Abstract
Orthodontics is a branch of dentistry that aims at the resolution of dental malocclusions. The specialist carries out the treatment using intraoral or extraoral orthodontic appliances that require forces of a given load level to obtain a tooth movement in a certain direction in dental arches. Orthodontic tooth movement is dependent on efficient remodeling of periodontal ligament and alveolar bone, correlated with several biological and mechanical responses of the tissues surrounding the teeth. A periodontal ligament placed under pressure will result in bone resorption whereas a periodontal ligament under tension results in bone formation. In the primary stage of the application of orthodontic forces, an acute inflammation occurs in periodontium. Several proinflammatory cytokines are produced by immune-competent cells migrating by means of dilated capillaries. In this paper we summarize, also through the utilization of animal models, the role of some of these molecules, namely, interleukin-1β and vascular endothelial growth factor, that are some proliferation markers of osteoclasts and osteoblasts, and the macrophage colony stimulating factor.
1. Orthodontic Movement and Inflammation
Orthodontic movement is correlated with an inflammatory process, that, in concert with the mechanical responses of periodontal and oral tissues, is essential for achieving tooth movement clinically. Early effects of orthodontic forces are of physical [1] and biological nature, and they involve extracellular matrix and cells of the alveolar bone, periodontal ligament, blood vessels, and neural elements. As consequence, many changes occur in these structures and various molecules are produced or inducted, as well as cytokines, growth factors, colony-stimulating factors, and neurotransmitters [2]. The first stages before the orthodontic force application are characterized by an acute inflammatory response. In the periodontium this process involves the vasodilatation of capillaries which allows the migration of leucocytes in the periodontal tissue, where they are induced by biochemical signals to synthesize and to secrete several proinflammatory cytokines and chemokines, growth factors and enzymes.
Orthodontic appliances impose forces on the teeth with a predetermined direction, and tooth movement occurs through different phases. The biological responses of hard tissue to mechanical loadings around the tooth are different between a tension area and a compression area (Figure 1). Mechanical forces are transduced to the cells triggering the biologic response by means of an aseptic transitory inflammatory process that involves several inflammatory mediators. This succession of local events is the base for achieving the remodeling of the parodontium, so to allow the tooth movement [3].
Figure 1.
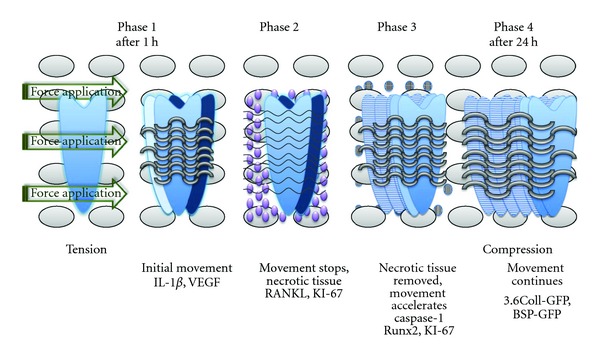
Phases of tooth movement associated with the application of orthodontic forces.
The following paragraphs of the paper are intended to focus on the role of various chemical and cellular factors primarily present after the application of orthodontic forces.
The goal of this paper is to provide to orthodontic clinicians and educators some basic information and the summary of some updated studies about the correlation between tooth movement and inflammatory process. Understanding of these molecular phenomena is crucial to share the notion that orthodontic tooth movement is “the biologic response to interference in the physiologic equilibrium of the dentofacial complex by an externally applied force” [4].
1.1. Interleukine-1β Induction at Primary Stage of Orthodontic Tooth Movement
Cytokine expression in the rat periodontal ligament at the initial stage of orthodontic tooth movement has been investigated to evaluate the change of periodontal ligament. Interleukin (IL)-1β is one of the most abundant cytokines in the periodontal environment during the initial stage of orthodontic tooth movement because of its direct implications in alveolar bone resorption induced in the pressure side by mechanical loading [5].
