. The New England Journal of Medicine. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 63.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomedicine and Pharmacotherapy. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Cadirci E, Oral A, Odabasoglu F, et al. Atorvastatin reduces tissue damage in rat ovaries subjected to torsion and detorsion: biochemical and histopathologic evaluation. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2010;381(5):455–466. doi: 10.1007/s00210-010-0504-y. [DOI] [PubMed] [Google Scholar]
- 65.Halici Z, Karaca M, Keles ON, et al. Protective effects of amlodipine on ischemia-reperfusion injury of rat ovary: biochemical and histopathologic evaluation. Fertility and Sterility. 2008;90(6):2408–2415. doi: 10.1016/j.fertnstert.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Figueroa XF, Poblete I, Fernandez R, Pedemonte C, Cortes V, Huidobro-Toro JP. NO production and eNOS phosphorylation induced by epinephrine through the activation of beta-adrenoceptors. American Journal of Physiology. 2009;297(1):H134–H143. doi: 10.1152/ajpheart.00023.2009. [DOI] [PubMed] [Google Scholar]
- 67.Priest RM, Hucks D, Ward JP. Potentiation of cyclic AMP-mediated vasorelaxation by phenylephrine in pulmonary arteries of the rat. British Journal of Pharmacology. 1999;127(1):291–299. doi: 10.1038/sj.bjp.0702525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Priest RM, Hucks D, Ward JPT. Noradrenaline, β-adrenoceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. British Journal of Pharmacology. 1997;122(7):1375–1384. doi: 10.1038/sj.bjp.0701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang HY. Role of nitric oxide in vasodilator response induced by salbutamol in rat diaphragmatic microcirculation. American Journal of Physiology. 1997;272(5):H2173–H2179. doi: 10.1152/ajpheart.1997.272.5.H2173. [DOI] [PubMed] [Google Scholar]
- 70.Cadirci E, Suleyman H, Aksoy H, et al. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chemico-Biological Interactions. 2007;170(1):40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 71.Saleh TS, Calixto JB, Medeiros YS. Effects of anti-inflammatory drugs upon nitrate and myeloperoxidase levels in the mouse pleurisy induced by carrageenan. Peptides. 1999;20(8):949–956. doi: 10.1016/s0196-9781(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 72.Odabasoglu F, Halici Z, Aygun H, et al. alpha-Lipoic acid has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced acute and cotton pellet-induced chronic inflammations. British Journal of Nutrition. 2011;105(1):31–43. doi: 10.1017/S0007114510003107. [DOI] [PubMed] [Google Scholar]
- 73.Lee CS, Jang YY, Song JS, Song JH, Han ES. Ambroxol inhibits peroxynitrite-induced damage of alpha1-antiproteinase and free radical production in activated phagocytic cells. Pharmacology & Toxicology. 2002;91(3):140–149. doi: 10.1034/j.1600-0773.2002.910309.x. [DOI] [PubMed] [Google Scholar]
- 74.Paino IM, Ximenes VF, da Fonseca LM, Kanegae MP, Khalil NM, Brunetti IL. Effect of therapeutic plasma concentrations of non-steroidal anti-inflammatory drugs on the production of reactive oxygen species by activated rat neutrophils. Brazilian Journal of Medical and Biological Research. 2005;38(4):543–551. doi: 10.1590/s0100-879x2005000400007. [DOI] [PubMed] [Google Scholar]
- 75.Halici Z, Dengiz GO, Odabasoglu F, Suleyman H, Cadirci E, Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. European Journal of Pharmacology. 2007;566(1–3):215–221. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 76.Odabasoglu F, Aygun H, Halici Z, et al. Effect of alpha-lipoic acid on myeloperoxidase activity and lipid peroxidation level in carrageenan-injected rats. The FEBS Journal. 2007;274:269–269. [Google Scholar]
- 77.García JJ, Reiter RJ, Guerrero JM, et al. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Letters. 1997;408(3):297–300. doi: 10.1016/s0014-5793(97)00447-x. [DOI] [PubMed] [Google Scholar]
- 78.Tanas S, Odabasoglu F, Halici Z, et al. Evaluation of anti-inflammatory and antioxidant activities of peltigera rufescens lichen species in acute and chronic inflammation models. Journal of Natural Medicines. 2010;64(1):42–49. doi: 10.1007/s11418-009-0367-z. [DOI] [PubMed] [Google Scholar]
- 79.Meister A, Anderson ME. Glutathione. Annual Review of Biochemistry. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 80.McCord JM. Superoxide production and human disease. In: Jesaitis A, Dratz E, editors. Molecular Basis of Oxidative Damage by Leukocytes. Boca Raton, Fla, USA: CRC; 1992. pp. 225–239. [Google Scholar]