IL-1β takes part in the survival, fusion, and activation of osteoclasts and exerts an important role since the amount of tooth movement correlates with the efficiency of bone remodeling in the alveolar process [6]. Higher levels of expression of inflammatory cytokines and of their respective receptors had been shown after an inducted inflammatory process by perforating the buccal cortical plate of orthodontically treated rats. The concentration of IL-1β mRNA in the rats' periodontal ligament is increased within 3 h after orthodontic force loading, particularly on pressure side [7]. The soft tissues surrounding the teeth are involved in orthodontic tooth shift such as the hard tissues, periodontal ligament, and alveolar bone, and changes in gingival contour always follow tooth movement. After the application of mechanical loadings, IL-1β mRNA in pressure side gingival is significantly increased in a rat model under orthodontic treatment [8].
IL-1β can bind two types of receptors, IL-1RI and IL-1RII [9]. Only the former is examined in this context [10, 11] since the latter acts only as a “decoy” target for the cytokine. The particular function as proinflammatory cytokine of IL-1β has been demonstrated by the administration of exogenous interleukin (IL)-1 receptor antagonist (IL-1Ra) in mice undergoing orthodontic treatment [12]. The level of IL-1β decreased by 66% in mice treated with IL-Ra therapy, and this associated with a reduction of the number of osteoclasts in the pressure side of periodontal tissues after histological characterization, and with a less rate of tooth displacement. These results showed that IL-1Ra has an anti-inflammatory role that leads to a downregulation of the orthodontic tooth movement.
The production of IL-1β is inducted from the processing and the activation of a pro-IL-1β by a protease, the caspase-1. Indeed, an apoptosis process occurs in conjunction with the inflammatory one that eliminates the hyalinized periodontal tissue formed during the early stages of orthodontic movement. Caspase-1 is the most important mediator of inflammation and apoptosis responses, activated by inflammatory signals as alterations in the intracellular ionic milieu. In a rat model under orthodontic treatment, caspase-1 expression is increased, and the level of caspase-1 changes with different temporal phases of orthodontic tooth movement [13]. If the local orthodontic application of force is excessive, or else if in the body there is an hyper-expression of caspase-1 by a kind of diseases like rheumatoid arthritis, an irreversible root resorption and a deformation of periodontal tissues might appear. Researchers propose that, because of the primary role of caspase-1 in inflammatory response due to orthodontic tooth movement, a method to preserve the structure of periodontal ligament may be the administration of the inhibitors of caspase-1 activity such as VX-765 [14] and Pralnacasan [15].
1.2. VEGF Localization during Orthodontic Tooth Movement in Animal Models
Vascular endothelial growth factor is the primary mediator of angiogenesis and it increases vascular permeability. This cytokine is involved in tissue neoformation that is strictly correlated with the presence of blood vessels. During orthodontic tooth movement, compressive forces induce angiogenesis of periodontal ligament together with the role of mediator of the VEGF. The localization of VEGF was analyzed in vivo in rat periodontal tissues during experimental tooth movement. In this analysis, 15 male Wistar rats were used. A compressive force at 150 mN was applied by means of a uniform standardized compressive spring placed between the right and left upper first molars in each rat's mouths. The maxillary bone was removed by the animals and it was analyzed with immunohistochemical staining. In the experimental animals, VEGF immunoreactivity was in vascular endothelial cells, osteoblasts, osteoclasts in resorption lacunae, in fibroblasts adjacent to hyalinized tissue, a local necrotic area in compressed zone, and in mononuclear cells in periodontal tissues from the animals [16]. VEGF mRNA was also detected in fibroblasts and osteoblasts in tension area of mice periodontal ligament during experimental tooth orthodontic movement [17]. The protocol included 10 mice, divided between experimental and control animals, and provided the analysis of premaxillary bone frontal sections [18]. Therefore, VEGF exerts a fundamental role in remodeling periodontal ligament and is also involved in bone resorption and formation.
1.3. Relation among Some Markers of Bone Cell Proliferation and M-CSF with Orthodontic Movement
There are other studies that examine a variety of proliferation markers expressed during orthodontic tooth movement. For instance, the high presence of the antigen KI-67, nuclear protein associated with cellular proliferation and ribosomal RNA transcription, and of RANKL, a key factor for osteoclast differentiation and activation [19, 20], indicates the recruitment of osteoclasts in compression areas [21], whereas, the expression of Runx2, a transcription factor associated with osteoblast differentiation, shows the increase of differentiated osteoblasts in tension areas [6]. In other studies, researchers analyzed the collagen type 1 (3.6Col1) and the bone sialoprotein (BSP) in periodontal ligament, using transgenic mice containing transgenes of these promoters fused with green fluorescent proteins (GFP), and they discovered that 3.6Col1-GFP and BSP-GFP cells have an increase on the tension side of the periodontal ligament [22, 23].
Another important role in tooth movement is played by the macrophage colony-stimulating factor (M-CSF), an early osteoclast differentiation factor, that increases the rate of osteoclastic recruitment and differentiation [24].
In particular, optimal dosages of M-CSF correlated with measurable changes in tooth movement and gene expression, providing potential for clinical studies in accelerating tooth movement.
2. Rats as Models for Orthodontic Movement
Up to now, a large number of studies in various species of animals, such as cats, dogs, and rats, have been done to enlighten the biological response to periodontal ligament. Rats are the most used animals for studying tooth movement, even if there are advantages and disadvantages [25]. Among the disadvantages, it must be remembered that the alveolar bone of rats is more dense than in humans, and there are no osteons. Indeed, the osteoid tissue along the alveolar bone surface in rats is less, their bone extracellular matrix has a few mucopolysaccharides, and, finally, the calcium concentration is more controlled by intestinal absorption. Disparities have been reported also in the arrangement of the peritoneal fibers and in the supporting structures, as in the root formations, which seem to be faster. Notwithstanding these disadvantages, rats are considered a good model to study orthodontic tooth movement. Indeed, they are relatively inexpensive, the histological preparation of their material is easier than other animals, and transgenic strains are almost exclusively developed in small rodents.
Clinical studies show that there are different phases in tooth movement. The application of force during orthodontic tooth movement results in bone resorption by osteoclasts and deposition by osteoblasts on the pressure and tension sides of the periodontal ligament. Recent studies in mice demonstrate that preosteoclasts, and not monocytes, may be recruited to the periodontal ligament during orthodontic tooth movement, and these cells may be targeted for acceleration of tooth movement.
3. Concluding Remarks
Knowledge regarding the biological mechanisms involved in orthodontic tooth movement appears to be of considerable importance for orthodontists that may modulate mechanoresponses and inflammatory process, accelerating or decelerating tooth movement, by adding various exogenous substances, taking also in consideration the condition of health of each orthodontically treated subject.
Acknowledgment
The authors thank R. De Lucia for her support in the drafting of the paper.
References
- 1.Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. Journal of Dental Research. 2009;88(7):597–608. doi: 10.1177/0022034509338914. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V. Cellular, molecular, and tissue-level reactions to orthodontic force. American Journal of Orthodontics and Dentofacial Orthopedics. 2006;129(4):469.e1–469.e32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. European Journal of Oral Sciences. 2007;115(5):355–362. doi: 10.1111/j.1600-0722.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 4.Proffit WR. Biologic basis of orthodontic therapy. In: Proffit WR, Fields HW, editors. Contemporary Orthodontics. 3rd edition. St. Louis, Mo, USA: Mosby; 2000. [Google Scholar]
- 5.Bletsa A, Berggreen E, Brudvik P. Interleukin-1 and tumor necrosis factor-α expression during the early phases of orthodontic tooth movement in rats. European Journal of Oral Sciences. 2006;114(5):423–429. doi: 10.1111/j.1600-0722.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira CC, Khoo E, Tran J, et al. Cytokine expression and accelerated tooth movement. Journal of Dental Research. 2010;89(10):1135–1141. doi: 10.1177/0022034510373764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba S, Kuroda N, Arai C, Nakamura Y, Sato T. Immunocompetent cells and cytokine expression in the rat periodontal ligament at the initial stage of orthodontic tooth movement. Archives of Oral Biology. 2011;56(5):466–473. doi: 10.1016/j.archoralbio.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Lee TY, Lee KJ, Baik HS. Expression of IL-1,MMP-9 and TIMP-1 on the pressure side of gingiva under orthodontic loading. Angle Orthodontist. 2009;79(4):733–739. doi: 10.2319/031308-145.1. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Jin HM, Kim K, et al. The mechanism of osteoclast differentiation induced by IL-1. Journal of Immunology. 2009;183(3):1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki LR, Haack JE, Nickel JC, Reinhardt RA, Petro TM. Human interleukin-1b and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Archives of Oral Biology. 2001;46(2):185–189. doi: 10.1016/s0003-9969(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki LR, Chandler JR, Marx DB, Pandey JP, Nickel JC. IL-1 gene polymorphisms, secretion in GCF, and speed of human tooth orthodontic movement. Orthodontics and Craniofacial Research. 2009;12(2):129–140. doi: 10.1111/j.1601-6343.2009.01446.x. [DOI] [PubMed] [Google Scholar]
- 12.Salla JT, Taddei SRA, Queiroz-Junior CM, et al. The effect of IL-1 receptor antagonist on orthodontic tooth movement in mice. Archives of Oral Biology. 2012;57(5):519–524. doi: 10.1016/j.archoralbio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Chen J, Hao Y, Wang Y, Zhu L. Changes of caspase-1 after the application of orthodontic forces in the periodontal tissues of rats. Angle Orthodontist. 2009;79(6):1126–1132. doi: 10.2319/100508-519R.1. [DOI] [PubMed] [Google Scholar]
- 14.Stack J, Beaumont K, Larsen PD, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. Journal of Immunology. 2005;175(4):2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 15.Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis and Cartilage. 2003;11(10):738–746. doi: 10.1016/s1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 16.Miyagawa A, Chiba M, Hayashi H, Igarashi K. Compressive force induces VEGF production in periodontal tissues. Journal of Dental Research. 2009;88(8):752–756. doi: 10.1177/0022034509341637. [DOI] [PubMed] [Google Scholar]
- 17.Kaku M, Motokawa M, Tohma Y, et al. VEGF and M-CSF levels in periodontal tissue during tooth movement. Biomedical Research. 2008;29(4):181–187. doi: 10.2220/biomedres.29.181. [DOI] [PubMed] [Google Scholar]
- 18.Kaku M, Kohno S, Kawata T, et al. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. Journal of Dental Research. 2001;80(10):1880–1883. doi: 10.1177/00220345010800100401. [DOI] [PubMed] [Google Scholar]
- 19.Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Archives of Oral Biology. 2007;52(3):244–250. doi: 10.1016/j.archoralbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthodontics and Craniofacial Research. 2009;12(2):113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PJ, Nilforoushan D, Manolson MF, Simmons CA, Gong SG. Molecular markers of early orthodontic tooth movement. Angle Orthodontist. 2009;79(6):1108–1113. doi: 10.2319/121508-638R.1. [DOI] [PubMed] [Google Scholar]
- 22.Uribe F, Kalajzic Z, Bibko J, et al. Early effects of orthodontic forces on osteoblast differentiation in a novel mouse organ culture model. Angle Orthodontist. 2011;81(2):284–291. doi: 10.2319/052410-279.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson C, Uribe F, Kalajzic Z, et al. Orthodontic tooth movement causes decreased promoter expression of collagen type-1, bone sialoprotein and alpha-smooth muscle actin in the periodontal ligament. Orthodontic Craniofacial Research. 2012;15:52–61. doi: 10.1111/j.1601-6343.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- 24.Brooks PJ, Heckler AF, Wei K, Gong SG. M-CSF accelerates orthodontic tooth movement by targeting preosteoclasts in mice. Angle Orthodontist. 2011;81(2):277–283. doi: 10.2319/051210-258.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement—a critical review and a proposed solution. European Journal of Orthodontics. 2004;26(5):483–490. doi: 10.1093/ejo/26.5.483. [DOI] [PubMed] [Google Scholar]


